Key Points
UCBT is an appropriate donor source when a matched sibling is not available for pediatric nonmalignant disorders.
UCBT conditioned without serotherapy could be lifesaving in immune deficiency disorders, even with active infections.
Abstract
There is no consensus on the best donor for children with nonmalignant disorders and immune deficiencies in the absence of a matched related donor (MRD). We evaluated the 2-year overall survival (OS) after umbilical cord blood transplantation (UCBT) in patients with nonmalignant disorders from 2009 to 2020 enrolled in a prospective clinical trial using either 5/6 or 6/6 UCB as the cell source. Patients receive a fully ablative busulfan, cyclophosphamide, and fludarabine without serotherapy. Fifty-five children were enrolled, median age 5 months (range, 1-111 months); primary immune deficiency (45), metabolic (5), hemophagocytic lymphohistiocytosis (1), and hematologic disorders (4). Twenty-six patients had persistent infections before transplant. Nineteen of them (34%) were 6/6 matched, and 36 (66%) were 5/6 human leukocyte antigen–matched. The OS at 2 years was 91% (95% cumulative incidence, 79-96), with a median follow-up of 4.3 years. The median time to neutrophil and platelet recovery were 17 days (range, 5-39 days) and 37 days (range, 20-92 days), respectively. All but one evaluable patient achieved full donor chimerism. The cumulative incidence of acute GVHD grades 2-4 on day 100 was 16% (n = 9). All patients with viral infections at the time of transplant cleared the infection at a median time of 54 days (range, 44-91 days). All evaluable patients underwent correction of their immune or metabolic defects. We conclude that in the absence of MRD, UCBT following myeloablative conditioning without serotherapy is an excellent curative option in young children with nonmalignant disorders. This trial has been registered at www.clinicaltrials.gov as NCT00950846.
Introduction
Hematopoietic stem cell transplantation is often the only curative option for patients with congenital nonmalignant diseases. Umbilical cord blood (UCB) has increasingly been used as the source of donor stem cells because UCB donors are promptly available for more than 90% of potential recipients.1 These cells may be less likely to produce graft-versus-host disease (in particular chronic GVHD) than other stem cell sources2, thereby reducing the required stringency of human leukocyte antigen (HLA)–matching.
Although multiple reports show the safety and efficacy of UCB to be comparable to other donor stem cell sources for the treatment of malignancy,3 a previous publication confirmed earlier reports that UCB transplant (UCBT) had inferior outcomes in infants with severe combined immunodeficiency (SCID) than transplantation using other stem cell sources, with an overall survival (OS) of 58%, declining to 40% when infection was present at the time of transplantation.4 Moreover, compared with other donor stem cell sources, patients undergoing UCBT have delayed neutrophil engraftment and T-cell reconstitution and an increase in opportunistic infections, which account for >50% non-relapse mortality in the first 3 months after transplantation.4,5 However, the incorporation of serotherapy with anti-thymocyte globulin (ATG; thymoglobulin) or alemtuzumab in the conditioning regimen was found to be the main reason for this poor T-cell reconstitution in cord blood recipients because of very efficient in vivo donor T-cell depletion resulting in very poor immune reconstitution in the months after CBT.6-9 The incidence or severity of post-transplant infection in immunodeficiency and other patients with nonmalignant disorders receiving UCBT may be reduced by omitting serotherapy from the conditioning regimen, thereby avoiding in vivo T-cell depletion. At present, there is little experience with this approach in the nonmalignant pediatric population. Chiesa et al described, for the first time, early immune reconstitution after omission of serotherapy after UCB in pediatric patients, 12 of whom had SCID. They reported a significant decrease in overall mortality because of infection (7%), but a high incidence of acute GVHD (grade 2-4, 50%; grade 3-4, 16%; chronic, 14%). The same group subsequently evaluated and reported on the timing of ATG therapy and the effects on immune reconstitution after UCBT.10 The authors concluded that the benefit of earlier immune reconstitution when ATG is omitted or limited needed to be balanced against the higher incidence of moderate and severe GVHD. However, the heterogeneity of diagnoses, conditioning regimens, and higher median age at transplant may have influenced the incidence of GVHD. They also reported that patients who underwent CBT without ATG/serotherapy had the lowest incidence of viral reactivation compared with bone marrow with and without ATG and peripheral blood stem cells with and without ATG.
We report a prospective single center clinical trial of UCBT omitting serotherapy in pediatric patients with nonmalignant diseases, the majority of whom had primary immune deficiencies, including patients with active infections. The main hypothesis of this study was that omitting serotherapy, which is currently not a standard of care for patients with nonmalignant disorders, could improve delayed T-cell reconstitution after cord transplant, resulting in a decrease in viral infection related mortality. To avoid the possible risk of increased rejection, serotherapy was substituted with fludarabine, a potent immunosuppressive agent with a very short half-life. We determined the safety and OS 2 years after transplant. We obtained encouraging OS with minimal post-transplant complications, even in patients with significant infections before transplant.
