Key Points
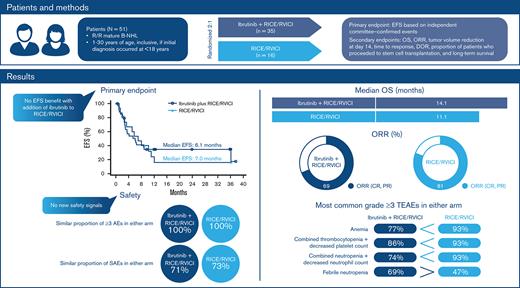
There was no benefit of ibrutinib added to RICE/RVICI in pediatric patients with relapsed/refractory mature B-cell non-Hodgkin lymphoma.
No new safety signals were observed.
Abstract
Part 1 results of the open-label, randomized, global phase 3 SPARKLE trial supported continued assessment of ibrutinib with either modified rituximab, ifosfamide, carboplatin, and etoposide (RICE) or rituximab, vincristine, ifosfamide, carboplatin, idarubicin, and dexamethasone (RVICI) in pediatric patients with relapsed/refractory (R/R) mature B-cell non-Hodgkin lymphoma (B-NHL). We report final results of Part 2 evaluating the efficacy of ibrutinib plus RICE or RVICI vs RICE/RVICI alone. Patients aged 1 to 30 years (initial diagnosis <18 years) were randomized 2:1 to receive ibrutinib with or without RICE/RVICI. Primary endpoint was event-free survival (EFS) based on independent committee-confirmed events. Fifty-one patients were enrolled. Median age was 15 years; Burkitt lymphoma, Burkitt leukemia, and Burkitt-like lymphoma (total: 45%) and diffuse large B-cell lymphoma/primary mediastinal B-cell lymphoma (51%) were the most common subtypes. At the preplanned interim analysis, median EFS was 6.1 vs 7.0 months with ibrutinib plus RICE/RVICI vs RICE/RVICI, respectively (hazard ratio, 0.9; 90% confidence interval, 0.5-1.6; P = .387); further enrollment was ceased. With ibrutinib plus RICE/RVICI vs RICE/RVICI, median overall survival was 14.1 vs 11.1 months, overall response rate was 69% vs 81%, and 46% vs 44% proceeded to stem cell transplantation. In both treatment arms, 100% of patients experienced grade ≥3 treatment-emergent adverse events. No EFS benefit was seen with ibrutinib. Salvage was generally poor in patients who received prior rituximab, regardless of treatment arm. No new safety signals were observed. Ibrutinib exposure in pediatric patients fell within the target range of exposure in adults. Trial is registered on www.clinicaltrials.gov (NCT02703272)
Introduction
Mature B-cell non-Hodgkin lymphoma (B-NHL) in children and young adults is the most common non-Hodgkin lymphoma (NHL) subtype.1,2 B-NHL accounts for ∼60% of all NHL in children and young adults.3-5 The most common types are Burkitt lymphoma (BL)/Burkitt leukemia and diffuse large B-cell lymphoma (DLBCL).3,5
Although event-free survival (EFS) rates in previously untreated pediatric BL and DLBCL reach 90% with short intensive multiagent chemotherapy, patients with relapsed/refractory (R/R) mature B-NHL have much poorer prognoses. Estimates of 1- to 8-year overall survival (OS) range from 22% to 34%,6-8 highlighting the need for more effective treatment.
Among chemoimmunotherapy regimens, rituximab plus ifosfamide, carboplatin, and etoposide (RICE) and, less frequently, rituximab plus vincristine, ifosfamide, carboplatin, idarubicin, and dexamethasone (RVICI) are used to treat pediatric patients with R/R B-NHL.9,10
Ibrutinib, a first-in-class oral covalent inhibitor of Bruton’s tyrosine kinase (BTK), is approved to treat adults with B-cell malignancies in 100 countries.11-13 In preclinical studies, ibrutinib was shown to inhibit BL tumor cell growth and enhance survival in BL xenografted mice14 and diminish DLBCL tumor cell growth.15 Combining ibrutinib with RICE or RVICI (RICE/RVICI) was considered to provide potential advantages for patients with pediatric B-NHL because of nonoverlapping mechanisms of action and toxicity profiles. A phase 1 study demonstrated safety and preliminary efficacy in adults with R/R DLBCL when ibrutinib was combined with RICE before autologous transplantation.16
Given the unmet clinical need in this population and preclinical data in BL, SPARKLE (NCT02703272), a 2-part, open-label, randomized, controlled phase 3 trial, was initiated to compare ibrutinib plus RICE/RVICI vs RICE/RVICI alone in children and young adults with R/R mature B-NHL.17
The run-in stage (Part 1) of SPARKLE determined safety, pharmacokinetics, and preliminary efficacy.17 The findings supported continued assessment of ibrutinib plus RICE/RVICI in Part 2. We report the efficacy, safety, pharmacokinetics, and pharmacodynamics from Part 2 of SPARKLE.
Methods
Study design and participants
This phase 3 study was conducted in 21 countries and 94 sites across North America, Latin America, Asia, and Europe from December 14, 2016, to June 11, 2021.
Eligible patients were 1 to 30 years of age and needed to have a diagnosis of mature B-NHL (initial diagnosis needed to have occurred at <18 years of age). Eligibility and exclusion criteria are described in the Appendix.
