Key Points
Sema7A controls neutrophil chemotaxis in vitro and the formation of platelet-neutrophil complexes in vivo.
Sema7A influences the early phase of inflammation, which is relevant to the outcome in murine experimental sepsis.
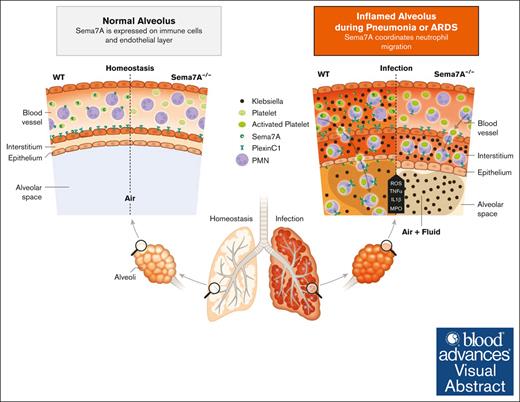
Visual Abstract
Pulmonary defense mechanisms are critical for host integrity during pneumonia and sepsis. This defense is fundamentally dependent on the activation of neutrophils during the innate immune response. Recent work has shown that semaphorin 7A (Sema7A) holds significant impact on platelet function, yet its role on neutrophil function within the lung is not well understood. This study aimed to identify the role of Sema7A during pulmonary inflammation and sepsis. In patients with acute respiratory distress syndrome (ARDS), we were able to show a correlation between Sema7A and oxygenation levels. During subsequent workup, we found that Sema7A binds to the neutrophil PlexinC1 receptor, increasing integrins, and L-selectin on neutrophils. Sema7A prompted neutrophil chemotaxis in vitro and the formation of platelet-neutrophil complexes in vivo. We also observed altered adhesion and transmigration of neutrophils in Sema7A−/−animals in the lung during pulmonary inflammation. This effect resulted in increased number of neutrophils in the interstitial space of Sema7A−/− animals but reduced numbers of neutrophils in the alveolar space during pulmonary sepsis. This finding was associated with significantly worse outcome of Sema7A−/− animals in a model of pulmonary sepsis. Sema7A has an immunomodulatory effect in the lung, affecting pulmonary sepsis and ARDS. This effect influences the response of neutrophils to external aggression and might influence patient outcome. This trial was registered at www.ClinicalTrials.gov as #NCT02692118.
Introduction
Sepsis remains one of the leading causes of death worldwide and one of the leading causes of hospitalization.1 Pneumonia is frequently the source of pulmonary sepsis, caused by the invasion of pathogens entering the lung and through the pulmonary barrier into the human circulation. In hospitalized and ventilated patients, gram-negative bacteria are frequently the cause of pneumonia, whereas gram-positive bacteria are more common in patients with community-acquired pneumonia.2 The therapy of pneumonia consists of treatment with supplemental oxygen if required, appropriate diagnostic testing, and appropriate antiviral and antimicrobial therapy; however, this approach is not always successful, and the severity of the disease progresses. Therefore, understanding the mechanisms that control, influence, or support the host response during pneumonia might help to improve the outcome of patients.3
Neutrophils are the forefront in the defense against pathogens that challenge the lung.4 After activation, neutrophils tether and roll along the endothelial surface. This process is dependent on L-selectin expression.5,6 L-selectin cleavage from the neutrophil surface is triggered by integrin engagement and is involved in neutrophil recruitment to the lung and bacterial clearance.7 P-selectin expression and MAC-1 are also important during the recruitment of neutrophils into the lung and correlate with outcome in patients with acute respiratory distress syndrome (ARDS).8,9 Subsequently, integrins play key roles in the transendothelial migration and activation of neutrophils. The differential context of the integrins is demonstrated by the fact that blocking antibodies against the β2 integrin CD18 results in an increased number of neutrophils in the alveolar space and a reduction in pulmonary injury. β2 integrin binds to ICAM-1 on endothelial cells, resulting in increased transendothelial neutrophil migration, an essential mechanism of pulmonary host defense.10
Recent work has shown that the neuronal guidance protein semaphorin 7A (Sema7A) is important throughout the initial stages of inflammation. During the inflammatory response, Sema7A can bind to integrin α1β1 to facilitate cytokine storm.11 We have shown in the past that Sema7A is induced during periods of hypoxia and activates platelets through the glycoprotein Ib-IX-V receptor complex.12,13 To highlight the role of Sema7A during the pulmonary immune response, we investigated the role of Sema7A during lipopolysaccharide (LPS)-induced and Klebsiella pneumoniae–induced pulmonary effects and in human samples from patients with ARDS.