Methods
Inclusion criteria
Children affected by congenital or acquired immunological, metabolic, or hematological disorders were eligible for inclusion in this study if they lacked 10/10 HLA-A, -B, -C, -DR, and –DQ-matched family donors. The study was approved by the Baylor College of Medicine (BCM) Institutional Review Board (protocol number H 25064), and written informed consent was obtained from the patients’ parents or legal guardians in each case according to the Declaration of Helsinki. Patients transplanted according to the study after 2018 were included in this manuscript and analyzed in a study approved by the BCM Institution Review Board.
Transplantation and supportive care
All patients received a fully ablative conditioning regimen consisting of busulfan at a total dose of 16 mg/kg targeted according to an area under the curve between 800 to 1200 μmol-min/L, starting on day −9 thru day −5; cyclophosphamide at 50 mg/kg per dose on days −5, −4, −3, −2, each administered over 2 to 4 hours; and fludarabine at 40 mg/m2 per dose on days −4, −3, −2, and −1 over 1 hour. If patients weighed less than 10 kg, fludarabine was administered at a dose of 1.3 mg/kg per dose. The UCB units were obtained from national and international cord blood banks and were required to be matched at 5/6 or more HLA A, B, and DRB1 loci. Low-resolution typing was performed for HLA-A, and -B and high-resolution allele typing was done for HLA DRB1. The minimum total nucleated cell dose (TNC) required for the transplant was 5 × 107 cells/kg.
GVHD prophylaxis consisted of cyclosporine (CSA from day −2, adjusted to a trough level of 150-250 ng/mL) and mycophenolate mofetil (MMF, starting on day 1) and was discontinued on day 45 unless GVHD was present. None of the patients received ATG or alemtuzumab.
Antimicrobial prophylaxis consisted of ciprofloxacin and penicillin from day −10 until the patient reached an absolute neutrophil count (ANC) >2500 cells/μL for 3 consecutive days. Acyclovir and fluconazole or caspofungin/micafungin were started on day +2 and discontinued at the time of neutrophil engraftment. We continued fungal prophylaxis in patients receiving steroids >0.5 mg/kg per day. Prevention of Pneumocystis jiroveci pneumonia (PJP), including INH or IV pentamidine at the time of admission, was changed to trimethoprim sulfa after neutrophil engraftment and maintained until 1 year after transplant, or the CD4 count was consistently above 200 103/UL. IV immunoglobulins were administered to maintain a level of >400 mg/dL or appropriate for age.
Phenytoin was added to all patients on the day of admission and continued until 48 hours after the completion of the final busulfan dose. Infants (patients less than 1 year old) received Keppra in addition to busulfan. Both anticonvulsants in this age group continued until day+2. Levels of phenytoin were checked daily and maintained at the therapeutic level.
We maintained strict fluid control and weight management. All the patients had <5% weight gain from admission to hospitalization. Infants were allowed to gain 20 g daily if they were fed >75% of the baseline. Aggressive diuresis was performed if patients were above the targeted weight, if necessary, using loop diuretics as a continuous infusion. Thiazide diuretics were added as a second diuretic agent if more diuresis was required to maintain the target weight. Albumin infusion (25%) was performed to maintain albumin levels above 3.0, improve oncotic pressure, and maximize diuresis. Fluid intake was maintained orally or intravenously, throughout transplant admission.
Granulocyte colony-stimulating factor was started at 10μg/kg per day on day +7 until ANC was >2500 cells/μL for 3 consecutive days.
Patients with engraftment syndrome were treated with steroids at 1 to 2 mg/kg per day for 5 to 7 days and weaned slowly by 10% to 20% weekly.
As a standard of care, Epstein-Barr virus (EBV) and cytomegalovirus (CMV) DNA levels were monitored weekly in the peripheral blood using quantitative polymerase chain reaction.
Outcomes of interest
The main outcome of interest and the primary endpoint were OS evaluated 100 days and 2 years after transplant. Other outcomes of interest and secondary endpoints were neutrophil and platelet engraftment, donor engraftment, graft versus host disease, engraftment syndrome, immune reconstitution, and functional analysis. Neutrophil engraftment was defined as the first day of ANC above 500×103 cells/uL for 3 consecutive days. Platelet engraftment was defined as an unsupported platelet count of 20,000×103/uL. Donor Engraftment was determined at the time of neutrophil engraftment and then monthly for 6 months, and then at months 9 and 12 by analyzing of X/Y chromosomes or short tandem repeats. Mixed chimerism was defined as the presence of >10% recipient DNA 1 year after UCBT. Graft failure was defined as the absence of neutrophil recovery > 500×10e6/L by day 42. Acute GVHD was diagnosed based on Seattle criteria. Chronic GVHD was assessed and scored using the National Institutes of Health criteria.7,8 Engraftment syndrome was defined as 2 or more of the following clinical symptoms: fever >38C, rash, fluid retention, tachypnea, irritability, hepatomegaly, and cholestasis occurring before or during engraftment.