Patients were randomized in a 2:1 ratio to ibrutinib plus RICE/RVICI or RICE/RVICI only for 3 cycles. Ibrutinib dose varied by age group, with patients aged ≥12 years receiving 329 mg/m2 per day and patients aged <12 years receiving 440 mg/m2 per day, as determined in Part 1. Investigators were permitted to select between RICE and RVICI as background therapy of choice. Randomization was balanced using randomly permuted blocks and stratified by histology (BL type vs non-BL type histology due to historically poorer reported outcomes with BL types6) and background therapy (RICE/RVICI).
After 3 combination therapy cycles, patients randomized to the ibrutinib plus RICE/RVICI arm with a partial response (PR) or better could continue on ibrutinib monotherapy at the same daily dose for up to 3 28-day cycles or until progressive disease or unacceptable toxicity, or until initiating subsequent antilymphoma therapy or a conditioning regimen for stem cell transplantation – whichever came first. Patients received ibrutinib in either a capsule (70 or 140 mg) or oral suspension to be self-administered at home.
The study was conducted according to the principles of the Declaration of Helsinki and Guidelines for Good Clinical Practice, with patients and/or their legally acceptable representatives providing written informed consent. The local Independent Ethics Committee and Institutional Review Board requirements at each participating center approved the study. This trial is registered at www.clinicaltrials.gov as #NCT02703272.
Procedures
The primary endpoint was EFS, based on independent review committee (IRC)-confirmed events, defined as time from randomization to death, progressive disease, or lack of complete response (CR) or PR after 3 ibrutinib treatment cycles in combination with RICE/RVICI background therapy or RICE/RVICI background therapy alone. Secondary endpoints included OS, overall response rate (ORR), tumor volume reduction at day 14, time to response, duration of response, proportion of patients who proceeded to stem cell transplantation, and long-term survival. Sensitivity, subgroup, and post-hoc EFS analyses are described in the data supplement.
Treatment-emergent adverse event (TEAE) severity in the safety population was assessed using the National Cancer Institute’s Common Terminology Criteria for Adverse Events, version 4.03.
Pharmacokinetic samples were collected from patients randomized to ibrutinib in combination with RICE/RVICI during Part 2 of the study to further characterize pharmacokinetics in pediatric patients. Blood samples were also collected for pharmacodynamic assessments (BTK occupancy). Further details are described in the data supplement.
Statistical analysis
EFS was compared between treatment groups using a nonstratified log-rank test. The estimated median EFS rate and 90% confidence interval (CI) for each treatment group are presented. The EFS curve is presented using the Kaplan-Meier method. The hazard ratio (HR) between the 2 treatment groups and associated 90% CI were computed using a nonstratified Cox proportional-hazards model. Further statistical analyses are described in the data supplement.
The preplanned interim analysis (IA) was conducted by the statistical support group independent of the study team and presented to the Independent Data Monitoring Committee (IDMC) when 31 EFS events were reached. The 1-sided P value required for early stopping for futility was ≥.341 and for superiority was ≤.006. This design employed the sequential testing approach, as described by O’Brien and Fleming, to preserve the Type-I error rate.20
Results
Patients
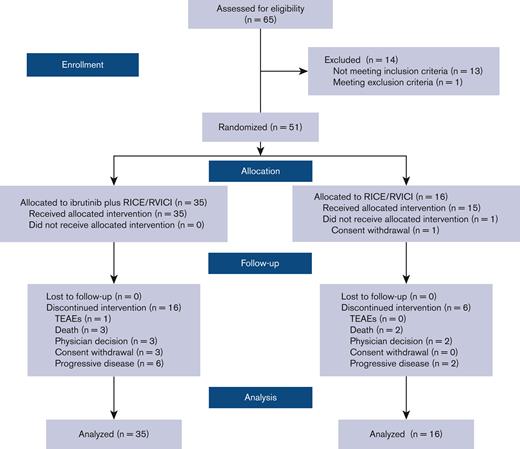
Sixty-five patients were assessed for eligibility (Figure 1). Between July 14, 2017, and July 14, 2020, 51 children and young adults were randomly assigned in Part 2 to receive either ibrutinib plus RICE/RVICI (n = 35) or RICE/RVICI alone (n = 16) and included in the intention-to-treat population (Figure 1).
Baseline disease characteristics were generally well balanced between treatment arms, although there was a numerically higher proportion of patients in the ibrutinib plus RICE/RVICI arm who had relapsed following prior rituximab exposure compared with the RICE/RVICI arm (86% vs 56%; Table 1). Following Part 1, the decision was made to restrict inclusion to those in first relapse or refractory to first-line therapy. The protocol was amended but while it was being approved in all sites, 2 patients in second relapse were included in the ibrutinib plus RICE/RVICI arm. In addition, there was a lower proportion of patients with parenchymal central nervous system involvement but a higher proportion of patients with cerebrospinal fluid involvement in the ibrutinib plus RICE/RVICI arm. The total proportion of patients with DLBCL/primary mediastinal large B-cell lymphoma (PMBCL) in Part 2 (51%) was numerically higher than Part 1 (19%). Also, the percentage of patients with DLBCL was higher in the RICE/RVICI arm, with 50% of these patients having DLBCL compared with only 34% in the ibrutinib plus RICE/RVICI arm. Of note, there were also no PMBCL cases in the RICE/RVICI arm, yet 17% of cases in the ibrutinib plus RICE/RVICI arm were PMBCL.
