Key Points
K1813 in the FVIII A3 domain, considered to be an FXa-binding site, is responsible for the A2 subunit dissociation of FVIIIa.
The FVIII-K1813A mutant showed higher global coagulation potential vs WT because of reduced A2 dissociation and increased FVIII stability.
Abstract
Factor VIII (FVIII) functions as a cofactor of FIXa for FX activation in the intrinsic tenase complex. The 1811-1818 region in the FVIII A3 domain was observed to contribute to FIXa binding, and the K1813A/K1818A mutant increased the binding affinity for FIXa. The current study aims to identify mutated FVIII protein(s) that increase FVIIIa cofactor activity in the 1811-1818 region. FVIII mutants with K1813A, K1818A, and K1813A/K1818A were expressed in baby hamster kidney cells and were followed by assessments using purified and global coagulation assays for mouse models with hemophilia A (HA). A surface plasmon resonance–based assay revealed that the Kd value of FVIII-K1813A for FIXa interaction was lower than that of the wild-type (WT) (3.9±0.7/6.3±0.3 nM). However, the Km value of FVIII-K1813A for FIXa on tenase activity was comparable with that of the WT, whereas the kcat of this mutant was significantly greater than that of the WT. Thrombin-catalyzed FVIII-K1813A activation was ∼1.3-fold more enhanced than that of the WT, and the spontaneous decay of activated FVIII-K1813A was ∼2.5-fold slower than that of WT. The heat stability assay revealed that the decay rate of FVIII-K1813A was ∼2.5-fold slower than that of WT. Thrombin generation assay and rotational thromboelastometry using blood samples from patients with HA demonstrated that the addition of FVIII-K1813A (0.5 nM) exhibited a coagulation potential compatible with that of WT (1 nM). In the tail clip assay of HA mice, FVIII-K1813A showed a two- to fourfold higher hemostatic potential than that of the WT. FVIII-K1813A, with higher a FIXa binding affinity, enhances the global coagulation potential because of the stability of FVIII/FVIIIa molecules.
Introduction
Factor VIII (FVIII), a plasma protein deficient or defective in the severe inherited bleeding disorder hemophilia A (HA), functions as a procofactor for the serine protease FIXa in the phospholipid (PL) surface–dependent conversion of FX to FXa.1 FVIII is synthesized as a multidomain, single-chain molecule (A1-A2-B-A3-C1-C2) consisting of 2332 amino acid residues with a molecular mass of about 300 kDa.2,3 FVIII is processed during secretion by furin cleavage, generating a variably proteolyzed-sized heavy chain (HCh) consisting of A1-A2-B domains, linked to a light chain (LCh) consisting of A3-C1-C2 domains.2-4
FVIII is activated by limited proteolytic cleavage at 3 sites of Arg372, Arg740, and Arg1689 by thrombin, followed by its conversion to FVIIIa.5 In intrinsic tenase assembly, FVIIIa molecules interact with FIXa and increase the kcat for FXa formation by ∼106-fold compared with that for FIXa alone.6,7 The A2, A3, and C2 domains of FVIIIa bind to the catalytic domain,8 the first epidermal growth factor domain,9 and the Gla domain10 of FIXa, respectively, through interactions with extended surface regions. Therefore, it is expected that modulation of the FVIIIa–FIXa association may lead to an increase in the potential for intrinsic tenase activity.
The FIXa-interactive sites in the A2 domain are located in at least 3 regions within residues 484-509,11 558-565,12,13 and 707-714.14 Although the affinity of the isolated A2 subunit for FIXa is low (Kd; ∼300 nM),15 it amplifies the enzymatic activity of FIXa by modulating an active site in the catalytic domain, and this interaction defines the cofactor activity of FVIIIa.8 In addition, FIXa stabilizes FVIIIa in the presence of calcium and PL membranes16 by enhancing the A2 subunit association with A1/A3C1C2 dimers.17,18 The FVIII mutant D519V/E665V reduced A2 subunit dissociation and enhanced cofactor activity, likely resulting in enhanced FVIIIa–FIXa binding affinity.19
In contrast, the high binding affinity (Kd; ∼15 nM)20 of the isolated LCh, in FVIIIa, for FIXa provides the majority of the binding energy for this interaction. To date, 1 region within the A3 domain, residues 1811-1818, has been identified as a major FIXa-interaction site.21,22 These residues are also involved in low-density lipoprotein receptor–related protein 1 binding in the regulation of the FVIII life cycle.23 Bloem et al22 reported that the Lys1813Ala/Lys1818Ala (K1813A/K1818A) double mutant of recombinant (r) FVIII showed an approximately twofold lower Kd value for FIXa interaction than that of the wild-type (WT). Thus, we hypothesized that FVIII molecules mutated within 1811-1818 residues because of an FIXa-interactive site that would affect the interaction with FIXa might contribute to enhancing functional FVIIIa cofactor activity. In this study, we focused on K1813 and K1818 residues and examined the coagulation function potential of FVIII K1813A, K1818A, and K1813A/K1818A mutants using both in vitro and in vivo experiments.
Materials and methods
Ethics
The experiments with whole blood samples were approved by the Medical Research Ethics Committee of Nara Medical University (Approval No. 2503). Blood samples were collected after obtaining informed consent, in accordance with the ethical guidelines of our university. All animal experiments were approved by the Animal Care and Use Committee of Nara Medical University (No. 13138), and all procedures followed the Policy on the Care and Use of Laboratory Animals of Nara Medical University.