For immune reconstitution, the absolute numbers of CD3, CD4, CD19, and natural killer cells were measured pretransplant and during follow-up at days 42, 60, 100, 6 months, 12 months, and 24 months after transplant. Lymphocyte proliferation following phytohemagglutinin (PHA) 10ug/mL, poke weed mitogen (PWM) 100ug/mL, and concanavalin A (ConA) 50ng/mL was measured. Adequate mitogen responses were defined as 120,000 CPM for PHA, 50,000 CPM for PWM, and 90,000 CPM for ConA. Serum concentrations of IgA, IgG, and IgM, as well as the response to the Prevnar vaccination, were measured to assess humoral immune function.
Additional functional analyses were performed when samples were available; we used enzyme-linked immunospot assays (ELISpot) to quantify T cells secreting interferon-γ in response to respiratory syncytial virus (RSV), HHV6, and CMV antigens. The antigen consisted of peptide libraries of 15 mers overlapping by 11 amino acids) spanning each virus (Genemed Synthesis, San Antonio, TX). Virus-specific activity was measured after the direct stimulation of donor peripheral mononuclear cells with each virus. Staphylococcus aureus was used as a positive control, whereas unstimulated cells served as a negative control. After 20 to 24 hour of incubation, ELIspot plates were developed according to the manufacturer’s instructions and sent to ZellNet Consulting (New York, NY) for quantification.
Statistical analysis
Patient and transplant characteristics and immune reconstitution were summarized using descriptive statistics. The OS was calculated from the time of UCBT to death from any cause. Patients who were alive at the last follow-up were censored. The Kaplan-Meier method was used to estimate and visualize survival. The cumulative incidence was calculated and plotted using the competing risk method, as described in Gray9. A P value less than .05 was considered statistically significant. All analyses were performed in R 3.6.1 and SAS version 9.4 statistical software (SAS Institute Inc).
Results
Transplant characteristics
A total of 55 consecutive patients who underwent UCBT within the Blood and Marrow Transplantation Unit at Texas Children’s Hospital, BCM, were enrolled in our prospective trial between September 2009 and February 2020. The patients were diagnosed with: primary immune deficiency (45), metabolic disorders (5), nonmalignant hematological disorders (4), and hemophagocytic lymphohistiocytosis (HLH) (1). Table 1 shows the baseline characteristics of the patients. Two-thirds of the patients had severe combined immunodeficiency (SCID), 37; with 11 of them being diagnosed before newborn screening (NBS) in Texas. NBS started in Texas in December 2012. The median age at transplantation was 5 months (range, 1-111 months). We treated 42 males and 13 females. Using the original HLA criteria (low to intermediate resolution molecular at class I A and B and high resolution at HLA class II DRB1), 35 (64%) patients received a 5/6 match and 20 (36%) received a 6/6 match graft. The median TNC dose was 15x107 cells/kg (range, 5-37). Forty-two patients had pretransplant comorbidities, including viral or PJP infections and disease with 22 patients developing pneumonias and 10 requiring assisted mechanical ventilation. Sixteen patients had active infection at the time of transplant.
Clinical features of study subjects
| Characteristics . | Number . | % . |
|---|---|---|
| Number of patients (male/female) | 55 (42/13) | |
| Age at transplant (mo), median (range) | 5 (1-111) | |
| Diagnosis | ||
| Primary immune deficiency | 45 | 82% |
| SCID | 37 | 67% |
| Pre-NBS | 11 | 30% |
| IL2RG | 10 | 28% |
| RAG1,2 | 7 | 19% |
| IL7R | 5 | 14% |
| CD3D | 2 | 6% |
| ADA/PNP | 4/1 | 14% |
| Zap70 | 1 | 3% |
| Jak3 | 1 | 3% |