Baseline demographics and characteristics in Part 2
| . | Ibrutinib plus RICE/RVICI (n = 35) . | RICE/RVICI (n = 16) . |
|---|---|---|
| Median age at randomization, y (range) | 15.0 (5.0-19.0) | 14.5 (3.0-18.0) |
| 1-5, n (%) | 2 (6) | 2 (13) |
| 6-11, n (%) | 5 (14) | 2 (13) |
| 12-17, n (%) | 23 (66) | 11 (69) |
| ≥18, n (%) | 5 (14) | 1 (6) |
| Male, n (%) | 23 (66) | 13 (81) |
| Race, n (%) | ||
| White | 22 (63) | 8 (50) |
| Black | 0 | 1 (6) |
| Asian | 8 (23) | 6 (38) |
| Other | 2 (6) | 1 (6) |
| Time in months from initial diagnosis to randomization, median (range) | 8.1 (3.0-69.0) | 6.6 (2.0-46.0) |
| BSA (m2) | ||
| Median (range) | 1.6 (0.7-2.1) | 1.5 (0.7-2.1) |
| Type of mature B-cell NHL at enrollment, n (%) | ||
| Burkitt-like lymphoma | 1 (3) | 1 (6) |
| BL | 12 (34) | 4 (25) |
| Burkitt leukemia | 4 (11) | 1 (6) |
| DLBCL | 12 (34) | 8 (50) |
| Primary mediastinal B-cell lymphoma | 6 (17) | 0 |
| Other pediatric mature B-cell NHL | 0 | 2 (13) |
| CNS involvement, n (%) | 1 (3) | 1 (6) |
| Bone marrow involvement, n (%) | 7 (20) | 5 (31) |
| First relapse, n (%) | ||
| Burkitt-like lymphoma | 1 (3) | 1 (6) |
| BL | 12 (34) | 4 (25) |
| Burkitt leukemia | 4 (11) | 1 (6) |
| DLBCL | 12 (34) | 8 (50) |
| Primary mediastinal B-cell lymphoma | 6 (17) | 0 |
| Other pediatric mature B-cell NHL | 0 | 2 (13) |
| Second relapse, n (%)∗ | 2 (6) | 0 |
| BL | 1 (3) | 0 |
| DLBCL | 1 (3) | 0 |
| CSF cytologic evaluation, n (%) | ||
| CSF-positive | 5 (14) | 1 (6) |
| CSF-negative | 30 (86) | 15 (94) |
| Prior rituximab treatment, n (%) | 30 (86) | 9 (56) |
| Time in months from start of latest prior line of therapy to progression on latest prior line of therapy, median (range) | 6.6 (1.3-68.6) | 5.6 (1.5-37.8) |
| . | Ibrutinib plus RICE/RVICI (n = 35) . | RICE/RVICI (n = 16) . |
|---|---|---|
| Median age at randomization, y (range) | 15.0 (5.0-19.0) | 14.5 (3.0-18.0) |
| 1-5, n (%) | 2 (6) | 2 (13) |
| 6-11, n (%) | 5 (14) | 2 (13) |
| 12-17, n (%) | 23 (66) | 11 (69) |
| ≥18, n (%) | 5 (14) | 1 (6) |
| Male, n (%) | 23 (66) | 13 (81) |
| Race, n (%) | ||
| White | 22 (63) | 8 (50) |
| Black | 0 | 1 (6) |
| Asian | 8 (23) | 6 (38) |
| Other | 2 (6) | 1 (6) |
| Time in months from initial diagnosis to randomization, median (range) | 8.1 (3.0-69.0) | 6.6 (2.0-46.0) |
| BSA (m2) | ||
| Median (range) | 1.6 (0.7-2.1) | 1.5 (0.7-2.1) |
| Type of mature B-cell NHL at enrollment, n (%) | ||
| Burkitt-like lymphoma | 1 (3) | 1 (6) |
| BL | 12 (34) | 4 (25) |
| Burkitt leukemia | 4 (11) | 1 (6) |
| DLBCL | 12 (34) | 8 (50) |
| Primary mediastinal B-cell lymphoma | 6 (17) | 0 |
| Other pediatric mature B-cell NHL | 0 | 2 (13) |
| CNS involvement, n (%) | 1 (3) | 1 (6) |
| Bone marrow involvement, n (%) | 7 (20) | 5 (31) |
| First relapse, n (%) | ||
| Burkitt-like lymphoma | 1 (3) | 1 (6) |
| BL | 12 (34) | 4 (25) |
| Burkitt leukemia | 4 (11) | 1 (6) |
| DLBCL | 12 (34) | 8 (50) |
| Primary mediastinal B-cell lymphoma | 6 (17) | 0 |
| Other pediatric mature B-cell NHL | 0 | 2 (13) |
| Second relapse, n (%)∗ | 2 (6) | 0 |
| BL | 1 (3) | 0 |
| DLBCL | 1 (3) | 0 |
| CSF cytologic evaluation, n (%) | ||
| CSF-positive | 5 (14) | 1 (6) |
| CSF-negative | 30 (86) | 15 (94) |
| Prior rituximab treatment, n (%) | 30 (86) | 9 (56) |
| Time in months from start of latest prior line of therapy to progression on latest prior line of therapy, median (range) | 6.6 (1.3-68.6) | 5.6 (1.5-37.8) |
BSA, body surface area; CNS, central nervous system; CSF, cerebrospinal fluid.