Reagents
Purified human FIXa, FX, and FXa (Hematologic Technologies, Essex Junction, VT); α-thrombin, r-hirudin, Glu-Gly-Arg (EGR)–chloromethylketone (Calbiochem, San Diego, CA), chromogenic substrate S-2222 (Sekisui Medical, Tokyo, Japan), Thrombocheck Aptt sla and FVIII-deficient plasma (Sysmex, Kobe, Japan), plasma of a patient with HA (George-King, Overland Park, KS), r-tissue factor (rTF; Innovin; Dade, Marburg, Germany), and thrombin-specific fluorogenic substrate (Bachem, Bubendorf, Switzerland) were purchased from the indicated vendors. A monoclonal antibody (mAbJR8) recognizing the C terminus (residues 563-740) in A2 was obtained from JR Scientific Inc (Woodland, CA).24,25 PL vesicles (phosphatidylserine/phosphatidylcholine/phosphatidylethanolamine 10/60/30%, respectively) were prepared using N-octylglucoside.26 Active site–blocked human EGR–bound FIXa (EGR-FIXa) was prepared as previously reported,10 and FIXa inactivation was considered complete when residual FIX activity was <0.2% in a FIX-specific assay (data not shown).
Patients’ samples
Whole blood samples were obtained by venipuncture from patients with HA (n=2) and healthy volunteers (n=20). One patient used an extended half-life FVIII product, and we drew whole blood samples 6 days after it was administered. The other 1 used a standard FVIII product, and we drew whole blood samples 1 week after it was administered. These samples were placed in test tubes containing a 1:9 volume of 3.2% (w/v) trisodium citrate without corn trypsin inhibitor. One week before blood sampling, none of the study participants had taken any additional medicine that could have affected their platelet or coagulation function.
Mutagenesis, expression, and purification of mutated FVIII
B domain–deleted WT-FVIII lacking Q744-S1637 and B domain–deleted FVIII mutants (single-mutated K1813A, K1818A, and double-mutated K1813A/K1818A) were purified and stably expressed in baby hamster kidney cells.27 The legacy numbering for FVIII residues was used to ensure consistency with an earlier study. The resultant FVIII forms were typically >90% pure, as determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and staining with GelCode Blue-Stain Reagent (Pierce), with albumin representing the major contaminant. The purified FVIII concentrations were measured using an enzyme-linked immunosorbent assay with 2 anti-FVIII mAbs (anti-FVIII C2 domain mAb ESH8 and anti-FVIII A2 domain mAb R8B12),28 and we could verify these concentrations by measuring the A280 absorbance (data not shown). FVIII activity (FVIII:C) levels were determined using a chromogenic FXa generation assay (Chromogenix). The samples were quickly frozen and stored at −80°C.
FXa generation assay
The rate of FX conversion to FXa was monitored using a purified system.29 FVIII (1 nM) in buffer (20 mM HEPES, 0.1 M NaCl, 5 mM calcium chloride [CaCl2], pH 7.2, 0.01% Tween 20) containing PL vesicles (20 μM) was activated by the addition of thrombin (30 nM). Thrombin activity was terminated after 30 seconds by the addition of hirudin (10 U/mL) and FXa generation was initiated by the addition of FIXa and FX at the indicated concentrations. The reactions were quenched after 1 minute by adding 50 mM ethylene-diamine-tetraacetic acid. The amount of FXa generated was determined by the addition of the chromogenic substrate S-2222 (final concentration: f.c. 0.46 mM), and the velocity rates of FXa generation were calculated. All reactions were performed at 23°C.
FVIII:C measurement
FVIII:C was measured in a 1-stage clotting assay using commercial FVIII-deficient plasma with a STart 4 hemostasis analyzer (Diagnostica Stago, Asnieres, France). All the reactions were performed at 37°C. FVIII (10 nM) was activated by the addition of thrombin (0.4 nM), at the indicated times, in 2-(4-(2-Hydroxyethyl)piperazin-1-yl)ethanesulfonic acid)-buffered saline containing 5 mM CaCl2 and 0.01% bovine serum albumin. Aliquots were removed from the mixtures, and thrombin was rapidly inactivated by the addition of hirudin (1 U/mL) and a 1500-fold dilution.27 The presence of thrombin and hirudin in the diluted samples did not affect FVIII:C levels in these assays (data not shown). To assess the spontaneous decay of FVIIIa activity, FVIII (1 nM) was activated for 30 seconds, by the addition of thrombin (30 nM), followed by the termination of the thrombin reaction by the addition of hirudin. To assess the heat stability of FVIII molecules, FVIII proteins (0.5 nM) were incubated at 55°C and their FVIII:C was measured at the indicated times in a 1-stage clotting assay.30
SPR-based assay
A surface plasmon resonance (SPR)–based assay using a Biacore T200TM instrument (Cytiva, Sheffield, UK) was used to determine the kinetics of the FVIII-EGR-FIXa interaction. EGR-FIXa was covalently coated onto a CM5 sensor chip at a coupling density of 2.2 ng/mm2. FVIII association was monitored in an HBS buffer containing 1 mM CaCl2 for 2 minutes at 10 μL/min. The dissociation of the bound ligand was recorded over a 2-minute period by replacing the ligand-containing buffer with only buffer. The level of nonspecific binding, corresponding to ligand binding to the uncoated chip, was subtracted from the obtained signal. The surface on the sensor chip was regenerated by washing with 50 mM NaOH. We conducted multiple FVIII injections/chip regeneration as previously described.10 The reactions were performed at 37°C. The rate constants for association (kass) and dissociation (kdiss) were determined using a nonlinear regression analysis using an evaluation software (Biacore AB). The dissociation constant (Kd) was calculated as kdiss/kass.
Thrombin generation assay (TGA)
TGA was performed as previously described.31 The samples (80 μL) were preincubated for 10 minutes with 20 μL of trigger reagent containing rTF and PL. The final concentrations (f.c.) of rTF and PL were 1 pM and 4 μM, respectively. After the addition of 20 μL of the reagent containing CaCl2 and fluorogenic substrate (f.c. 16.7 mM and 2.5 mM, respectively), the development of fluorescent signals was monitored using a Fluoroscan Ascent microplate reader (Thermo Fisher Scientific, MA). Data analyses were performed using the manufacturer's software to derive the standard parameters: peak thrombin, time-to-peak thrombin (time-to-peak), and endogenous thrombin potential.
