TO THE EDITOR:
β-thalassemia is a hereditary disease caused by the β-globin gene mutations. Blood transfusion,1,2 stem cell transplantation,3,4 and gene therapy5-8 have demonstrated good effects on patients with β-thalassemia but with significant limitations and complications. microRNA-144/451 (miR-144/451) gene locus encodes miR-451 and miR-144, the 2 most abundantly expressed microRNAs (miRNAs) in erythrocytes.9 We have previously demonstrated that miR-144/451 safeguards normal erythrocyte formation because of its overall protective roles against oxidative stress,10 apoptosis,11 and differentiation defect.12 However, whether miR-144/451 also protects erythropoiesis in β-thalassemia is unclear. Here, we discovered a marked beneficial (rather than deleterious) effect of miR-144/451 depletion in β-thalassemic mice, and miR-144 depletion dominantly mediates this effect.
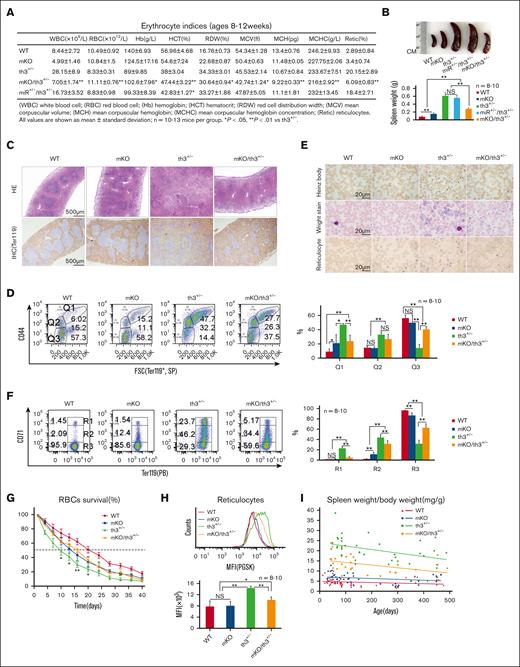
In th3+/− mice, the levels of miR-144 and miR-451 in peripheral blood and in erythroid precursors from hematopoietic organs were higher than those in wild-type mice (supplemental Figure 1A-D). The elevation of miR-144 and miR-451 in th3+/− blood was not due to the large numbers of reticulocytes because mature red blood cells (RBCs) and reticulocytes contain similar levels of miR-144 and miR-451 (supplemental Figure 1E). Accordingly, we crossed miR-144/451 knockout (mKO) mice (with mild anemia)10 with th3+/− mice (with severe anemia)13 to investigate whether the ablation of miR-144/451 is deleterious for th3+/− mice. Unexpectedly, mKO/th3+/− double-mutant mice exhibited dramatic improvement in anemia, evidenced by increased RBC/hemoglobin/hematocrit levels, decreased red cell distribution width (RDW) and reticulocyte count (Figure 1A; supplemental Figure 2A), smaller spleens (Figure 1B), relatively normal spleen architecture (Figure 1C), and decreased number of erythroblasts (Q1, Ter119+/CD44+/FSCHi) in spleens and bone marrows (BMs) (Figure 1D; supplemental Figure 2B). mKO/th3+/− mice also presented lower numbers of Heinz bodies and reticulocytes in peripheral blood (Figure 1E-F; supplemental Figure 2A). RBCs in mKO/th3+/− mice survived longer than those in th3+/− mice (Figure 1G). Moreover, mKO/th3+/− mice had lower cellular iron levels in reticulocytes (Figure 1H), a low transferrin receptor in immature splenic erythroid cells, low iron levels in sera (supplemental Figure 3A-C), and less iron precipitation in liver tissues (supplemental Figure 3D) compared with th3+/− mice. Iron levels in BM and spleen tissues were not affected (supplemental Figure 3E-F). To further verify the role of miR-144/451 in β-thalassemia, we crossed mKO mice with β-654 mice, another mouse model of β-thalassemia.14 An ameliorative effect was observed in β-654 mice after knocking out miR-144/451 (supplemental Figure 4). To investigate whether this effect is age-dependent, we examined older mKO/th3+/− mice (14-15 months old) and found that they still exhibited significant improvement in anemia compared with th3+/− mice (Figure 1I; supplemental Figure 5). Together, our findings indicate that the ablation of miR-144/451 reverses ineffective erythropoiesis in β-thalassemic mice. All animal experiments were approved by the animal ethics committee at Yangzhou University.