Following Part 1, the decision was made to restrict inclusion to those in first relapse or refractory to first-line therapy. The protocol was amended but whilst the protocol was being approved in all sites, 2 patients in second relapse were included.
Efficacy
At the prespecified IA cutoff date of July 20, 2020, the IDMC recommended stopping enrollment for Part 2 as the futility boundary for early stopping was crossed.
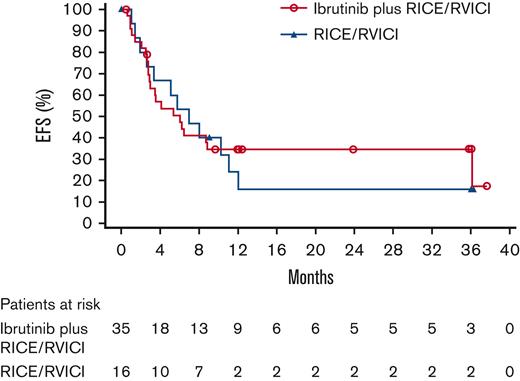
Per IRC event determination, the HR of ibrutinib plus RICE/RVICI vs RICE/RVICI was 0.9 (90% CI, 0.5-1.6; P = .387). Median EFS was 6.1 months for patients in the ibrutinib plus RICE/RVICI arm vs 7 months for patients in the RICE/RVICI arm (Figure 2). Sensitivity analyses are presented in the data supplement. Overall concordance for EFS per investigator and IRC event determination was 94% in the ibrutinib plus RICE/RVICI arm and 88% in the RICE/RVICI arm. The subgroup analysis showed that EFS was similar between treatment arms, regardless of age, histology, background chemoimmunotherapy, baseline lactate dehydrogenase level, and bone marrow involvement status (supplemental Figure 1). Fourteen percent in the ibrutinib plus RICE/RVICI arm and 13% in the RICE/RVICI arm remained event free at 2 years post-randomization (P = 1.000).
Kaplan-Meier curve for EFS in ITT population in Part 2. ITT, intention to treat.
Kaplan-Meier curve for EFS in ITT population in Part 2. ITT, intention to treat.
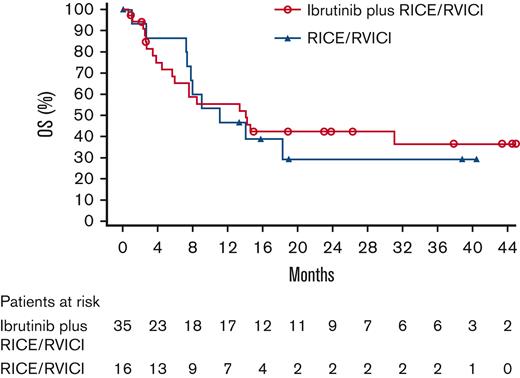
Median OS for patients in the ibrutinib plus RICE/RVICI arm vs patients in the RICE/RVICI arm was 14.1 (90% CI, 6.0-non-estimable [NE]) vs 11.1 months (90% CI, 7.4-NE), respectively (Figure 3). Similarly, 3-year OS rates for patients in the ibrutinib plus RICE/RVICI and the RICE/RVICI arm were 36% (90% CI, 21-52) and 30% (90% CI, 11-50), respectively. The HR of ibrutinib plus RICE/RVICI vs RICE/RVICI was 0.9 (90% CI, 0.5-1.7; P = .789).
ORR (CR, CR biopsy-negative, CR unconfirmed, PR) was seen in 69% of patients in the ibrutinib plus RICE/RVICI arm (6 [17%] and 18 [51%] patients had a CR and PR, respectively) and 81% (3 [19%] and 10 [63%] patients had a CR and PR, respectively) of patients in the RICE/RVICI arm (odds ratio, 0.5; 90% CI, 0.2-1.7; P = .347) (Table 2).
| . | Ibrutinib plus RICE/RVICI (n = 35) . | RICE/RVICI (n = 16) . |
|---|---|---|
| Best ORR (CR, CRb, CRu, PR), n (%)‡ | 24 (69) | 13 (81) |
| Best OR, n (%) | ||
| CR§ | 6 (17) | 3 (19) |
| PR | 18 (51) | 10 (63) |
| MR | 4 (11) | 0 |
| SD | 3 (9) | 2 (13) |
| Progressive disease | 1 (3) | 0 |
| No evidence of disease‖ | 1 (3) | 0 |
| Unknown/missing | 2 (6) | 1 (6) |
| . | Ibrutinib plus RICE/RVICI (n = 35) . | RICE/RVICI (n = 16) . |
|---|---|---|
| Best ORR (CR, CRb, CRu, PR), n (%)‡ | 24 (69) | 13 (81) |
| Best OR, n (%) | ||
| CR§ | 6 (17) | 3 (19) |
| PR | 18 (51) | 10 (63) |
| MR | 4 (11) | 0 |
| SD | 3 (9) | 2 (13) |
| Progressive disease | 1 (3) | 0 |
| No evidence of disease‖ | 1 (3) | 0 |
| Unknown/missing | 2 (6) | 1 (6) |
CRb, complete response biopsy-negative; CRu, complete response unconfirmed; ITT, intention-to-treat; MR, minor response; SD, stable disease.