| RAC | 1 | 3% |
| other | 5 | 10% |
| IPEX syndrome | 2 | 4% |
| Wiskott-Aldrich syndrome | 2 | 4% |
| Leukocyte adhesion defect 1 | 2 | 4% |
| Chronic granulomatous disease | 2 | 4% |
| Metabolic disorders | 5 | 9% |
| Krabbes | 2 | |
| Alpha-mannosidosis | 1 | |
| Adrenoleukodystrophy | 1 | |
| Hurler | 1 | |
| HLH | 1 | 2% |
| Hematological diseases | 4 | 7% |
| Diamond-Blackfan Anemia | 2 | |
| Bone marrow failure | 2 | |
| Degree of HLA matching | ||
| 5/6 | 35 | 64% |
| 6/6 | 20 | 36% |
| Total nucleated cell dose median | 13×107/kg (5.1-27) | |
| Number of patients with pretransplant infections (active at time of transplant) | 46 (16) | |
| Viral | 26 (13)∗ | |
| CMV | 8 (5) | |
| RSV | 6 (3) | |
| Paraflu3 | 4 (3) | |
| VZV | 1 (2) | |
| Adv. | 1 (1) | |
| Rhino | 5 | |
| Norovirus/sapovirus | 3 (1) | |
| PJP | 5 | |
| Bacterial | 9 | |
| Mycobacterial | 1(1) | |
| Fungal | 5 (1) | |
| Number of patients with pretransplant pneumonia | 22 | 40% |
| Number of patients with pretransplant used of assisted mechanical ventilation | 10 | 18% |
| Characteristics . | Number . | % . |
|---|---|---|
| Number of patients (male/female) | 55 (42/13) | |
| Age at transplant (mo), median (range) | 5 (1-111) | |
| Diagnosis | ||
| Primary immune deficiency | 45 | 82% |
| SCID | 37 | 67% |
| Pre-NBS | 11 | 30% |
| IL2RG | 10 | 28% |
| RAG1,2 | 7 | 19% |
| IL7R | 5 | 14% |
| CD3D | 2 | 6% |
| ADA/PNP | 4/1 | 14% |
| Zap70 | 1 | 3% |
| Jak3 | 1 | 3% |
| RAC | 1 | 3% |
| other | 5 | 10% |
| IPEX syndrome | 2 | 4% |
| Wiskott-Aldrich syndrome | 2 | 4% |
| Leukocyte adhesion defect 1 | 2 | 4% |
| Chronic granulomatous disease | 2 | 4% |
| Metabolic disorders | 5 | 9% |
| Krabbes | 2 | |
| Alpha-mannosidosis | 1 | |
| Adrenoleukodystrophy | 1 | |
| Hurler | 1 | |
| HLH | 1 | 2% |
| Hematological diseases | 4 | 7% |
| Diamond-Blackfan Anemia | 2 | |
| Bone marrow failure | 2 | |
| Degree of HLA matching | ||
| 5/6 | 35 | 64% |
| 6/6 | 20 | 36% |
| Total nucleated cell dose median | 13×107/kg (5.1-27) | |
| Number of patients with pretransplant infections (active at time of transplant) | 46 (16) | |
| Viral | 26 (13)∗ | |
| CMV | 8 (5) | |
| RSV | 6 (3) | |
| Paraflu3 | 4 (3) | |
| VZV | 1 (2) | |
| Adv. | 1 (1) | |
| Rhino | 5 | |
| Norovirus/sapovirus | 3 (1) | |
| PJP | 5 | |
| Bacterial | 9 | |
| Mycobacterial | 1(1) | |
| Fungal | 5 (1) | |
| Number of patients with pretransplant pneumonia | 22 | 40% |
| Number of patients with pretransplant used of assisted mechanical ventilation | 10 | 18% |
Adv, Adenovirus.
One patient had 2 viruses.
Outcomes: OS, engraftment, engraftment syndrome, GVHD, lung injury, infections, and immune reconstitution
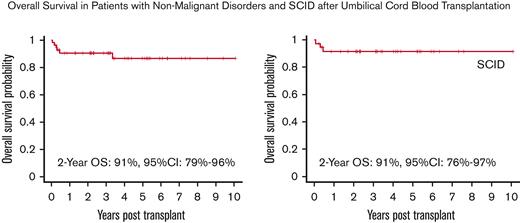
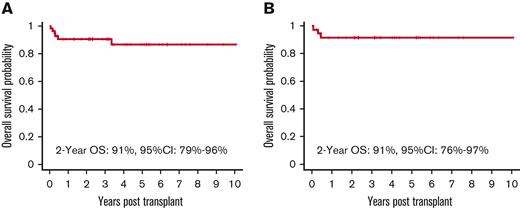
The OS at 2 years was 91% (95% confidence interval [CI], 79%-96%), with a median follow-up of 4.3 years (range, 0.5 to 11 years). The OS for patients with a SCID diagnosis was 91% (95% CI, 76%-97%) at 2 years (Figure 1 A, B). Five patients died whose diagnoses were IPEX (1 patient), HLH (1 patient), and SCID (3 patient). One patient with IPEX died of complications of severe colitis that were present before transplant, but whose subsequent course could not be distinguished from gut GVHD. The other patient had RAG1 deficiency SCID who developed mixed donor chimerism and severe inflammatory autoimmune liver disease. Three patients died of multiorgan failure: familial HLH (1) and SCID (2, one with an unknown mutation and the other with a purine nucleoside phosphorylase [PNP] gene mutation). The HLH and PNP patients were transplanted early in the study before reduced intensity stem cell transplant preparative regimens were demonstrated to be preferable for the treatment of these diseases.