Rotational thromboelastometry (ROTEM)
Previous studies have shown that nonactivated TEM in ROTEM reflects the physiological coagulation status.32 Therefore, this method was adopted using a whole blood hemostasis analyzer (Pentapharm, Munich, Germany). Citrated whole blood samples (280 μL) were left for 30 minutes at room temperature before the addition of 20 μL CaCl2 (f.c. 12.5 mM) to initiate coagulation. Clot formation was evaluated using 3 parameters: clotting time (CT, the period until reaching a 2mm amplitude), clot formation time (CFT, the period until reaching a 20mm amplitude), and CT+CFT.
Animals and the tail clip assay
HA mice with targeted destruction of exon 16 on a 129×C57BL/6 background were kindly gifted by Yoichi Sakata (Jichi Medical University, Shimotsuke, Japan) and backcrossed with C57BL/6 mice (CLEA Japan, Tokyo, Japan). The in vivo tail clip assay was performed on male and female mice aged 8 to 12 weeks (body weight: 20-25 g). All the animals were maintained under specific pathogen-free conditions. The tail clip assay was performed as previously described.19 C57BL/6 and the HA mice were anesthetized with a mixture of medetomidine, midazolam, and butorphanol and then placed on a hot plate (Tokyo Garasu Kikai, Tokyo, Japan) at 37°C for at least 10 minutes. After prewarming, the HA mice were intravenously infused with the K1813A mutant (1 and 2 μg/kg), K1818A (2 μg/kg), K1813A/K1818A (2 μg/kg), FVIII-WT (2 and 4 μg/kg), or saline (Otsuka Pharmaceutical, Tokyo, Japan). Five minutes after administration, the tail was cut 5 mm from the tip and immediately placed in a conical tube containing 10 mL of saline (prewarmed to 37°C). The volume of blood loss collected over 40 minutes was quantified gravimetrically.19
Data analysis
The Km and Vmax values for FVIIIa/FIXa–catalyzed activation of FX were calculated by fitting the data to a nonlinear least-squares regression using Equation 1.
The intermolecular or intramolecular stability of FVIII/FVIIIa activity was determined using Equation 2. The rate constant (k) for FVIII/FVIIIa activity was calculated using the following equation:
Results
Interactions between FIXa and FVIII mutants within the A3 1813-1818 residues
A previous study reported that FVIIIa mutated with K1813A/K1818A exhibits a higher affinity for the FIXa interaction relative to that by the WT.22 However, the relationship between FIXa and individual single mutants remains unclear. We prepared rFVIII mutant in which single Lys was converted to alanine (K1813A and K1818A) and double Lys were converted to Ala (K1813A/K1818A). In a 1-stage clotting assay, the specific activities of K1813A, K1818A, and K1813A/K1818A mutants were 240%, 170%, and 230% of the WT, respectively. In an FXa generation assay, they were 210%, 75%, and 150% of the WT, respectively, indicating that K1813A and K1813A/K1818A mutants had high specific activities of FVIII in both assays.
We examined the direct interaction between FVIII mutants and FIXa by an SPR-based assay. Because FIXa proteolyzes FVIII,18 an active site–blocked EGR-FIXa was used in this method.10 Representative curves of the binding of FVIII mutants to immobilized EGR-FIXa are shown (supplemental Figure 1), and the data were comparatively fitted by nonlinear regression using a 1:1 binding model with a drifting baseline. The Kd value (3.9±0.7 nM) in the K1813A mutant interaction for EGR-FIXa was significantly lower than that in the WT (6.3±0.3 nM) (P<.05). In contrast, the interaction for EGR-FIXa was comparable in K1818A (5.1±1.8 nM) and K1813A/K1818A (4.7±1.2 nM) mutants.
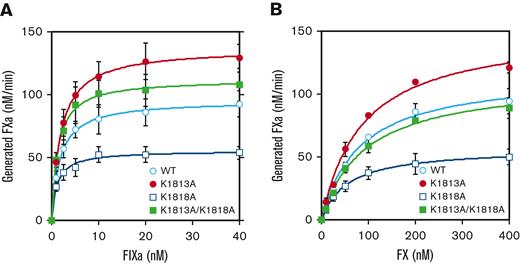
To investigate the relationship between these interactions and the intrinsic tenase complex, the effect of these mutants on FIXa-catalyzed FX activation was assessed using a purified FXa generation assay (Figure 1). The kinetic parameters of the FVIII mutants for FIXa or FX association are summarized in Table 1. The Km values for FIXa in K1818A (1.2±0.3 nM) and K1813A/K1818A (1.3±0.1 nM) mutants were significantly lower than those in the WT (1.7±0.1 nM), but those in K1813A (1.9±0.1 nM) were similar to those in the WT (Figure 1A). On the contrary, the kcat value for FIXa in K1813A (137±17 minutes−1) and K1818A (56±7.2 minutes−1) was significantly greater and lower than that in the WT (95±14 minutes−1), but that in K1813A/K1818A was not significantly different from that of the WT. The K1813A and K1813A/K1818A mutants showed a 1.3- to 1.5-fold higher catalytic efficiency than the WT but were not significantly different from that of the WT. In contrast, the kinetic parameters of FX in these mutants appeared to be comparable to those of the WT (Figure 1B). These results indicate that the catalytic function potential of FIXa in the K1813A mutant on the tenase complex was increased, despite the lack of influence of the binding potential for FIXa.