Based on independent committee-confirmed events.
Efficacy based on all randomized patients.
Best overall response will only consider tumor assessment up to subsequent anticancer therapy.
There were no other CR categories.
If no disease observed at baseline, follow-up assessments were described by this classification.
The least-squares mean difference for percentage change from baseline in tumor volume in the ibrutinib plus RICE/RVICI vs the RICE/RVICI arm per IRC assessment was 9% (90% CI, −11.0 to 29.5; P = .444).
Median time to response was 0.89 months in the ibrutinib plus RICE/RVICI arm vs 0.82 months with RICE/RVICI alone (HR, 0.7; 90% CI, 0.4-1.3; P = .354). Median duration of response was 6.0 months for patients in the ibrutinib plus RICE/RVICI arm vs 6.5 months for patients in the RICE/RVICI arm.
Sixteen (46%) patients in the ibrutinib plus RICE/RVICI arm and 7 (44%) patients in the RICE/RVICI arm proceeded to stem cell transplantation based on physician decision. Thirteen patients with IRC-assessed response of PR or better proceeded to stem cell transplantation in the ibrutinib plus RICE/RVICI arm (7 allogeneic and 6 autologous) and 7 patients in the RICE/RVICI arm (2 allogeneic and 5 autologous) (odds ratio, 0.8; 90% CI, 0.3-2.1; P = .760).
The post-hoc analyses of EFS in patients with and without prior rituximab exposure who received ibrutinib plus RICE/RVICI and RICE/RVICI alone are shown in supplemental Figure 2. In the ibrutinib plus RICE/RVICI arm, 7 of 30 patients with prior rituximab exposure and 2 of 5 patients without prior rituximab exposure were alive and event free at 12 months. In the RICE/RVICI arm, 1 of 9 patients with prior rituximab exposure and 1 of 9 patients without prior rituximab exposure were alive and event free at 12 months.
EFS in patients in both arms with and without prior rituximab exposure is shown in supplemental Figure 3. The median EFS of all patients treated with prior rituximab (N = 39) was 5.1 months (90% CI, 3.0-8.1), and in patients without prior rituximab (N = 12) was 10.3 months (90% CI, 2.8-NE).
As the median investigator-assessed EFS at 18-month median follow-up in Part 1 of the study was unreached in the ibrutinib plus modified RICE group, we performed a post-hoc analysis of the EFS of ibrutinib plus RICE from Part 1 only and ibrutinib plus RICE from Part 1 plus 2 in both BL types and DLBCL/PMBCL (supplemental Figure 4). For BL types, the median investigator-assessed EFS in Part 1 only and Part 1 plus 2 was NE (90% CI, 0.8-NE) and 4.7 months (90% CI, 2.8-NE), respectively. For DLBCL/PMBCL types, the median EFS in Part 1 only and Part 1 plus 2 was NE (90% CI, NE-NE) and 8.7 months (90% CI, 2.8-NE), respectively.
Safety
All patients reported at least 1 grade ≥3 TEAE (Table 3). The most common (≥30%) grade ≥3 TEAEs seen in the ibrutinib plus RICE/RVICI and RICE/RVICI arms, respectively, included anemia (77% vs 93%), combined thrombocytopenia and decreased platelet count (86% vs 93%), combined neutropenia and decreased neutrophil count (74% vs 93%), and febrile neutropenia (69% vs 47%) (Table 4).
| . | Ibrutinib plus RICE/RVICI (n = 35) . | RICE/RVICI (n = 15) . |
|---|---|---|
| TEAEs, n (%) | 35 (100) | 15 (100) |
| Grade ≥3 | 35 (100) | 15 (100) |
| Study drug related‡ | 29 (83) | NA |
| Treatment-emergent SAEs, n (%) | 25 (71) | 11 (73) |
| Grade ≥3 | 25 (71) | 11 (73) |
| Study drug related‡ | 18 (51) | NA |
| TEAEs leading to ibrutinib discontinuation,‡ n (%) | 1 (3) | NA |
| TEAEs leading to RICE/RVICI discontinuation,§ n (%) | 4 (11) | 0 |
| TEAEs with outcome of death, n (%) | 4 (11) | 2 (13) |
| . | Ibrutinib plus RICE/RVICI (n = 35) . | RICE/RVICI (n = 15) . |
|---|---|---|
| TEAEs, n (%) | 35 (100) | 15 (100) |
| Grade ≥3 | 35 (100) | 15 (100) |
| Study drug related‡ | 29 (83) | NA |
| Treatment-emergent SAEs, n (%) | 25 (71) | 11 (73) |
| Grade ≥3 | 25 (71) | 11 (73) |
| Study drug related‡ | 18 (51) | NA |
| TEAEs leading to ibrutinib discontinuation,‡ n (%) | 1 (3) | NA |
| TEAEs leading to RICE/RVICI discontinuation,§ n (%) | 4 (11) | 0 |
| TEAEs with outcome of death, n (%) | 4 (11) | 2 (13) |
NA, not applicable.