Overall Survival. (A) OS of the entire cohort. (B) OS of patients with SCID. The Kaplan-Meier survival curve showing that omission of serotherapy leads to excellent OS in patients receiving a fully myeloablative transplant regimen for nonmalignant disorders. OS for the entire cohort was 91% (95% CI, 79-96) at 2 years with OS for SCID patients being 91% (95% CI, 76-97), with a median follow-up of 4.3 years (range, 0.5 to 11 yrs).
Overall Survival. (A) OS of the entire cohort. (B) OS of patients with SCID. The Kaplan-Meier survival curve showing that omission of serotherapy leads to excellent OS in patients receiving a fully myeloablative transplant regimen for nonmalignant disorders. OS for the entire cohort was 91% (95% CI, 79-96) at 2 years with OS for SCID patients being 91% (95% CI, 76-97), with a median follow-up of 4.3 years (range, 0.5 to 11 yrs).
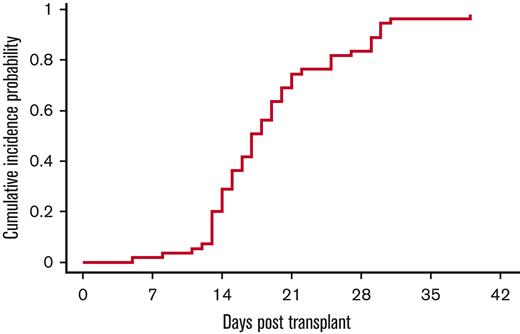
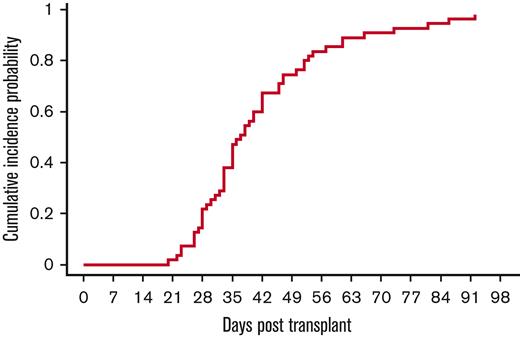
All except 1 patient were engrafted by day 42, with a median time to neutrophil engraftment of 17 days (range, 5-39 days). The cumulative incidence of neutrophil engraftment was 98% (95% CI, 73%-100%) on day 42 (Figure 2). The single patient who failed to engraft died of multiorgan failure and cardiac arrest on day 21. With regard to platelets, except 1 patient had platelet engraftment at a median of 37 days (range, 20-92 days) (Figure 3). The cumulative incidence of platelet engraftment was 98% (95% CI, 74%-100%) on day 92.
Neutrophil engraftment. 54 of 55 patients engrafted at a median time of 17 days (range, 5-30 days). The exception was a patient who died of multiorgan failure on day 16 before engraftment.
Neutrophil engraftment. 54 of 55 patients engrafted at a median time of 17 days (range, 5-30 days). The exception was a patient who died of multiorgan failure on day 16 before engraftment.
Platelet engraftment. 53 of 55 patients had platelet engraftment at a median time of 37 days (range, 23-86 days). The exceptions were 2 patients with multiorgan failure before platelet engraftment.
Platelet engraftment. 53 of 55 patients had platelet engraftment at a median time of 37 days (range, 23-86 days). The exceptions were 2 patients with multiorgan failure before platelet engraftment.
The median donor engraftment was 93.5% (range, 65%-100%) and 96.6% (range, 62%-100%) at 100 days and 1 year, respectively. Three patients had mixed chimerism, with a median donor engraftment of 62%, 68%, and 84%, but with complete correction of their disease, as determined by enzyme concentration or functional lymphocyte recovery in patients with metabolic disorders or immune deficiencies, respectively. A patient with IPEX syndrome had progressive loss of donor engraftment 2 years after transplant, requiring a second stem cell transplant with a mismatched unrelated donor, but subsequently died from complications of severe GVHD after his second transplant.
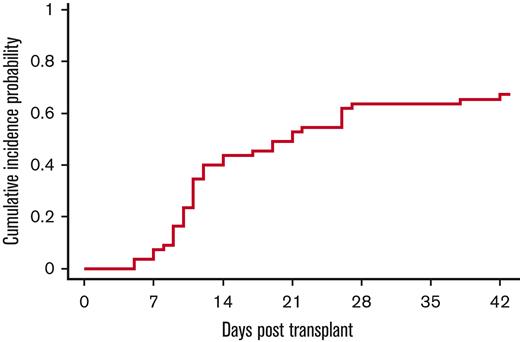
Thirty-seven (67%; 95% CI, 53%-78%) of the 55 patients developed engraftment syndrome at a median of 11 days (range, 5-28 days; Figure 4). None of the patients met criteria of acute GVHD. All patients were successfully treated.
