Key Points
Empiric reduction or delay of doxorubicin in patients with disease-related hyperbilirubinemia may reduce disease control.
Pharmacokinetic analyses show doxorubicin plasma concentrations and toxicity are within the range of patients without hepatic impairment.
Abstract
Aggressive lymphomas are curable with doxorubicin-based chemotherapy. In patients presenting with elevated serum bilirubin, doxorubicin is commonly dose reduced or delayed based on limited pharmacokinetic data. We evaluated plasma pharmacokinetics of doxorubicin and its metabolite doxorubicinol as well as toxicity in 59 patients with normal bilirubin levels and 10 patients with elevated bilirubin levels. Patients received full-dose EPOCH +/−rituximab. Median (range) age was 51 (18-75) years. Patients with elevated bilirubin levels had higher international prognostic index and poorer performance status. Although median doxorubicin clearance was lower and median plasma doxorubicin and doxorubicinol concentrations were higher in patients with elevated bilirubin levels, values were within the concentration range observed in patients with normal levels. Rates of febrile neutropenia were similar between groups, but there was greater grade 4 neutropenia and thrombocytopenia during the first but not subsequent treatment cycles in patients with elevated bilirubin. More grade 3/4 gastrointestinal and neurotoxicity occurred in patients with elevated bilirubin during the first but not subsequent cycles. Although toxicity was greater on cycle 1, the adverse effects were managed safely. These results show that empiric dose reductions of continuous infusion doxorubicin may not be necessary in patients with elevated bilirubin levels. This trial was registered at www.clinicaltrials.gov as #NCT00001337, #NCT00069238, and #NCT00005780.
Introduction
Doxorubicinis essential for the curative treatment of aggressive lymphoma.1 Doxorubicin is metabolized in the liver, with approximately 80% excreted in the bile, and patients with cholestasis are reported to have delayed clearance with greater systemic toxicity.2-4 Although infrequent, patients with lymphoma may present with disease-related hepatic dysfunction, resulting in empiric dose reductions or delays due to concern over delayed metabolism and enhanced toxicity.5 The reasoning for these empiric dose modifications has not been prospectively examined or considered in relapsed/refractory patients.5-7 We performed a detailed doxorubicin pharmacokinetic (PK) and toxicity analysis in patients with and without hepatic dysfunction who received dose-adjusted (DA) EPOCH+/−R (etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin and +/−rituximab) for newly diagnosed aggressive lymphomas. DA-EPOCH+/−R contains all antineoplastic agents present in standard R-CHOP but administers doxorubicin over 96 hours compared with several minutes and dose adjusts based on the absolute neutrophil count (ANC) nadir.8
Methods
Patients with untreated aggressive lymphomas were enrolled in 1 of 3 phase 2 studies of DA-EPOCH+/− R between July 1999 and August 2004.8 Among 403 patients, 69 had PK sampling. All patients received full dose DA-EPOCH (doxorubicin 40 mg/m2) infused over 96 hours on cycle 1 regardless of hepatic function, with subsequent doses adjusted according to a standard algorithm (see supplement Table 1).8 Daily granulocyte-colony stimulating factor at 5 μg/kg per day rounded to 300 μg or 480 μg was started on day 6 and continued until ANC > 5 × 109/L. Pneumocystis jirovecii prophylaxis was required in all patients. Samples were collected during cycle 1 and all subsequent cycles: 0 hours (baseline), 22 hours, and 94 hours. Patients provided written informed consent in accordance with the Declaration of Helsinki, and the studies were approved by the Institutional Review Board.
Doxorubicin and its metabolite doxorubicinol were quantified by high-performance liquid chromatography with fluorescence detection after solid phase extraction. Doxorubicin clearance, doxorubicin steady-state concentrations (Css), and doxorubicinol Css were analyzed as previously described.9 Toxicity was graded on each cycle using CTCAE version 2.0.
Results and discussion
Of 69 patients with PK samples, 59 had normal and 10 had elevated total bilirubin levels (>1.0 mg/dL, the cutoff for normal at our institution). Patient characteristics were similar between the 2 groups except for a higher proportion of Eastern Cooperative Oncology Group performance status >2 and high/high-intermediate international prognostic index in the elevated bilirubin group (Table 1). Of the 10 patients, 2 had large extrahepatic periportal masses, 2 had nonspecific liver abnormalities, 1 had massive liver metastasis, 1 had numerous small hepatic lesions, 1 had hepatosplenomegaly without focal masses, and 4 had no computed tomography imaging evidence of liver abnormalities. All in all, 6 of the 10 patients had imaging signs of hepatic/perihepatic abnormality related to lymphoma. Of the 4 patients with normal liver findings on computed tomography, 2 had had a history of autoimmune hepatitis, 1 had Gilberts syndrome, and 1 had no other known cause of elevated bilirubin level and so was presumed to be related to lymphoma. Hepatitis B and C was checked; there were no cases of hepatitis C, and 1 patient with chronic hepatitis B was on lamuvidine. No concomitant medications were given that affected hepatic clearance.