TEAEs are defined as adverse events with onset or worsening on or after date of first dose of study treatment up to and including 30 days after date of last dose of study medication.
Based on all patients who received at least 1 dose of study treatment.
This row is applicable to ibrutinib only.
Refers to discontinuation of any component of RICE/RVICI.
Incidence of TEAEs ≥30% patients in either arm by preferred term in safety population∗in Part 2
| . | Ibrutinib plus RICE/RVICI (n = 35) . | RICE/RVICI (n = 15) . | ||||
|---|---|---|---|---|---|---|
| All Grades . | Grade 3-4 . | Grade 5 . | All Grades . | Grade 3-4 . | Grade 5 . | |
| Patients with TEAE, n (%) | 35 (100) | 31 (89) | 4 (11) | 15 (100) | 13 (87) | 2 (13) |
| Anemia | 29 (83) | 27 (77) | 0 | 14 (93) | 14 (93) | 0 |
| Febrile neutropenia | 24 (69) | 24 (69) | 0 | 7 (47) | 7 (47) | 0 |
| Combined thrombocytopenia and platelet count decreased | 31 (89) | 30 (86) | 0 | 14 (93) | 14 (93) | 0 |
| Vomiting | 25 (71) | 7 (20) | 0 | 4 (27) | 0 | 0 |
| Nausea | 21 (60) | 2 (6) | 0 | 2 (13) | 0 | 0 |
| Abdominal pain | 13 (37) | 0 | 0 | 2 (13) | 0 | 0 |
| Combined neutropenia and neutrophil count decreased | 26 (74) | 26 (74) | 0 | 14 (93) | 14 (93) | 0 |
| White blood cell count decreased | 8 (23) | 8 (23) | 0 | 6 (40) | 6 (40) | 0 |
| Alanine aminotransferase increased | 7 (20) | 5 (14) | 0 | 6 (40) | 4 (27) | 0 |
| Aspartate aminotransferase increased | 6 (17) | 3 (9) | 0 | 5 (33) | 2 (13) | 0 |
| Pyrexia | 14 (40) | 2 (6) | 0 | 6 (40) | 1 (7) | 0 |
| Hypokalemia | 12 (34) | 11 (31) | 0 | 7 (47) | 4 (27) | 0 |
| Hypomagnesemia | 6 (17) | 2 (6) | 0 | 5 (33) | 2 (13) | 0 |
| Headache | 16 (46) | 3 (9) | 0 | 3 (20) | 0 | 0 |
| Infections and infestations† | 19 (54) | 12 (34) | 2 (6) | 10 (67) | 6 (40) | 2 (13) |
| . | Ibrutinib plus RICE/RVICI (n = 35) . | RICE/RVICI (n = 15) . | ||||
|---|---|---|---|---|---|---|
| All Grades . | Grade 3-4 . | Grade 5 . | All Grades . | Grade 3-4 . | Grade 5 . | |
| Patients with TEAE, n (%) | 35 (100) | 31 (89) | 4 (11) | 15 (100) | 13 (87) | 2 (13) |
| Anemia | 29 (83) | 27 (77) | 0 | 14 (93) | 14 (93) | 0 |
| Febrile neutropenia | 24 (69) | 24 (69) | 0 | 7 (47) | 7 (47) | 0 |
| Combined thrombocytopenia and platelet count decreased | 31 (89) | 30 (86) | 0 | 14 (93) | 14 (93) | 0 |
| Vomiting | 25 (71) | 7 (20) | 0 | 4 (27) | 0 | 0 |
| Nausea | 21 (60) | 2 (6) | 0 | 2 (13) | 0 | 0 |
| Abdominal pain | 13 (37) | 0 | 0 | 2 (13) | 0 | 0 |
| Combined neutropenia and neutrophil count decreased | 26 (74) | 26 (74) | 0 | 14 (93) | 14 (93) | 0 |
| White blood cell count decreased | 8 (23) | 8 (23) | 0 | 6 (40) | 6 (40) | 0 |
| Alanine aminotransferase increased | 7 (20) | 5 (14) | 0 | 6 (40) | 4 (27) | 0 |
| Aspartate aminotransferase increased | 6 (17) | 3 (9) | 0 | 5 (33) | 2 (13) | 0 |
| Pyrexia | 14 (40) | 2 (6) | 0 | 6 (40) | 1 (7) | 0 |
| Hypokalemia | 12 (34) | 11 (31) | 0 | 7 (47) | 4 (27) | 0 |
| Hypomagnesemia | 6 (17) | 2 (6) | 0 | 5 (33) | 2 (13) | 0 |
| Headache | 16 (46) | 3 (9) | 0 | 3 (20) | 0 | 0 |
| Infections and infestations† | 19 (54) | 12 (34) | 2 (6) | 10 (67) | 6 (40) | 2 (13) |
Patients with multiple events for the same system organ class or preferred term are counted only once per row. If a patient had TEAEs more than once and with different toxicity grades, he or she was counted once in the highest toxicity grade. Patients who had events with missing toxicity grades were counted in the “all grades” column but not summarized separately. TEAEs were coded using MedDRA Version 23.1. Verbatim terms are displayed for any TEAEs not coded at the time of the database cutoff.
MedDRA, Medical Dictionary for Regulatory Activities; SOC, system, order, class.
Based on all patients who received at least 1 dose of study treatment.