Effective erythropoiesis in th3+/− mice upon the depletion of miR-144/451. (A) Automated hematology analysis showing the elevated RBC, Hb, and HCT levels, and the reduced RDW in mKO/th3+/− mice. Reticulocytes were detected using flow cytometer. (B) Statistical analysis of the spleen weight. n = 8 to 10. Representative images of spleens from each group are shown. (C) HE staining and IHC staining of cell surface marker Ter119 in spleen sections of 10-week-old mice. Relatively normal splenic architecture in mKO/th3+/− mice is shown. (D) Flow cytometric analysis of the frequencies of splenic erythroblasts, reticulocytes, and mature RBCs identified using Ter119, CD44, and forward scatter channel (FSC). Effective erythropoiesis in mKO/th3+/− mice is evidenced by the significantly declined numbers of erythroblasts (Q1, Ter119+/CD44+/FSCHi) and increased proportion of mature erythrocytes (Q3, Ter119+/CD44-/FSCLow). (E) Observation of Heinz bodies, erythrocyte morphology, and the frequency of reticulocytes in peripheral blood from different groups of mice. Heinz bodies, reflecting precipitated α-globin on erythrocyte membranes, are reduced in mKO/th3+/− erythrocytes. Wright-Giemsa stain shows the significantly decreased anisocytosis and polychromasia (blue tinge) in peripheral blood from mKO/th3+/− mice compared with th3+/− mice. mKO/th3+/− mice exhibit less reticulocytes in circulating blood compared with th3+/− mice. (F) Flow cytometry analysis of the percentages of reticulocytes in peripheral blood from 4 genotypic mice. The frequency of both early-stage (R1, Ter119HiCD71Hi) and late-stage reticulocytes (R2, Ter119HiCD71Med) are declined, and the percentage of mature RBCs (R3, Ter119HiCD71Neg) is increased in the circulating blood from mKO/th3+/− mice compared with that in the blood from th3+/− mice. (A-F) All experiments were repeated at least 3 times. (G) Erythrocytes in mKO/th3+/− mice survive longer than erythrocytes in th3+/− mice. In vivo biotin labeling was used to follow erythrocyte survival kinetics over a 40-day period. The x-axis shows the days after biotin labeling; the y-axis shows the percentages of the biotinylated RBCs in mice at the age of 3 months. The time required for loss of 50% of the labeled RBCs in mKO/th3+/− mice is 6 days longer than in th3+/− mice. Experiments were repeated twice. (H) PGSK stain showing iron levels in reticulocytes of peripheral blood. mKO/th3+/− reticulocytes contain less iron. Experiments were repeated at least 3 times. (I) Aged mKO/th3+/− mice still possess low spleen weight to body weight ratio, suggesting a persistent alleviation of thalassemia because of the loss of miR-144/451. ∗P < .05; ∗∗P < .01. Hb, hemoglobin; HCT, hematocrit; HE, Hematoxylin/eosin; IHC, immunohistochemical staining; PGSK, phen green SK diacetate; RDW, red cell distribution width.