Patient characteristics and toxicities in patients with elevated bilirubin and normal bilirubin levels prior to therapy
| Patient characteristics . | Elevated bilirubin . | Normal bilirubin . | P . | ||
|---|---|---|---|---|---|
| N = 10 . | N = 59 . | ||||
| Median age, y | 39 (18-56) | 52 (20-75) | .24 | ||
| Male sex (%) | 5/10 (50) | 30/59 (51) | 1.00 | ||
| Lymphoma type (%) | |||||
| B cell | 5/10 (50) | 52/59 (88) | .011 | ||
| T cell | 5/10 (50) | 7/59 (12) | |||
| Stage III/IV (%) | 10/10 (100) | 44/59 (75) | .10 | ||
| Elevated lactate dehydrogenase (%) | 7/10 (70) | 27/59 (46) | .19 | ||
| Involvement > 1 extranodal site (%) | 6/10 (60) | 30/59 (51) | .74 | ||
| Performance status ≥ 2 (%) | 7/10 (70) | 12/59 (20) | .0032 | ||
| IPI (intermediate high/high risk) (%) | 7/10 (70) | 21/59 (36) | .078 | ||
| Bilirubin, total (median) | 2.7 mg/dL | 0.5 mg/dL | n/a | ||
| Bilirubin, total (range) | 1.2-22.5 mg/dL | 0.2-1 mg/dL | |||
| Toxicity (390 cycles assessed) | |||||
| Cycle 1 | All cycles | Cycle 1 | All cycles | P (C1) | |
| ANC < 500/mm3 (% cycles) | 9/10 (90) | 29/44 (66) | 30/59 (51) | 195/337 (58) | .035 |
| Platelet < 25 × 103/mm3 (% cycles) | 4/10 (40) | 12/44 (27) | 4/59 (7) | 21/337 (6) | .013 |
| Febrile neutropenia (% cycles) | 2/10 (20) | 4/44 (9) | 5/59 (8) | 36/337 (11) | .27 |
| G3/4 GI toxicity and neurotoxicity (% cycles) | 3/10 (30) | 5/44 (11) | 8/59 (14) | 36/337 (11) | .19 |
| Response (%) | |||||
| Complete response | 7/10 (70) | 49/59 (83) | .38 | ||
| Partial response | 0/10 (0) | 3/59 (5) | 1.00 | ||
| Stable disease | 1/10 (10) | 1/59 (2) | .27 | ||
| Progressive disease | 2/10 (20) | 5/59 (8) | .27 | ||
| Patient characteristics . | Elevated bilirubin . | Normal bilirubin . | P . | ||
|---|---|---|---|---|---|
| N = 10 . | N = 59 . | ||||
| Median age, y | 39 (18-56) | 52 (20-75) | .24 | ||
| Male sex (%) | 5/10 (50) | 30/59 (51) | 1.00 | ||
| Lymphoma type (%) | |||||
| B cell | 5/10 (50) | 52/59 (88) | .011 | ||
| T cell | 5/10 (50) | 7/59 (12) | |||
| Stage III/IV (%) | 10/10 (100) | 44/59 (75) | .10 | ||
| Elevated lactate dehydrogenase (%) | 7/10 (70) | 27/59 (46) | .19 | ||
| Involvement > 1 extranodal site (%) | 6/10 (60) | 30/59 (51) | .74 | ||
| Performance status ≥ 2 (%) | 7/10 (70) | 12/59 (20) | .0032 | ||
| IPI (intermediate high/high risk) (%) | 7/10 (70) | 21/59 (36) | .078 | ||
| Bilirubin, total (median) | 2.7 mg/dL | 0.5 mg/dL | n/a | ||
| Bilirubin, total (range) | 1.2-22.5 mg/dL | 0.2-1 mg/dL | |||
| Toxicity (390 cycles assessed) | |||||
| Cycle 1 | All cycles | Cycle 1 | All cycles | P (C1) | |
| ANC < 500/mm3 (% cycles) | 9/10 (90) | 29/44 (66) | 30/59 (51) | 195/337 (58) | .035 |
| Platelet < 25 × 103/mm3 (% cycles) | 4/10 (40) | 12/44 (27) | 4/59 (7) | 21/337 (6) | .013 |
| Febrile neutropenia (% cycles) | 2/10 (20) | 4/44 (9) | 5/59 (8) | 36/337 (11) | .27 |
| G3/4 GI toxicity and neurotoxicity (% cycles) | 3/10 (30) | 5/44 (11) | 8/59 (14) | 36/337 (11) | .19 |
| Response (%) | |||||
| Complete response | 7/10 (70) | 49/59 (83) | .38 | ||
| Partial response | 0/10 (0) | 3/59 (5) | 1.00 | ||
| Stable disease | 1/10 (10) | 1/59 (2) | .27 | ||
| Progressive disease | 2/10 (20) | 5/59 (8) | .27 | ||
IPI, international prognostic index; n/a, there is no legitimate p-value since the categories are formed based on the bilirubin being within the normal or elevated range.