Incidence of engraftment syndrome. The cumulative incidence of engraftment syndrome was 67% (95% CI, 53-78), occurring at a median time of 11 days (range, 5 to 28 days).
Incidence of engraftment syndrome. The cumulative incidence of engraftment syndrome was 67% (95% CI, 53-78), occurring at a median time of 11 days (range, 5 to 28 days).
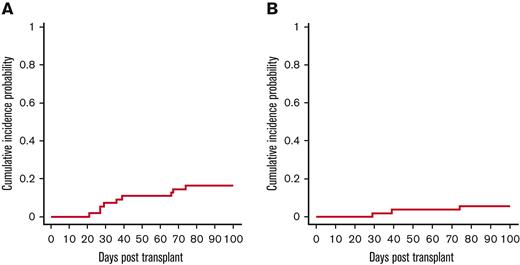
The cumulative incidence of acute GVHD grades 2-4 and 3-4 on day 100 was 16% (95% CI, 7%-25%) and 5% (95% CI, 1%-14%), respectively (Figure 5A,B). Nine patients developed acute GVHD, and 3 of them had Grade 3-4 GVHD. Five patients developed skin GVHD grade 2; 1 patient with IPEX syndrome and a history of bloody enterocolitis before transplant developed possible stage IV gut GVHD, but gut biopsy was unable to distinguish between GVHD and his original colitis, and 2 patients developed stage III skin GVHD. None of the patients developed chronic GVHD (0%).
Cumulative Incidence of Acute GvHD. (A) Acute GVHD 2-4. (B) Acute GVHD 3-4. A total of 9 patients developed grade 2 or more GVHD of skin or gastrointestinal tract, with only 1 patient developing stage IV disease.
Cumulative Incidence of Acute GvHD. (A) Acute GVHD 2-4. (B) Acute GVHD 3-4. A total of 9 patients developed grade 2 or more GVHD of skin or gastrointestinal tract, with only 1 patient developing stage IV disease.
Eight patients developed inflammatory lung disease after transplant. Four patients had a history of lung disease that required mechanical ventilation before transplantation admission. In 3 patients (1 Krabbe; 1 IPEX; and 1 RAG1 SCID mutation), lung inflammation was associated with the early withdrawal of immunosuppression to overcome mixed donor chimerism. All patients were successfully treated with steroids administered at 2 mg/kg per day and weaned by 10% to 20% weekly for ∼6 weeks.
Forty-six patients had a history of infections before transplant, including PJP (5), viral (26), bacterial (9), fungal (5), and mycobacterial (1) infections. Twenty-two patients developed pneumonia before transplant, 10 of whom required mechanical ventilation. Nine of the 11 patients with SCID before NBS had infection (80%), but even after the introduction of screening in Texas, 9/25 (36%) infants had developed viral infection before transplant. A total of 28 new viral infections were noted, including RSV, 5; Parainfluenza 3, 5; VZV,1; Influenza A, 1; Metapneumovirus, 1; HHV6 reactivation, 4; EBV reactivation, 4; adenovirus viremia, 3; CMV reactivation, 2; rotavirus/norovirus, 2. There were only 2 patients with viral disease (disseminated VZV and RSV pneumonia), but both patients were infected with these viruses before transplantation. One patient developed disseminated candidemia in the setting of multiorgan failure. Infection related mortality was not observed in our group. Viral infections before transplant were all cleared by a median of 54 days after transplant (range, 44-91 days). Three patients received donor derived trivirus CTLs for prophylaxis. No other CTLs were administered to the rest of the group.
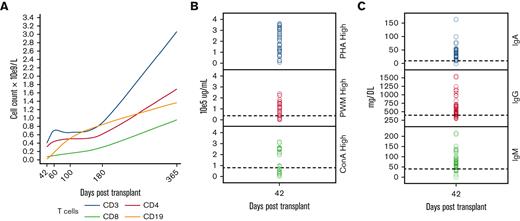
Lymphocyte reconstitution was prompt after transplant. The total T-cell numbers were above 500 × 106/L by day 60. CD19+ B-cell engraftment was observed as early as 60 days after UCBT (Table 2; Figure 6A). T-cell responses to PHA, ConA, and PWM were adequate in 75%, 65%, and 82% of patients, respectively, by day 42 after UCBT (Figure 6B). Specific T-cell responses to CMV (4), parainfluenza (2), and RSV (2) were assessed by interferon gamma release assay, and responses were observed at a median of 60 days after transplant (range, 38-112 days) (Figure 6C). Regarding the humoral immune system, more than 80% of the patients produced immunoglobulins as determined by IgM production and immunoglobulin class switching, as determined by the production of IgA at 6 months post-UCBT (Figure 6D). The median duration of IVIG administration for the entire cohort was 3 months (range, 2-24 months), whereas the median time to start immunizations was 14 months (range, 10-24 months).