Effective erythropoiesis in th3+/− mice upon the depletion of miR-144/451. (A) Automated hematology analysis showing the elevated RBC, Hb, and HCT levels, and the reduced RDW in mKO/th3+/− mice. Reticulocytes were detected using flow cytometer. (B) Statistical analysis of the spleen weight. n = 8 to 10. Representative images of spleens from each group are shown. (C) HE staining and IHC staining of cell surface marker Ter119 in spleen sections of 10-week-old mice. Relatively normal splenic architecture in mKO/th3+/− mice is shown. (D) Flow cytometric analysis of the frequencies of splenic erythroblasts, reticulocytes, and mature RBCs identified using Ter119, CD44, and forward scatter channel (FSC). Effective erythropoiesis in mKO/th3+/− mice is evidenced by the significantly declined numbers of erythroblasts (Q1, Ter119+/CD44+/FSCHi) and increased proportion of mature erythrocytes (Q3, Ter119+/CD44-/FSCLow). (E) Observation of Heinz bodies, erythrocyte morphology, and the frequency of reticulocytes in peripheral blood from different groups of mice. Heinz bodies, reflecting precipitated α-globin on erythrocyte membranes, are reduced in mKO/th3+/− erythrocytes. Wright-Giemsa stain shows the significantly decreased anisocytosis and polychromasia (blue tinge) in peripheral blood from mKO/th3+/− mice compared with th3+/− mice. mKO/th3+/− mice exhibit less reticulocytes in circulating blood compared with th3+/− mice. (F) Flow cytometry analysis of the percentages of reticulocytes in peripheral blood from 4 genotypic mice. The frequency of both early-stage (R1, Ter119HiCD71Hi) and late-stage reticulocytes (R2, Ter119HiCD71Med) are declined, and the percentage of mature RBCs (R3, Ter119HiCD71Neg) is increased in the circulating blood from mKO/th3+/− mice compared with that in the blood from th3+/− mice. (A-F) All experiments were repeated at least 3 times. (G) Erythrocytes in mKO/th3+/− mice survive longer than erythrocytes in th3+/− mice. In vivo biotin labeling was used to follow erythrocyte survival kinetics over a 40-day period. The x-axis shows the days after biotin labeling; the y-axis shows the percentages of the biotinylated RBCs in mice at the age of 3 months. The time required for loss of 50% of the labeled RBCs in mKO/th3+/− mice is 6 days longer than in th3+/− mice. Experiments were repeated twice. (H) PGSK stain showing iron levels in reticulocytes of peripheral blood. mKO/th3+/− reticulocytes contain less iron. Experiments were repeated at least 3 times. (I) Aged mKO/th3+/− mice still possess low spleen weight to body weight ratio, suggesting a persistent alleviation of thalassemia because of the loss of miR-144/451. ∗P < .05; ∗∗P < .01. Hb, hemoglobin; HCT, hematocrit; HE, Hematoxylin/eosin; IHC, immunohistochemical staining; PGSK, phen green SK diacetate; RDW, red cell distribution width.
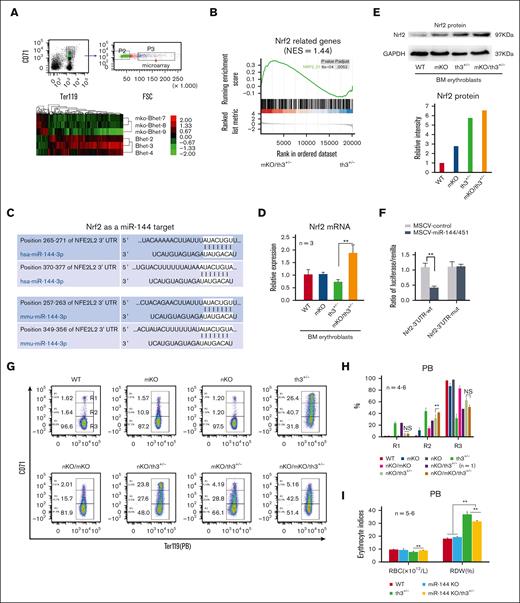
Reactive oxygen species (ROS) accumulation in peripheral blood cells was markedly lesser in mKO/th3+/− mice than in th3+/− mice (supplemental Figure 6). To explore the mechanism of anemia improvement in mKO/th3+/− mice, we fractionated erythroblasts from BM for microarray analysis (Figure 2A). Gene set enrichment analysis15 suggests significant activation of Nrf2 and superoxide dismutase (SOD) signaling (Figure 2B; supplemental Figure 7). Nrf2 and SOD are recognized as 2 important cytoprotective systems. The bioinformatics algorithm Targetscan predicts Nrf2 to be a potential miR-144 target (Figure 2C). Nrf2 messenger RNA and protein levels were elevated in mKO/th3+/− mice compared with that in th3+/− mice (Figure 2D-E). Luciferase reporter assay verified that Nrf2 messenger RNA is directly targeted by miR-144 (Figure 2F). Nrf2 is a key regulator of the antioxidant response,16,17 and its gene knockout leads to oxidant-induced hemolytic anemia.18,19 Thereby, we assessed the effects of inactivation of Nrf2 on mKO/th3+/− mice by crossing Nrf2 KO mice. Anemia in Nrf2 KO/mKO/th3+/− triple-mutant mice was aggravated, as evidenced by increased reticulocytosis (Figure 2G-H; supplemental Figure 8). Of note, this finding may be underestimated regarding the extent of anemia aggravation. We have recently found that high Nrf2 activity inhibits early-stage erythroid differentiation (unpublished data). To define the function of SOD in β-thalassemia, we crossed th3+/− mice with SODTg mice. High SOD activity in SODTg/th3+/− RBCs was confirmed (supplemental Figure 9A). However, ROS levels and the anemic indices remained the same as those in th3+/− mice (supplemental Figure 9B-G). These findings demonstrate that increased Nrf2 after depletion of miR-144/451 plays a partial role in the improvement of anemia in mKO/th3+/− mice.