The pretreatment median total bilirubin was 2.7 mg/dL (1.5-22.5) and 0.5 mg/dL (0.2-1) in patients with and without hepatic dysfunction, respectively. Among patients with elevated bilirubin levels, median direct bilirubin was 1 mg/dL (0.3-16.5) and median indirect bilirubin was 2.1 mg/dL (0.5-6). The median bilirubin level improved to 1.6 mg/dL (0.4-8.1) after cycle 1. Nine of 10 patients with elevated bilirubin level improved after cycle 1, and all normalized by cycle 4 (supplement Table 2).
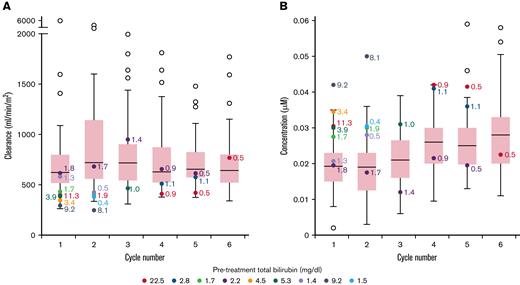
The median (range) doxorubicin clearance in patients with elevated vs normal bilirubin was 393 mL/min/m2 (294-616) vs 622 mL/min/m2 (262-5950) (P = .0048) during cycle 1. The median (range) of plasma doxorubicin Css at 96 hours were 0.029 (0.020-0.042) vs 0.019 (0.002-0.035) μM (P = .0036) (Figure 1), and doxorubicinol Css were 0.030 (0.018-0.050) vs 0.019 (0.003-0.036) μM (P = .0050) in patients with elevated vs normal bilirubin levels during cycle 1 (supplement Table 3). Notably, among patients with elevated bilirubin level, there was a significant correlation between pretreatment bilirubin levels and Css, indicating the degree of bilirubin elevation is a surrogate for these parameters. In patients with elevated bilirubin level, median doxorubicin clearance increased on subsequent cycles whereas patients with normal bilirubin level had similar clearance on all cycles. Over the course of treatment, the median plasma doxorubicin Css was higher in patients with elevated bilirubin level compared with patients with normal bilirubin level.
Box plot showing doxorubicin clearance and steady-state concentrations across all cycles. Range of doxorubicin clearance (A) and doxorubicin steady-state concentration (B) per cycle in all patients. For patients with normal bilirubin, each box represents 50% of the data, with the median value displayed as a horizontal line. The line extending from the box represents the normal range (± 1.5 times the interquartile distance). Open circles represent patients with normal bilirubin that are outside the reference range. Patients with elevated bilirubin are represented with colored circles. Numerical values beside each colored circle are bilirubin levels at the start of that cycle. The number of determinations for patients at each cycle is C1 = 49; C2 = 52; C3 = 45; C4 = 52; C5 = 50; C6 = 47.
Box plot showing doxorubicin clearance and steady-state concentrations across all cycles. Range of doxorubicin clearance (A) and doxorubicin steady-state concentration (B) per cycle in all patients. For patients with normal bilirubin, each box represents 50% of the data, with the median value displayed as a horizontal line. The line extending from the box represents the normal range (± 1.5 times the interquartile distance). Open circles represent patients with normal bilirubin that are outside the reference range. Patients with elevated bilirubin are represented with colored circles. Numerical values beside each colored circle are bilirubin levels at the start of that cycle. The number of determinations for patients at each cycle is C1 = 49; C2 = 52; C3 = 45; C4 = 52; C5 = 50; C6 = 47.