SOC term.
Rates of treatment-emergent serious adverse events (SAEs) were similar in the ibrutinib plus RICE/RVICI and RICE/RVICI arms (71% and 73%). All-cause deaths and TEAEs leading to death are summarized in supplemental Table 1. There was a numerically higher rate of major hemorrhage TEAEs in the ibrutinib plus RICE/RVICI arm compared with the RICE/RVICI arm (6 [17%] vs 1 [7%]). No patient had a COVID-19–related TEAE or death.
Other measures
Pharmacokinetics
The pharmacokinetic analysis revealed that ibrutinib exposure in pediatric patients fell within the target range of adults when receiving a daily dose of 329 mg/m2 or 440 mg/m2 for children ≥12 years and children <12 years, respectively. Further details are shown in the data supplement.
Pharmacodynamics
Pharmacodynamic data are described in the data supplement. Where measurable, full BTK occupancy was achieved at the prescribed dose of 329 mg/m2/day for patients ≥12 years and 440 mg/m2/day for patients <12 years of age.
Palatability and acceptability
Palatability and acceptability of the ibrutinib formulations were generally favorable and are described in the data supplement.
Discussion
Part 2 of SPARKLE investigated the efficacy, safety, pharmacokinetics, and pharmacodynamics of ibrutinib plus RICE/RVICI vs RICE/RVICI alone. Despite terminating enrollment early, our study reports efficacy data from the only international randomized study of pediatric patients with R/R B-NHL undergoing reinduction therapy.
Baseline demographics and disease characteristics were largely comparable between treatment arms, with some exceptions. These include the numerically higher proportion of patients in the ibrutinib plus RICE/RVICI arm who had previously relapsed on rituximab compared with the RICE/RVICI arm. There were also 2 patients included in the ibrutinib plus RICE/RVICI arm who had experienced a second relapse. As progression during initial therapy is associated with poor outcomes,9 the inclusion of these patients may have limited the efficacy seen in the ibrutinib plus RICE/RVICI arm.
Among all patients was a numerically higher than expected proportion with DLBCL/PMBCL in Part 2 compared with Part 1, with a higher percentage of DLBCL/PMBCL than BL types, suggesting an over-representation of DLBCL/PMBCL histology in the study population. In Part 2, the percentage of patients with DLBCL was higher in the RICE/RVICI arm. In addition, there were no PMBCL cases in the RICE/RVICI arm; however, 17% of patients in the ibrutinib plus RICE/RVICI arm had PMBCL.
At the ibrutinib dose established in Part 1,17 the pharmacokinetic analysis revealed that the expected ibrutinib exposure fell within that of the adult target range in these pediatric patients.
No significant difference in EFS was seen between the treatment arms, showing that even in the presence of ibrutinib, patients failed to improve. These results confirm the previously reported poor prognosis of children and young adults with R/R mature B-NHL,6,8,9,21 suggesting chemoimmunotherapy-resistant disease.
Taking into consideration differences in response criteria among studies, best ORR in the ibrutinib plus RICE/RVICI arm was within the range previously reported with most rituximab-based regimens.10,22,23 However, compared with those same studies, the ORR in the RICE/RVICI arm of our study (81%) was higher. As with the EFS rates, the CR rate in both arms was low (17% and 18%, respectively), suggesting CR may be a better predictor of EFS. The CR rate reported in our study was also lower than reported in other studies (43% to 70%).10,22,23 These same studies had higher proportions of patients with Burkitt-type lymphomas vs DLBCL,22,23 whereas our study appears to have a numerically higher proportion of patients with DLBCL/PMBCL than with BL types. Among these other studies, only 1 (Griffin et al)10 cites the response criteria (Response Criteria for Non-Hodgkin’s Lymphomas, 1999)24 used at the time. The response criteria used in the SPARKLE study are internationally validated.18
In our study, 46% of patients in the ibrutinib plus RICE/RVICI arm and 44% of patients in the RICE/RVICI arm continued to stem cell transplantation, which was numerically higher than previously reported in the evaluation of the RICE regimen with R/R B-NHL and Burkitt leukemia (6/20 [30%]).10 However, the reported OS in that study is similar (38% at 2-3 years) to the 3-year OS reported in our study, suggesting that the proportion of patients going to transplant is also not a robust indicator of future OS or EFS.
The numerically higher proportion of patients in the ibrutinib plus RICE/RVICI arm who had prior exposure to rituximab compared with the RICE/RVICI arm (86% and 56%) might have partly contributed to this poor outcome. When this study was planned, rituximab was not standard frontline therapy, and there were no data on treatment efficacy in patients who were refractory to rituximab therapy. It has since been established that patients with BL and DLBCL who are refractory to rituximab have dismal survival outcomes.9,25 From the post-hoc analysis, regardless of treatment arm and considering the small patient numbers, fewer patients with prior rituximab exposure benefited from rituximab at second line compared with those without prior rituximab exposure. This finding might be attributed to some of the many mechanisms that cause rituximab resistance.26 In addition, post-hoc analyses of median EFS in all patients in both treatment arms who received prior rituximab showed that salvage was poor.