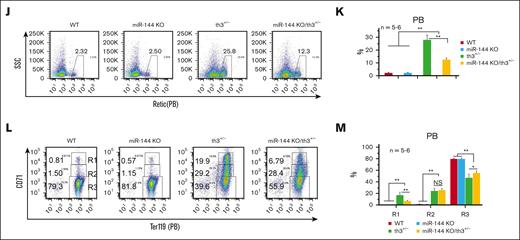
miR-144-Nrf2 regulatory axis plays a partial role in the alleviation of anemia in th3+/− mice. (A) Microarray transcriptome analysis of BM erythroblasts (Ter119+/CD71+/FSCHi) from th3+/− (βhet) and mKO/th3+/− (mKO/βhet) mice. Samples were analyzed for messenger RNA (mRNA) expression using Affymetrix Gene Chips. (B) Gene set enrichment analysis showing that Nrf2-related genes are upregulated in mKO/th3+/− BM erythroblasts compared with that in th3+/− BM erythroblasts. (C) Targetscan nucleotide sequence alignments showing the complementarity of 3' untranslated region (3′UTR) of Nrf2 mRNA and miR-144. There are 2 miR-144–binding sites in both mouse and human Nrf2 mRNAs. (D) Nrf2 mRNA levels in BM erythroblasts from mKO/th3+/− mice quantitated via real-time polymerase chain reaction. Experiments were repeated at least 3 times. (E) Western blot showing Nrf2 protein levels in BM erythroblasts from different groups of mice. Experiments were repeated at least 3 times. (F) Firefly luciferase reporter assay showing the direct binding of miR-144 to Nrf2 mRNA. 3′UTR of Nrf2 (WT) or its mutant version (mut, an 8-base pair replacement within the region complementary to miR-144 seed sequence) were cloned to luciferase reporter construct. Luciferase activities were determined 24 hours after transfection in 293T cells. (G-H) Flow cytometry analysis of the percentages of reticulocytes in peripheral blood from different group of mice. R1 (Ter119+CD71Hi), early-stage reticulocytes; R2 (Ter119+CD71Med), late-stage reticulocytes. (I) Automated hematology analysis revealing more RBCs in miR-144 KO/th3+/− blood and lower RDW in comparison to th3+/− mice. (J-K) Flow cytometry analysis of the percentages of peripheral blood reticulocytes by Retic-count (thiazole orange) reagent. (L-M) Flow cytometry analysis showing the frequencies of peripheral blood reticulocytes from WT, miR-144 KO, th3+/−, and miR-144 KO/th3+/− mice. The percentage of early-stage reticulocytes (R1) declines and mature RBCs (R3) increases in miR-144 KO/th3+/− blood compared with that in th3+/− mice. (I-M) All experiments were repeated at least 3 times. ∗P < .05; ∗∗P < .01. RDW, red cell distribution width; WT, wild-type; PB, peripheral blood; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
miR-144-Nrf2 regulatory axis plays a partial role in the alleviation of anemia in th3+/− mice. (A) Microarray transcriptome analysis of BM erythroblasts (Ter119+/CD71+/FSCHi) from th3+/− (βhet) and mKO/th3+/− (mKO/βhet) mice. Samples were analyzed for messenger RNA (mRNA) expression using Affymetrix Gene Chips. (B) Gene set enrichment analysis showing that Nrf2-related genes are upregulated in mKO/th3+/− BM erythroblasts compared with that in th3+/− BM erythroblasts. (C) Targetscan nucleotide sequence alignments showing the complementarity of 3' untranslated region (3′UTR) of Nrf2 mRNA and miR-144. There are 2 miR-144–binding sites in both mouse and human Nrf2 mRNAs. (D) Nrf2 mRNA levels in BM erythroblasts from mKO/th3+/− mice quantitated via real-time polymerase chain reaction. Experiments were repeated at least 3 times. (E) Western blot showing Nrf2 protein levels in BM erythroblasts from different groups of mice. Experiments were repeated at least 3 times. (F) Firefly luciferase reporter assay showing the direct binding of miR-144 to Nrf2 mRNA. 3′UTR of Nrf2 (WT) or its mutant version (mut, an 8-base pair replacement within the region complementary to miR-144 seed sequence) were cloned to luciferase reporter construct. Luciferase activities were determined 24 hours after transfection in 293T cells. (G-H) Flow cytometry analysis of the percentages of reticulocytes in peripheral blood from different group of mice. R1 (Ter119+CD71Hi), early-stage reticulocytes; R2 (Ter119+CD71Med), late-stage reticulocytes. (I) Automated hematology analysis revealing more RBCs in miR-144 KO/th3+/− blood and lower RDW in comparison to th3+/− mice. (J-K) Flow cytometry analysis of the percentages of peripheral blood reticulocytes by Retic-count (thiazole orange) reagent. (L-M) Flow cytometry analysis showing the frequencies of peripheral blood reticulocytes from WT, miR-144 KO, th3+/−, and miR-144 KO/th3+/− mice. The percentage of early-stage reticulocytes (R1) declines and mature RBCs (R3) increases in miR-144 KO/th3+/− blood compared with that in th3+/− mice. (I-M) All experiments were repeated at least 3 times. ∗P < .05; ∗∗P < .01. RDW, red cell distribution width; WT, wild-type; PB, peripheral blood; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
We have previously reported that loss of miR-144/451 affects normal erythropoiesis but is mediated largely by miR-451.10-12 These findings raise the possibility that miR-144 depletion attenuates anemia in th3+/− mice because miR-451 KO should worsen anemia. To test this hypothesis, we generated miR-144 and miR-451 single KO mice (supplemental Figure 10A-B). Homozygous miR-144 or miR-451 null mice were born at a normal Mendelian ratio, bred normally, and without obvious physical abnormalities. miR-144 KO did not affect the expression of mature miR-451 and vice versa (supplemental Figure 10C). As predicted, we obtained miR-144 KO/th3+/− double-mutant mice without any difficulty compared with th3+/− mice, suggesting the ameliorative effect of miR-144 deletion on anemia in th3+/− mice. miR-144 KO/th3+/− mice exhibited an increased number of RBCs and lower RDW (Figure 2I), decreased reticulocytosis (Figure 2J-M), and more mature RBCs (Figure 2L-M) in peripheral blood. miR-144 KO/th3+/− mice also possessed smaller spleens (although without statistical difference; supplemental Figure 11A) and a lower degree of reticulocytosis in spleens (supplemental Figure 11B). miR-144 single depletion derepressed Nrf2 expression (supplemental Figure 11C). These results demonstrated that the alleviation of anemia in β-thalassemic mice because of the deletion of the miR-144/451 gene locus is attributed partially to the KO of miR-144, which is consistent with in vitro findings that the miR-144/Nrf2 axis might associate with human sickle cell disease and thalassemia.16,20,21
We failed to generate adult miR-451–deficient th3+/− mice. At a normal Mendelian ratio, we should obtain approximately 25 miR-451 KO/th3+/− newborns (16.7%) in 148 offspring by crossing miR-451+/−/th3+/− with miR-451+/−/th3+/− mice (supplemental Figure 12A). However, we only obtained 1 miR-451 KO/th3+/− mouse (0.68%). Moreover, that mouse was much smaller than its littermates (supplemental Figure 12B) and eventually died at the age of 1 month. miR-451+/−/th3+/− double-heterozygous mice survived with enlarged spleens similar to the spleens of th3+/− mice (supplemental Figure 12C) and with similar frequencies of reticulocytosis in peripheral blood (supplemental Figure 12D-E). The frequencies of immature CD71+Ter119+FSCHi erythroblasts in both bone marrows and spleens from miR-451+/−/th3+/− double-heterozygous mice were also similar to the ones from th3+/− mice (supplemental Figure 12F-G). These results indicate that depletion of miR-451 failed to reverse the anemic condition in th3+/− mice and might even accelerate anemia in th3+/− mice. However, we should not ignore the fact that simultaneous depletion of both miR-144 and miR-451 does synergize to make β-thalassemia milder than miR-144 single depletion, indicating a coordination of miR-144 knocking out with miR-451 ablation with undefined mechanisms. However, we believe that, in the absence of miR-144, miR-451 deletion might also protect erythroid cells in β-thalassemia mice.