Although doxorubicin clearance was lower and Css of doxorubicin and doxorubicinol was higher in patients with elevated bilirubin level during cycle 1, these values were nonetheless within the range of values observed in patients with normal bilirubin concentrations.7,10 Furthermore, on subsequent cycles, the drug clearance increased in these patients, commensurate with normalization of bilirubin concentrations due to disease response.
To assess the clinical relevance of doxorubicin clearance, we analyzed toxicity on cycle 1 in patients with elevated vs normal pretreatment bilirubin concentrations (Table 1). The incidence of grade 4 neutropenia was higher on cycle 1 in patients with elevated bilirubin level but comparable over all subsequent cycles in the 2 groups. Febrile neutropenia was not statistically different between the 2 groups on cycle 1 (20% vs 8%) and over all cycles (9% vs 11%) for patients with and without elevated bilirubin levels. A difference in cycle 1 cannot be excluded secondary to low numbers. In contrast, the incidence of grade 4 thrombocytopenia was higher on cycle 1 (40% vs 7%) and over all cycles (27% vs 6%) in patients with elevated bilirubin level. However, this was not associated with an increased incidence of bleeding. Grades 3 and 4 gastrointestinal toxicity and neurotoxicity combined were also higher in patients with elevated bilirubin level (30% vs 14%) but only on cycle 1. Gastrointestinal toxicities included stomatitis and constipation; neurotoxicity included syncope and confusion, which are attributable to vincristine. Toxicities were assessed prior to each cycle, and every 3 months after completing treatment until death or loss to follow-up.
One patient in the elevated bilirubin group had a dose increase in chemotherapy from level 1 to level 2 based on protocol parameters for cycle 2, but all other patients stayed at the same dose as cycle 1 (dose level 1). In contrast, 45% of patients with normal bilirubin were able to escalate to dose level 2 on cycle 2. For subsequent cycles in the elevated bilirubin group, approximately half of patients continued to dose escalate, except for 1 patient who had a dose decrease. Most patients with normal bilirubin were able to dose escalate on cycles 3 to 6 (75% of patients during cycle 3 and approximately 80% on cycles 4-6). Although there were significantly more dose increases in patients with normal bilirubin levels, the complete remission rates between the 2 groups were similar (Table 1). Notably, among the patients with elevated pretreatment bilirubin levels, there was no correlation with the ANC nadir (r = 0.38; Spearman Correlation) or the occurrence of fever and neutropenia (P = .29; Wilcoxon rank sum test), indicating that the degree of bilirubin elevation is not predictive of toxicity.
Because we did not adjust the doses of other drugs in DA-EPOCH for bilirubin, we cannot rule out their contribution to the toxicity observed on cycle 1. Notably, however, we previously reported that the plasma clearance and Css of etoposide are unaffected in patients with elevated bilirubin level,11 as has been reported for cyclophosphamide.12
Although we observed an association between PK parameters and elevated bilirubin, the parameters were within the range measured in patients with normal bilirubin levels, and more severe toxicity was manageable in patients with elevated bilirubin levels. Although there was a significant correlation between pretreatment bilirubin levels and doxorubicin PK on cycle 1, the absence of correlation between bilirubin level and toxicity does not support adjusting the doxorubicin dose based on the bilirubin concentration. Thus, these results indicate that patients with secondary hepatic impairment due to lymphoma should be considered for full-dose infusional doxorubicin. Indeed, empiric dose reductions or delays of doxorubicin must be carefully weighed against adverse effects on the curative potential of treatment. These results cannot be extrapolated to R-CHOP, which used bolus dose doxorubicin. Although doxorubicin metabolism is not saturable, indicating clearance is unaffected by schedule, higher sustained concentrations associated with bolus vs infusional schedules may further increase toxicity in patients with elevated bilirubin levels.13
Authorship
Contribution: C.L. and W.H.W. conceived the idea for the paper; C.L., D.E.C., E.D., and N.L. completed data collection; C.L., S.M.S., E.D., B.C.W., and W.H.W. contributed to data analysis and interpretation; C.L. and W.H.W. wrote the initial draft; and all authors contributed to critical revision and final approval of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Catherine Lai, Leukemia Clinical Research Unit, Perelman Center for Advanced Medicine, University of Pennsylvania, 12 South Pavilion, 3400 Civic Center Blvd, Philadelphia, PA 19104; e-mail: catherine.lai@pennmedicine.upenn.edu.
References
Author notes
The data that support the findings of this study are available from the National Cancer Institute of the National Institutes of Health. Restrictions apply to the availability of these data as a government facility. Readers may also contact the corresponding author, Catherine Lai (catherine.lai@pennmedicine.upenn.edu).
The full-text version of this article contains a data supplement.