Effects of FVIII K1813A, K1818A, and K1813A/K1818A mutants on the kinetics of FIXa-catalyzed FX activation. (A) FIXa association. FVIII WT or mutants (1 nM) were activated by thrombin (30 nM) for 30 seconds in the presence of PL vesicles (20 μM). FXa generation was initiated by the addition of FX (300 nM) and various concentrations of FIXa (0-40 nM). (B) FX association. The WT or mutant FVIII (1 nM) was activated by thrombin (30 nM) for 30 seconds in the presence of PL vesicles (20 μM). FXa generation was initiated by the addition of FIXa (40 nM) and various concentrations of FX (0-400 nM). WT, open circles; K1813A, closed circles; K1818A, open squares; and K1813A/K1818A, closed squares. Experiments were performed 3 separate times, and the average and standard deviation values are shown. The initial rates of FXa generation were plotted as a function of FIXa or FX concentration and fitted to Equation 1 using nonlinear least-squares regression.
Effects of FVIII K1813A, K1818A, and K1813A/K1818A mutants on the kinetics of FIXa-catalyzed FX activation. (A) FIXa association. FVIII WT or mutants (1 nM) were activated by thrombin (30 nM) for 30 seconds in the presence of PL vesicles (20 μM). FXa generation was initiated by the addition of FX (300 nM) and various concentrations of FIXa (0-40 nM). (B) FX association. The WT or mutant FVIII (1 nM) was activated by thrombin (30 nM) for 30 seconds in the presence of PL vesicles (20 μM). FXa generation was initiated by the addition of FIXa (40 nM) and various concentrations of FX (0-400 nM). WT, open circles; K1813A, closed circles; K1818A, open squares; and K1813A/K1818A, closed squares. Experiments were performed 3 separate times, and the average and standard deviation values are shown. The initial rates of FXa generation were plotted as a function of FIXa or FX concentration and fitted to Equation 1 using nonlinear least-squares regression.
Kinetic parameters for the interaction of K1813A, K1818A, and K1813A/K1818A mutants with FIXa or FX by FXa generation
| FVIII mutant . | FIXa association . | FX association . | ||||
|---|---|---|---|---|---|---|
| Km . | kcat . | kcat/Km . | Km . | kcat . | kcat/Km . | |
| nM . | min−1 . | (fold) . | nM . | min−1 . | (fold) . | |
| WT | 1.7 ± 0.1 | 95 ± 14 | 55 ± 9.1 (1.0) | 82 ± 21 | 118 ± 20 | 1.5 ± 0.14 (1.0) |
| K1813A | 1.9 ± 0.1 | 137 ± 17∗ | 71.8 ± 11 (1.3) | 89 ± 19 | 154 ± 11 | 1.8 ± 0.3 (1.2) |
| K1818A | 1.2 ± 0.3∗ | 56 ± 7.2∗ | 50 ± 15 (0.91) | 55 ± 3.8 | 57 ± 10∗∗ | 1.1 ± 0.2 (0.73) |
| K1813A/K1818A | 1.3 ± 0.1∗ | 112 ± 16 | 85 ± 18 (1.5) | 86 ± 10 | 128 ± 28 | 1.5 ± 0.5 (1.0) |
| FVIII mutant . | FIXa association . | FX association . | ||||
|---|---|---|---|---|---|---|
| Km . | kcat . | kcat/Km . | Km . | kcat . | kcat/Km . | |
| nM . | min−1 . | (fold) . | nM . | min−1 . | (fold) . | |
| WT | 1.7 ± 0.1 | 95 ± 14 | 55 ± 9.1 (1.0) | 82 ± 21 | 118 ± 20 | 1.5 ± 0.14 (1.0) |
| K1813A | 1.9 ± 0.1 | 137 ± 17∗ | 71.8 ± 11 (1.3) | 89 ± 19 | 154 ± 11 | 1.8 ± 0.3 (1.2) |
| K1818A | 1.2 ± 0.3∗ | 56 ± 7.2∗ | 50 ± 15 (0.91) | 55 ± 3.8 | 57 ± 10∗∗ | 1.1 ± 0.2 (0.73) |
| K1813A/K1818A | 1.3 ± 0.1∗ | 112 ± 16 | 85 ± 18 (1.5) | 86 ± 10 | 128 ± 28 | 1.5 ± 0.5 (1.0) |
FVIII (1 nM) was activated with thrombin (30 nM) for 30 seconds in the presence of 20 μM PL vesicles. FXa generation was initiated by the addition of FX (300 nM) and various concentrations of FIXa (0-40 nM) or by the addition of FIXa (40 nM) and various concentrations of FX (0-400 nM), as described in Methods. Significant differences between the WT and FVIII mutants were investigated using the Student t test. Experiments were performed 3 separate times, and the mean values are shown.
P <.05.
P <.01.
Thrombin activation of FVIII mutants within the 1813-1818 residues
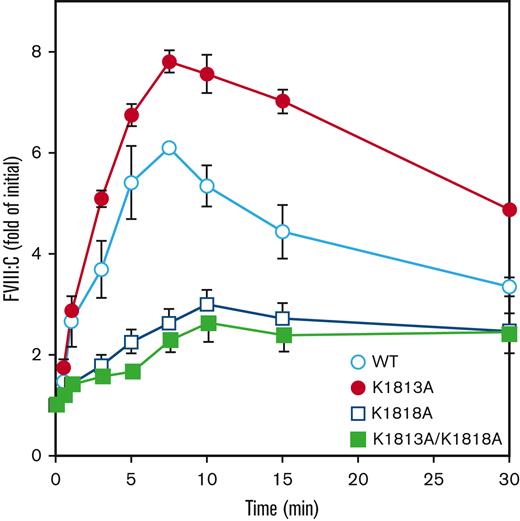
The influence on FVIII and thrombin association or the intramolecular stability of FVIII/FVIIIa molecules could be considered as the mechanism(s) of the increased catalytic function potential for FIXa in the K1813A mutant. Therefore, FVIII mutants (10 nM) were first examined for activation by thrombin (0.4 nM), as described in Methods. After a 7.5-minute reaction, the peak value of FVIII-WT increased approximately sixfold over the initial level, followed by a time-dependent decrease in FVIII:C. However, the K1813A mutant exhibited peak activity with an approximately eightfold increase in the initial level, supporting the enhanced increase of FVIIIa activity compared with that in the WT. On the contrary, peak values and the time of peak thrombin activation in both K1818A and K1813A/K1818A mutants were markedly decreased and delayed, respectively, compared with those of the WT (Figure 2).