Post-hoc EFS analysis of ibrutinib plus RICE only from Part 1 plus 2 revealed that patients with DLBCL/PMBCL have approximately twice the investigator-assessed median EFS of patients with BL types, suggesting that this treatment regimen may have better efficacy in patients with DLBCL/PMBCL. This suggestion is supported by the higher OS previously reported in pediatric patients with DLBCL/PMBCL (35%/29%) compared with BL (19%) who received chemoimmunotherapy.6
The patients in the SPARKLE study were stratified by BL types vs non-BL types, which led to grouping PMBCL with DLBCL. However, it should be noted that these are distinct histologies27 and patients with relapsed PMBCL have additional proven salvage options, such as radiation therapy28 or checkpoint inhibitors.29 Such additional therapy may influence OS when PMBCL is combined with DLBCL, as in our study.
The most common TEAEs in both treatment arms were consistent with the reported ibrutinib safety profile in adults, background therapies, and underlying disease.10,30 The most common SAE reported across both arms was febrile neutropenia. Griffin et al10 reported the RICE regimen was likewise associated with severe but reversible myelosuppression in almost all patients. The hematologic toxicities reported in our study were nonetheless manageable, expected, and did not prevent timely protocol completion. Despite the small number of patients, the rates of certain TEAEs were numerically higher with RICE/RVICI alone than with ibrutinib plus RICE/RVICI (combined thrombocytopenia and decreased platelet count [93% vs 89%], neutropenia and decreased neutrophil count [93% vs 74%], and infections [67% vs 54%]).
There was a higher rate of major hemorrhage TEAEs in the ibrutinib plus RICE/RVICI arm. Griffin et al10 reported that grade ≥3 hemorrhagic TEAEs occurred in 2 of 20 patients treated, indicating that hemorrhagic TEAEs are expected in this treatment setting. Furthermore, hemorrhage and major hemorrhage are ibrutinib-associated TEAEs in adults.12,13 All major hemorrhage TEAEs in the ibrutinib plus RICE/RVICI arm were in the presence of thrombocytopenia, and 3 were considered not related or doubtfully related to ibrutinib. Together these data show that it is possible to add ibrutinib to RICE or RVICI, but the underlying toxicity of the regimen needs to be observed in future studies.
The study was stopped early for futility, as the addition of ibrutinib did not improve EFS in this population. Given the apparent lack of efficacy when adding ibrutinib to standard chemotherapy in this population, we would recommend that alternative strategies be explored in future studies, such as those proposed by the ACCELERATE Paediatric Strategy forum on R/R B-NHL, that prioritize bispecific antibodies, antibody-drug conjugates, and CAR-T cells in this patient population.31
Nevertheless, our data show that a large international study from a very rare population of patients with R/R B-NHL is possible, leading to better therapies for children and young adults with mature B-NHL. However, the choice of backbone chemotherapy needs consideration. The current study showed a shift in the proportion of patients with DLBCL/PMBCL for reasons that are not clear; future studies should consider the balance of B-NHL subtypes. Our data show salvage is poor in a subset of patients who have received prior rituximab although chemoimmunotherapy-resistant disease is prevalent and requires new treatment strategies.
Acknowledgments
This study was funded by Janssen Research and Development. The authors thank Xavier Woot de Trixhe for his contributions to the study and all of the patients and their families who were included in this analysis. Writing assistance was provided by Ian Phillips of Parexel, and was funded by Janssen Research and Development.
Authorship
Contribution: G.A.A.B., V.M-C., B.B., M.Ca., and K.N. conceived and designed the study; K.N. provided administrative support; G.A.A.B., A.U., F.L., V.M-C., B.B., M.Ca., and K.N. provided study materials or patients; all authors provided manuscript writing, final approval of the manuscript, and were accountable for all aspects of their work.
Conflicts-of-interest disclosure: G.A.A.B.’s institution received consultancy fees from Janssen, Merck, Takeda, Roche, and Oxford Immune Algorithmics. H.J.K. participated on an advisory board for Amgen, Novartis, and Cartexell and received research funding from Amgen. F.L.’s institution received consultancy fees from Janssen. V.M-C.’s institution received consultancy fees from Janssen, Roche, BMS, Novartis, and Celgene and research funding from Roche and BMS. B.B.’s institution received consultancy fees from Janssen, Roche, ADC Therapeutics, Novartis, and Celgene. M.T. and S. Srinivasan are employed by Janssen. M. Curtis S.D., K.N., A.H., and S. Sun are employed by Janssen and hold J&J stock. M. Cairo had research funding from Janssen, participated on a speakers bureau for Jazz Pharmaceuticals, Sobi, Amgen, Servier, and Sanofi, and was a consultant for Jazz Pharmaceuticals, Novartis, AstraZeneca, and Omeros. L.V., E.K., A.B., N.T., A.U., D.B., and R.N. have nothing to disclose.
Correspondence: G. A. Amos Burke, Department of Paediatric Haematology, Oncology and Palliative Care, Cambridge University Hospitals NHS Foundation Trust, Addenbrooke’s Hospital, Cambridge CB2 0QQ, United Kingdom; e-mail: amos.burke@nhs.net.
References
Author notes
The data sharing policy of the Janssen Pharmaceutical Companies of Johnson & Johnson is available at www.janssen.com/clinical-trials/transparency. Requests for access to data from select studies can be submitted through the Yale Open Data Access (YODA) Project site at yoda.yale.edu. Individual participant data will not be shared.
The full-text version of this article contains a data supplement.