Taken together, our findings provide evidence that the existence or abnormal buildup of miR-144/451 exacerbates murine β-thalassemia. This effect is partially mediated by miR-144-Nrf2-controlled redox modulation. In addition, there may be ROS-independent mechanisms that contribute to the improvement of anemia in mKO/th3+/− mice (unpublished data). Our findings also demonstrate that miR-144 and miR-451 commit overlapping and independent gene regulations in erythroblasts, but the net effect of miR-144 and miR-451 coordination and which miRNA is dominant is stress type– or disease-dependent. Our studies also show that globin gene mutation is the fundamental cause of β-thalassemia, but the severity of the anemia also depends on epigenetic modifiers such as miRNAs. The limitation of our study is that murine models may not truly recapitulate human erythropoietic responses.
Acknowledgments: The authors thank Mitch Weiss (St. Jude Children’s Research Hospital) for providing th3+/− β-thalassemic and miR-144/451 KO mice. The authors are also grateful to Yitao Zeng (Shanghai Jiao Tong University School of Medicine) and Depei Liu (Peking Union Medical College/Chinese Academy of Medical Sciences) for β-654 thalassemic mice and Masayuki Yamamoto (Tohoku University Graduate School of Medicine) and Depei Liu for Nrf2 KO mice. The authors thank Lilyin Yu for language editing.
This work was supported by the National Natural Science Foundation of China (81670186 and 81870096) (D.Y.). This work was also supported by funds from Guangxi Science and Technology Project (21-220-22,GuikeAD22035121, and GuikeZy1949016) (S.H.), Guangxi Medical High-level backbone Talents "139" Plan (G202003023) (S.H.), Open Project from Guangxi Key Laboratory of Reproductive Health and Birth Defects Prevention (GXWCH-ZDKF-2022-01) (D.Y.), and the Postgraduate Research & Practice Innovation Program of Jiangsu Province (KYCX21_3294) (L.L.) and (KYCX22_3565) (Lei Yang).
Contribution: D.Y., L.L., and F. Wang designed the study; L.L., F. Wang, Y.L., S.H., F. Wu, Lei Yang, L.X., T.W., S.Z., F.Y., Z.W., Lan Yang, Z.Y., Y.Z., J.X., and X.Y. conducted the experiments; X.F. and Z.W. performed the bioinformatics analyses; H.W. interpreted data and provided the suggestions and consultation; and D.Y. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Duonan Yu, Jiangsu Key Laboratory of Experimental & Translational Non-coding RNA Research, Yangzhou University Medical College, Yangzhou, Jiangsu Province 225009, China; email: dnyu@yzu.edu.cn; and Hongwei Wei, Guangxi Zhuang Autonomous Region Women and Children Care Hospital, Nanning, China; email: weihongwei1965@163.com.
References
Author notes
L.L., F. Wang, Y.L., and S.H. contributed equally to this work.
Microarray data reported in the study are available at Gene Expression Omnibus (accession number GSE208608).
Data are available on request from the corresponding author, Duonan Yu (dnyu@yzu.edu.cn).
The full-text version of this article contains a data supplement.