Thrombin-catalyzed activation of FVIII mutants of K1813A, K1818A, and K1813A/K1818A. FVIII WT or mutants (10 nM) were incubated with thrombin (0.4 nM) before measuring FVIII activity at the indicated times in a 1-stage clotting assay, as described in the Methods section. The symbols used are defined in Figure 1. The initial FVIII activities of WT, K1813A, K1818A, and K1813A/K1818A mutants were 7.8, 16.1, 14.3, and 15.1 IU/mL at t=0, respectively. FVIII activity was expressed as a fold of initial (t=0) and was plotted as a function of incubation time. Experiments were performed 3 separate times, and the average and standard deviation values are shown.
Thrombin-catalyzed activation of FVIII mutants of K1813A, K1818A, and K1813A/K1818A. FVIII WT or mutants (10 nM) were incubated with thrombin (0.4 nM) before measuring FVIII activity at the indicated times in a 1-stage clotting assay, as described in the Methods section. The symbols used are defined in Figure 1. The initial FVIII activities of WT, K1813A, K1818A, and K1813A/K1818A mutants were 7.8, 16.1, 14.3, and 15.1 IU/mL at t=0, respectively. FVIII activity was expressed as a fold of initial (t=0) and was plotted as a function of incubation time. Experiments were performed 3 separate times, and the average and standard deviation values are shown.
Thrombin-induced activation of FVIII is mainly governed by the cleavage of Arg372 in HCh. Thus, the time course of HCh cleavage determined by western blotting using anti-A2 mAbJR8 was investigated. The K1813A mutant did not affect thrombin cleavage at Arg372, similar to the thrombin cleavage of the WT. The K1818A mutant had significantly delayed cleavage at Arg372 as compared with that of the WT, but the K1813A/K1818A mutant had slightly delayed cleavage, indicating a difference between thrombin-catalyzed FVIII activation and cleavage at Arg372 (supplemental Figure 2). Our results suggest that the enhancement of thrombin-catalyzed activation of the K1813A mutant might be due to the reduced dissociation of the A2 subunit from FVIIIa rather than from the WT.
Assessment of stability of FVIII/FVIIIa with K1813A mutant
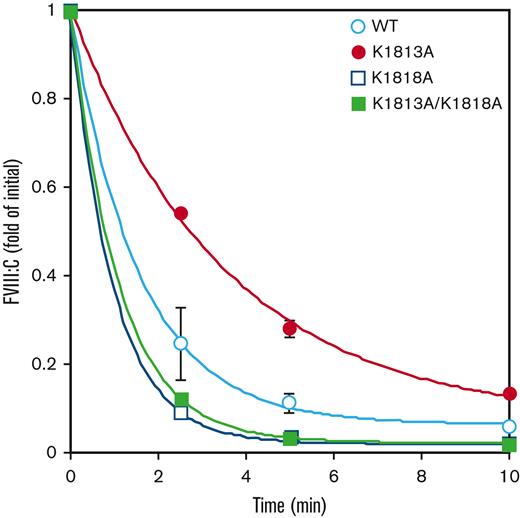
Next, we compared the differences in the spontaneous decay of FVIIIa molecules between the WT, K1813A, K1818A, and K1813A/K1818A mutants. FVIII (1 nM) was examined for activation by thrombin (30 nM), followed by the measurement of FVIII activity, reflecting A2 dissociation–dependent FVIIIa activity. The decay rates in K1818A alone (1.05±0.03) and K1813A/K1818A (0.91±0.05) were 1.3- to 1.5-fold higher than those in the WT (0.70±0.26), consistent with a previous report.22 In contrast, the decay rate in FVIIIa activity of the K1813A mutant was low by ∼2.4-fold compared with that of the WT (0.29±0.01 and 0.70±0.26, respectively), indicative of the A2 subunit stability of FVIIIa with K1813A, relative to that with the WT, and the enhancement of FVIIIa cofactor activity (Figure 3).
Comparison with FVIIIa stability of WT and K1813A mutant. FVIII WT or mutant (1 nM) were incubated with thrombin (30 nM) before measuring FVIII activity at the indicated times in a 1-stage clotting assay. The symbols used are defined in Figure 1. FVIIIa activity was expressed as the fold change of the initial value and was plotted as a function of incubation time. The experiments were performed 3 times, and the average and standard deviation calculations are shown. The data were fitted to the formula given in Equation 2.
Comparison with FVIIIa stability of WT and K1813A mutant. FVIII WT or mutant (1 nM) were incubated with thrombin (30 nM) before measuring FVIII activity at the indicated times in a 1-stage clotting assay. The symbols used are defined in Figure 1. FVIIIa activity was expressed as the fold change of the initial value and was plotted as a function of incubation time. The experiments were performed 3 times, and the average and standard deviation calculations are shown. The data were fitted to the formula given in Equation 2.
A previous study reported that the temperature-dependent decrease in FVIII activity resulted from the instability of interactions between the HCh and LCh, indicating the structural stability of FVIII.33 To further assess the structural stability of the K1813A mutant, we evaluated temperature-dependent decreases in FVIII activity.30,33 FVIII (0.5 nM) was incubated at 55°C, and FVIII activity was measured at the indicated times. The FVIII decay rate of K1813A was ∼2.3-fold lower than that of the WT (∼0.01, and ∼0.023, respectively), in keeping with the results of the FVIIIa degradation rate as mentioned above. In contrast, the decay rates of the K1818A and K1813A/K1818A mutants (∼0.031 and ∼0.032, respectively) were modestly increased relative to those of the WT, indicating that the K1813A mutant also enhanced the structural stability of FVIII (Figure 4).