Lymphocyte reconstitution was prompt after transplant. The median CD3 and /CD3CD4 absolute numbers were above 500 ×106/L by day 60. Median CD19 was above 400 ×106 by day 100, correlating with a lower need for IVIG infusions
| Lymphocyte subtype . | Day 42 Median (range) . | Day 60 Median (range) . | Day 100 Median (range) . | 6 mo Median (range) . | 12 mo Median (range) . |
|---|---|---|---|---|---|
| CD3+ | 362 (233-1314) | 733 (110-3693) | 614 (145-2844) | 966 (199-5876) | 3360 (640-8232) |
| CD3+/CD8+ | 71 (14-262) | 115 (9-1195) | 112 (8-2123) | 295 (33-3468) | 1006 (150-2364) |
| CD3+/CD4+ | 296 (51-1077) | 506 (48-2620) | 421 (133-1536) | 694 (92-3861) | 1695 (4.6-6259) |
| CD16+/56+ | 142 (61-358) | 213 (70-449) | 249 (128-781) | 265 (100-1499) | 335 (70-2058) |
| CD19 | 5.5 (0-860) | 172 (0-1562) | 525 (15-3201) | 879 (56-3763) | 1346 (313-3777) |
| Lymphocyte subtype . | Day 42 Median (range) . | Day 60 Median (range) . | Day 100 Median (range) . | 6 mo Median (range) . | 12 mo Median (range) . |
|---|---|---|---|---|---|
| CD3+ | 362 (233-1314) | 733 (110-3693) | 614 (145-2844) | 966 (199-5876) | 3360 (640-8232) |
| CD3+/CD8+ | 71 (14-262) | 115 (9-1195) | 112 (8-2123) | 295 (33-3468) | 1006 (150-2364) |
| CD3+/CD4+ | 296 (51-1077) | 506 (48-2620) | 421 (133-1536) | 694 (92-3861) | 1695 (4.6-6259) |
| CD16+/56+ | 142 (61-358) | 213 (70-449) | 249 (128-781) | 265 (100-1499) | 335 (70-2058) |
| CD19 | 5.5 (0-860) | 172 (0-1562) | 525 (15-3201) | 879 (56-3763) | 1346 (313-3777) |
Immune reconstitution after UCBT. (A) Median absolute number of CD3, CD4, CD8, CD19 lymphocytes. (B) T-cell proliferation responses to mitogens. Most patients had normal T-cell proliferative responses to mitogen stimulation. (C) Immunoglobulin production and class switching after CBT. Dashed line is 50th percentile of normal for age.
Immune reconstitution after UCBT. (A) Median absolute number of CD3, CD4, CD8, CD19 lymphocytes. (B) T-cell proliferation responses to mitogens. Most patients had normal T-cell proliferative responses to mitogen stimulation. (C) Immunoglobulin production and class switching after CBT. Dashed line is 50th percentile of normal for age.
Discussion
In this large single center trial studying the outcomes after MAC UCBT without ATG, for nonmalignant disorders we found high engrafted survival rates (>90%) even in patients with active infections and underlying SCID. This high survival rate was associated with a fast functional immune reconstitution in all patients. Our current data suggest that this transplant platform is a good alternative for patients with nonmalignant disorders who lack a matched sibling donor. Although we understand that the myeloablative regimen platform could be controversial in some genotypes of patients with SCID, it allows for better cell line engraftment, prompt immune recovery, and decreased infection related mortality, with a very low incidence of GVHD.
Previous experience with UCBT has shown that delayed lymphocyte recovery is associated with impaired immune reconstitution, which correlates with increased morbidity and mortality secondary to infections early after transplant.5,11-13 The use of in vivo T-cell depletion using ATG or alemtuzumab within the conditioning regimen was found to be the main predictor of delayed immune recovery.10,14 In recent years, some centers have omitted ATG from their conditioning regimen to promote early immune recovery and increase the graft vs leukemia effect. These studies clearly showed an excellent immune recovery after UCBT when ATG is excluded.15 However, there is still an increased incidence of acute GVHD and graft failure without an advantage in the OS. Moreover, by individualizing ATG in a prospective clinical trial has shown a significant improvement of immune reconstitution after cord blood transplantation, while not impacting GVHD probability.16
To the best of our knowledge, this is the first prospective single center study in the US to show that avoiding serotherapy after UCBT for pediatric nonmalignant disorders, including a large group of patients with immune deficiencies and a very high risk of rejection, can produce a marked improvement in survival without an increased incidence of GVHD or graft failure. All patients were engrafted with a cumulative incidence of GVHD 2-4 of 16%, with only 3 patients developing severe acute GVHD. Moreover, there was an absence of chronic GVHD compared with other reports.7,11,17,18 This improved outcome without increased GVHD may be attributable to the younger median age of our children, in addition to the use of highly matched units (5/6 and 6/6), and improved engraftment secondary to a higher cell dose per kilogram compared with other reports of UCBT in nonmalignant pediatric patients. The naïve T-cell phenotype could also influence the reduced incidence of acute and chronic GVHD, making it an ideal source to preserve the quality of life of these young patients. Alternatively, the high percentage of engraftment syndrome (75%) associated with early steroid use after UCBT may have contributed to the low GVHD rates. The naïve population may also play a role in the development of early and functional anti-infectious immunity. Patients who developed new viral diseases after UCBT were able to clear these infections, whereas those who had viral reactivation or viremia did not develop end-organ disease. Notably, none of our engrafted patients died secondary to viral, bacterial, or fungal infections. Although virus-specific T-cell responses were not observed in patients in the absence of viral reactivation or infection, all evaluable patients with an infection showed detectable virus-specific T-cell responses early after transplants. Chiesa et al were the first to show early induction of virus-specific T cells after CBT, which is in line with other study findings, and now correlates with our findings in this prospective trial.7,19,20 Given the absence of significant viral illness, it seems likely that naïve lymphocytes initially appearing after UCB can adopt an effector phenotype following exposure to viral agents, even when this exposure occurs early after UCBT.