FVIII stability of K1813A, K1818A, and K1813A/K1818A mutants. FVIII WT or mutant (0.5 nM) were incubated at 55°C before measuring FVIII activity at the indicated times in a 1-stage clotting assay. The symbols used are defined in Figure 1. FVIII activity was expressed as the fold change of the initial value and was plotted as a function of incubation time. The experiments were performed 3 times, and the average and standard deviation calculations are shown. The data were fitted to the formula given in Equation 2.
FVIII stability of K1813A, K1818A, and K1813A/K1818A mutants. FVIII WT or mutant (0.5 nM) were incubated at 55°C before measuring FVIII activity at the indicated times in a 1-stage clotting assay. The symbols used are defined in Figure 1. FVIII activity was expressed as the fold change of the initial value and was plotted as a function of incubation time. The experiments were performed 3 times, and the average and standard deviation calculations are shown. The data were fitted to the formula given in Equation 2.
Evaluation of global coagulation function of K1813A mutant
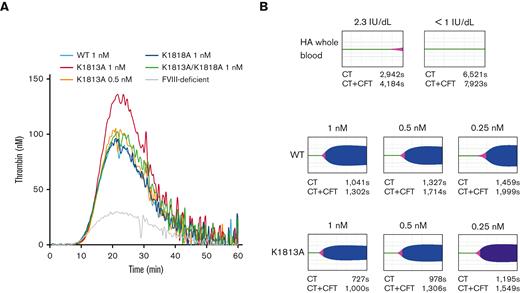
A previous study demonstrated that A2 stabilization could enhance global coagulation function.19 Thus, we assessed the global coagulation potential of the K1813A mutant using plasma-based TGA and whole blood–based ROTEM assays. We performed the TGA using FVIII-deficient plasma spiked with the K1813A, K1818A, or K1813A/K1818A mutants. The FVIII mutants were adjusted to a concentration of 0.5 or 1 nM. A representative TGA curve is illustrated in Figure 5A. The time-to-peak values in all FVIII mutants were not different from those in the WT. The peak thrombin and endogenous thrombin–potential values in K1813A (0.5 nM) were similar to those in the WT (1 nM), but the coagulation potential in K1813A (1 nM) was greater than that in the WT (1 nM). In contrast, those in K1818A (1 nM) and K1813A/K1818A (1 nM) were comparable to those in the WT (1 nM) (supplemental Table 1). It indicates that the coagulation potential in K1813A exhibited at least a twofold increase relative to that in the WT.
Global coagulation function potentials on FVIII K1813A, K1818A, and K1813A/K1818A mutants. (A). FVIII WT (1 nM) or mutants (K1813A; 0.5 and 1 nM; K1818A, 1 nM; K1813A/K1818A, 1 nM) were added to commercial FVIII-deficient plasma. These samples were incubated with TF (1 pM) and PL vesicles (4 μM) before the addition of fluorogenic substrate and CaCl2 at the start of the assay. Experiments were performed 3 separate times, and the mean values are shown thrombin generation curves are shown (black; WT 1 nM, red; K1813A 1 nM, tangerine; K1813A 0.5 nM, blue; K1818A 1 nM, green; K1813A/K1818A 1 nM, gray; buffer). (B) ROTEM. CaCl2 was added to citrated whole blood sample (residual FVIII activity of 2.3 and <1 IU/dL) in 2 patients with severe HA, together with various concentrations of FVIII mutants (WT and K1813A), followed by a ROTEM assay. The raw blood data in 2 patients and the representative curves in 1 patient are shown. The average parameters (CT and CT+CFT) in 2 patients are shown below each figure. The CT and CT+CFT obtained from normal controls (n=20) were 938±128 seconds and 1273±217 seconds, respectively. CT, clot time; CFT, clot formation time.
Global coagulation function potentials on FVIII K1813A, K1818A, and K1813A/K1818A mutants. (A). FVIII WT (1 nM) or mutants (K1813A; 0.5 and 1 nM; K1818A, 1 nM; K1813A/K1818A, 1 nM) were added to commercial FVIII-deficient plasma. These samples were incubated with TF (1 pM) and PL vesicles (4 μM) before the addition of fluorogenic substrate and CaCl2 at the start of the assay. Experiments were performed 3 separate times, and the mean values are shown thrombin generation curves are shown (black; WT 1 nM, red; K1813A 1 nM, tangerine; K1813A 0.5 nM, blue; K1818A 1 nM, green; K1813A/K1818A 1 nM, gray; buffer). (B) ROTEM. CaCl2 was added to citrated whole blood sample (residual FVIII activity of 2.3 and <1 IU/dL) in 2 patients with severe HA, together with various concentrations of FVIII mutants (WT and K1813A), followed by a ROTEM assay. The raw blood data in 2 patients and the representative curves in 1 patient are shown. The average parameters (CT and CT+CFT) in 2 patients are shown below each figure. The CT and CT+CFT obtained from normal controls (n=20) were 938±128 seconds and 1273±217 seconds, respectively. CT, clot time; CFT, clot formation time.
To further evaluate the global coagulation function of the K1813A mutant, the ex vivo effects of different concentrations of K1813A and the WT in whole blood samples obtained from 2 patients with severe HA receiving regular FVIII prophylaxis (trough FVIII:C 2.3 and <1 IU/dL) were examined using calcium-triggered ROTEM assays. A representative thromboelastogram is shown in Figure 5B. The average CT and CT+CFT parameters of 2 patients are shown in each figure. Whole blood samples from patients with HA showed longer CT and CT+CFT. The ex vivo addition of K1813A and the WT resulted in dose-dependent improvements in both parameters at each concentration. The parameters in the WT (0.5 and 1 nM, respectively) were equivalent to those in K1813A (0.25 and 0.5 nM, respectively), suggesting that the coagulation potential of K1813A in the whole blood samples was approximately increased by twofold compared with that of the WT. Overall, these results demonstrated that K1813A enhanced the coagulation potential of the WT by approximately twofold.
In vivo hemostatic efficacy of K1813A mutant for HA mice
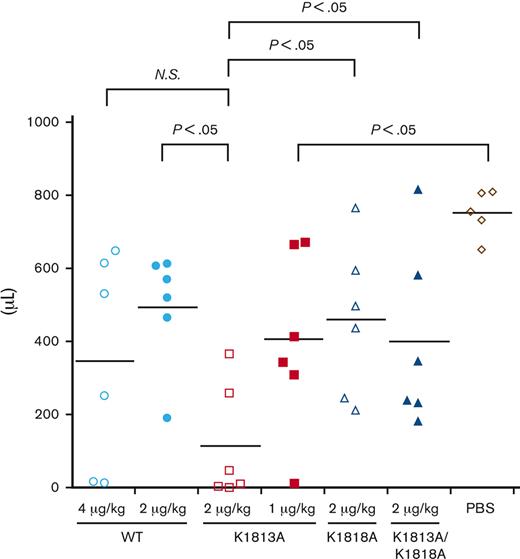
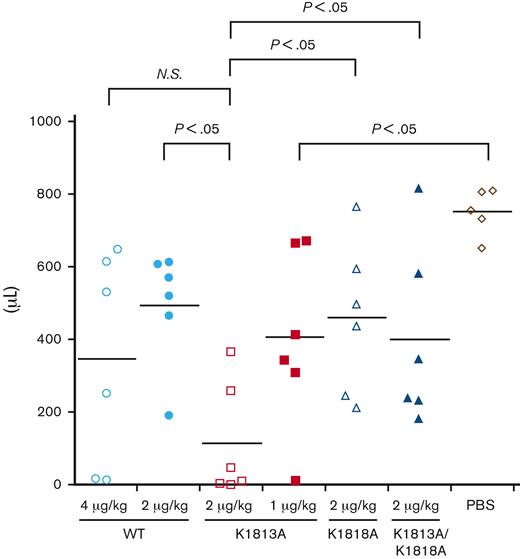
To investigate the in vivo efficacy of the K1813A mutant, either the WT or the K1813A mutant was infused into HA mice, and the tail clip assay was performed (Figure 6). The blood loss volume for the K1813A infusion (2 μg/kg) was lower than that for the WT (4 μg/kg), although no significant difference was observed. The blood loss volume for the K1813A infusion (2 μg/kg) was lower than that for the WT (2 μg/kg) with a significant difference (P<.05); however, that for the K1813A (1 μg/kg) was not significantly different from that for the WT (2 and 4 μg/kg). The blood loss volume for the K1813A infusion (2 μg/kg) was lower than that for the K1818A (2 μg/kg) or K1813A/K1818A (2 μg/kg) with significant difference (P<.05), indicating that the tail clip assay validated the in vitro data. Overall, K1813A exhibited an approximately two- to fourfold enhanced coagulation potential relative to the WT in the tail clip assay. We concluded that the K1813A mutant can enhance coagulation potential in an HA mouse model.
In vivo hemostatic effect of FVIII K1813A in HA mice by a tail clip assay. HA mice were infused with PBS, 1 and 2 μg/kg FVIII K1813A, 2 μg/kg K1818A, 2 μg/kg K1813A/K1818A, or 2 and 4 μg/kg WT. Five minutes after infusion, the terminal 5 mm of the tail was amputated, and shed blood was collected for 40 minutes. Each point represents a mouse. The short horizontal lines in each graph indicate the average values. A one-way ANOVA with Dunnett multiple comparison test was used to determine significance relative to HA mice PBS controls. A Student t test was used to compare between each group. ANOVA, analysis of variance; NS, not significant; PBS, phosphate buffered saline.
In vivo hemostatic effect of FVIII K1813A in HA mice by a tail clip assay. HA mice were infused with PBS, 1 and 2 μg/kg FVIII K1813A, 2 μg/kg K1818A, 2 μg/kg K1813A/K1818A, or 2 and 4 μg/kg WT. Five minutes after infusion, the terminal 5 mm of the tail was amputated, and shed blood was collected for 40 minutes. Each point represents a mouse. The short horizontal lines in each graph indicate the average values. A one-way ANOVA with Dunnett multiple comparison test was used to determine significance relative to HA mice PBS controls. A Student t test was used to compare between each group. ANOVA, analysis of variance; NS, not significant; PBS, phosphate buffered saline.
Discussion
In this study, we demonstrated that the K1813A mutant located in the FIXa-binding region could increase the global coagulation potential because of the FVIII/FVIIIa stability. In both in vitro and in vivo experiments, this result shows that K1813A contributed to the reduced decay rate of FVIIIa molecules and improved global hemostatic function.