B-cell recovery was also rapid, with a median time to reach normal counts being 2 months after transplant and the appearance of immunoglobulin class switching, consistent with early CD4+ T-cell recovery. Finally, natural killer cell recovery was prompt, consistent with other studies,7 with a median time to achieve normal counts at 1-month post-UCBT. Fernandes et al also showed in a registry analysis (EUROCORD/EBMT) that patients after UCBT are more likely be off IVIG compared with Haplo identical transplant.21
Our early and rapid immune recovery in a very young population of patients with nonmalignant disorders has translated to an improved survival rate of over 90% without an increased risk of treatment related morbidity, including GVHD, or the need for subsequent transplants. Encouragingly, these benefits extend to patients with a history of pretransplant infections. Early availability with a decreased risk of GVHD or graft failure makes UCBT without serotherapy a safe and effective option for young patients with nonmalignant diseases, especially those with SCID and other immune deficiencies.
Authorship
Contribution: C.M. designed and conducted the study, analyzed the data, and wrote the manuscript; R.A.K., M.K.B., H.E.H., I.C.H., A.L. designed the study and reviewed the manuscript; P.A.-H., N.C., A.P., L.F., F.S., C.D., J.C., L.N., and S. Naik. collected the data and reviewed the manuscript; N.R., S. Nicholas, and I.C. reviewed the manuscript; H.R., B.O., T.J., K.Y., J.C., S.B., E.D., A.G., B.S., B.F., G.S., D.S., M.H., N.A., C.A., and M.W. prepared the figures and performed statistical analysis.
Conflict-of-interest disclosure: I.C.: consultant for Pharming and receives royalties from Wolters Kluwer (UpToDate) ; C.A.: advisory board of Sobi, research support from Genentech; N.A.: named inventor on patents and patent applications owned by BCM. N.A.: received one-time royalties from Celgene and Cell Medica, consulted in the past for Adaptimmune, and continues to consult for Equillium (pro bono) and The Children’s Cancer Hospital Egypt 5735; A.L.: equity holder of Allovir and Marker Thepapeutics; H.E.H.: equity holder of AlloVir, Marker Therapeutics, Fresh Wind Biotechnologies, CoRegen Inc., scientific advisory board of Gilead Biosciences, Novartis, Tessa Therapeutics, Marker Therapeutics, Kiadis, PACT Pharma, Mesoblast, Ankyra Therapeutics, research support of Kuur Therapeutics and Tessa Therapeutics; M.K.B.: equity holder of AlloVir, Marker Therapeutics, Tessa Therapeutics, scientific advisory board of Tessa Therapeutics, Marker Therapeutics, Allogene, Kuur, Walking Fish, Turnstone Biologics, Posedia, Tscan, Bluebird Bio, Adaptimmune Therapeutics PLC, Adintus, Onkimure, Triumvira Immunologics, Bellicum Pharmaceuticals, Memgen LLC, Brooklyn Immunotherapeutics, Coya, AstraZeneca Pharmaceuticals, royalties: Takeda, Bellicum, Maker Therapeutics; stock: Turnstone, Blue Bird Bio, Allogene, Walking Fish, AlloVir, Tessa, Tscan. The remaining authors declare no competing financial interests.
Correspondence: Caridad Martinez, Center for Cell and Gene Therapy, Baylor College of Medicine, Texas Children's Hospital, 1102 Bates Ave, Suite 1630, Houston, TX 77030; e-mail: camartin@texaschildrens.org.
References
Author notes
Data are available on request from the corresponding author, Caridad Martinez (camartin@texaschildrens.org).