It is generally known that FVIIIa is inactivated by 2 mechanisms: spontaneous A2 dissociation and activated protein C (APC) cleavage.34 Previous studies have suggested that A2 dissociation predominantly contributes to FVIIIa inactivation and APC cleavage does not have a major impact on the rate of FVIIIa inactivation.16,35,36 A recent study demonstrated that an APC-resistant FVIII mutant enhanced the hemostatic effect in an HA mouse model.37 In addition, FVIII mutants (R1645H and D519VE665V) that showed the slower A2 dissociation increased the global coagulation potential in in vivo models,19,38 indicating that suppression of FVIIIa inactivation could lead to improved hemostatic function. Our results showed that A2 dissociation in K1813A was 2.3-fold slower than that in the WT. On comparing the coagulation potential in K1813A (2 μg/kg) with that in R1645H (2 μg/kg), in the tail clip assay, the blood loss for the R1645H infusion was significantly reduced than that for 2 μg/kg of the WT infusion (P<.05), but that for the R1645H was not significantly different from that for K1813A (data not shown). These results confirmed that the stabilization of the A2 domain contributed to the improved hemostatic efficacy as efficiently as the previous results.19,38
The FIXa affinity of K1813A in the SPR-based assay was superior to that of the WT. However, the FIXa affinity of the K1813A mutant in the FXa generation assay did not differ from that of the WT. The reason for the discrepancy between these results remains unclear, but 1 reason may be the difference in the assay. The results of the SPR-based assay directly reflect FVIII and FIXa interactions. However, the result of the FXa generation assay shows the interaction between FVIIIa and FIXa in assembling FXase on the PL surface. Overall, the FIXa affinity of K1813A could improve in the absence of the PL surface, but the FIXa affinity of K1813A under the physiological state on the PL surface was not different from that of the WT.
A recent mass spectrometry–assisted study demonstrated that lysine residues with a change in surface exposure on A2 domain dissociation contribute to the retention of the A2 domain.39 In addition, K1813 was partially protected from chemical modification in FVIII, indicating that this lysine residue might affect the A1/A3-C1-C2 dimer and the A2 domain interaction.39 Our data showed the spontaneous decay of the thrombin-activated K1813A was slower because of less dissociation of the A2 domain from the A1/A3-C1-C2 dimer, indicating that this single mutation results in conformational change of the A3 domain affecting its interface contacting the A2 and even possibly A1 domains. In contrast, K1813 is located on the A3 domain surface and far from the A2 domain, based on the FVIII crystal structure.40 Considering some information, we would speculate that allosteric conformational change due to the replacement of K1813 with a hydrophobic amino acid and a small size does not weaken the interaction with the A1/A3-C1-C2 dimer and the A2 domain but may rather contribute to the slower A2 dissociation, affecting its interface contacting the A2 domain (even possibly A1). However, the precise mechanism(s) by which K1813A affects the affinity of the A1/A3-C1-C2 dimer for the A2 domain remains unclear.
By producing continuous steady-state levels of FVIII, gene therapy for patients with HA would provide the potential for an attractive alternative treatment for patients with HA.41 However, the expression of human FVIII is much more inefficient than that of other proteins of similar size.42 Regarding hemophilia B, gene therapy has been developed using a hyperactive FIX mutant, FIX-R338L.43 An approximately eightfold increase in the specific activity of FIX-R338L enables the use of a lower therapeutic vector dose to avoid adeno-associated virus–related hepatotoxicity.44 Recently, some studies have focused on designing inactivation-resistant FVIII molecules and creating various hyperactive FVIII mutants possessing a two- to fivefold increased coagulation function in HA mouse models.19,37 The hemostatic function of R336Q/R562Q was fivefold higher than that of the WT and showed the highest FVIII potency to date.37 Our gain-of-function FVIII mutant, K1813A, appeared to exhibit a two- to fourfold enhanced coagulation potential in the HA mouse model relative to WT. We have to consider the possibility that our enzyme-linked immunosorbent assay data using anti-FVIII monoclonal antibodies could overestimate or underestimate the FVIII mutants’ concentrations because of conformational change; however, we could confirm the validity of the protein concentrations using A280 absorbance measurements, suggesting that inserting a single mutation into FVIII could provide similar hemostatic efficacy to R336Q/R562Q. Although the mutated FVIII may result in heightened immunogenicity, a single mutation could be helpful in decreasing immunogenicity. The enhanced global coagulation potential in K1813A may increase the thrombogenic potential. Further investigations are needed, although K1813A could be an attractive FVIII molecule for improving hemostatic efficacy and decreasing immunological risks.
Some limitations have to be discussed. We did not ensure the status of immobilized proteins in an SPR assay. In addition, the precise mechanism(s) by which K1813A affects A2 dissociation remains unclear. Nevertheless, the FVIII-K1813A mutant showed superior FVIIIa cofactor activity compared with that of FVIII–WT in both in vitro and in vivo studies. To our knowledge, this is the first FVIII mutant with a 1-point mutation carrying an increase greater than fourfold in the coagulation potential. We expect that, in the future, the K1813A mutant could be a suitable FVIII molecule for both protein and gene-based therapies for patients with HA.
Acknowledgments
The authors are especially grateful to Kana Sasai and Kaoru Horiuchi for their technical assistance. This research was financially supported by the Japan Agency for Medical Research and Development under grant 21fk0410037 and was partly supported by a grant-in-aid for scientific research (KAKENHI) from the Ministry of Education, Culture, Sports, Science and Technology (21K07804 [K.N.]).
Authorship
Contribution: Y.N. carried out the experiments, analyzed the data, created the figures, wrote the manuscript, and approved the final version for publication; M.T. designed the study, carried out the experiments, and interpreted the data; A.O. carried out the experiments; N.S. interpreted the data; and K.N. designed the study, interpreted the data, and wrote and edited the manuscript.
Conflict-of-interest disclosure: Y.N. and K.N. received a grant from Takeda Pharmaceutical Co. A.O. received a grant from Sanofi. N.S. taught the course endowed by CSL Behring. M.T. declares no competing financial interests.
Correspondence: Yuto Nakajima, Department of Pediatrics, Nara Medical University, 840 Shijo-cho, Kashihara, Nara 634-8522, Japan; e-mail: nakajima-yamanashi@naramed-u.ac.jp.
References
Author notes
∗Y.N. and M.T. contributed equally to this study.
Presented in abstract form at the ISTH 2022 Congress, London, England, UK, 9-13 July 2022.
Data are available on request from the corresponding author, Yuto Nakajima (nakajima-yamanashi@naramed-u.ac.jp).
The full-text version of this article contains a data supplement.