Methods
Mice
Sema7A−/− mutant mice were generated, characterized, and validated as described in Pasterkamp et al.14 We generated the Sema7A-floxed mouse line (Sema7AloxP/loxP),12 which was then crossbred with the listed Cre recombinase-positive mouse lines to generate mice with the following tissue-specific gene deletions: immune cell-specific LysMCre+; megakaryocyte- and thrombocyte-specific PF4Cre+; and endothelial-specific Tie2Cre+. As internal experimental controls, Sema7AloxP/loxP Cre– littermates were used. WT animals were obtained from inbreeding colonies maintained by operational researchers and animal facility staff.
Murine lung injury model with LPS inhalation
Murine lung injury model with K pneumoniae instillation
Briefly, mice were anesthetized with a 3-component fentanyl mixture applied intraperitoneally, followed by a small skin incision and exposure of the trachea. Direct tracheal instillation of 4 × 107K pneumoniae (ATCC strain 43816) in 50 μL of phosphate-buffered saline was performed using a 30-gauge needle to minimize tracheal damage. Additional information is provided in the supplemental Data.
In vivo migration assay
Twenty-four hours after K pneumoniae instillation, a fluorescent (APC)–conjugated Ly6G antibody (clone 1A8) was injected via the tail vein to label intravascular PMNs. The lungs were incubated with anti-CD45 PerCP-Cy5·5 (clone 30-F11) and anti-Ly6G PE/Cy7 (clone 1A8). The absolute cell counts in the BALF and lungs were determined. We differentiated between interstitial PMNs (CD45-PerCP-Cy5·5+; Ly6G-PE-Cy7+; and Ly6G-APC–) and intravascular PMNs (CD45-PerCP-Cy5·5+; Ly6G-PE-Cy7+; and Ly6G-APC+) (all antibodies from BioLegend) by flow cytometry (FACS Canto II; BD Biosciences).
Chemotaxis assay
For detailed information, please see the supplemental Data.
Intravital microscopic analysis of cremaster microcirculation
Mouse cremaster preparation was performed as previously described.17 Additional information is provided in the supplemental Data.
Lung intravital microscopy
Anesthesia was performed with ketamine-xylazine (100 mg/kg-16 mg/kg), and an antibody cocktail composed of 7 μg of anti-CD31-A647, 5 μg of Ly6G-A488, and 5 μg of GPIX-A546 was administered IV in a bolus of 100 μL. For correct microscopy of the lung, the mouse was intubated by tracheotomy, and breathing was normalized for 5 minutes after the instillation of 5 μg/g BW LPS (O26:B6). For murine lung microscopy, a Leica Stellaris 8 resonance microscope with an original magnification 25× objective and a resolution of 232.72 μm2 was used. Videos were analyzed with Leica software. Additional information is provided in the supplemental Data.
Flow cytometry
For detailed information, please see the supplemental Data.
Staining of murine PNCs
For detailed information on staining of murine platelet-neutrophil complexes (PNCs), please see the supplemental Data.
Human fibrinogen binding assay and human ICAM-1 binding assay
For detailed information, please see the supplemental Data.
Regulation of adhesion receptors
For detailed information, please see the supplemental Data.
Immunofluorescent staining of purified murine PMNs, human PMNs, and lung tissue
For detailed information, please see the supplemental Data.
Enzyme-linked immunosorbent assay
All enzyme-linked immunosorbent assay kits were from R&D and used the DuoSet principle. The absorbance of the developed color was measured at 450 nm in a plate reader (Tecan, Männedorf, Switzerland).
Respiratory burst assay
For detailed information, please see the supplemental Data.
Phagocytosis assay
For detailed information, please see the supplemental Data.
Proteomics analysis
For detailed information, please see the supplemental Data.
Immunohistochemistry
For detailed information, please see the supplemental Data.
H&E staining and evaluation
For detailed information, please see the supplemental Data.
Statistical analysis
The data are presented as bar graphs using the mean ± standard deviation. Statistical analysis was performed using Student t tests to compare the 2 groups. When comparing several groups with each other, 1-way analyses of variances and Dunnett tests were performed. For comparisons that are considered statistically significant, the P values are displayed as ∗P < .05; ∗∗P < .01; and ∗∗∗P < .001.
All animal procedures conformed to the German guidelines for the use of living specimens and were approved by the institutional animal care and the Regierungspräsidium Tübingen and Würzburg.
Approval for the processing of human samples was given by the ethics committee (institutional review board) of the University of Tuebingen (approval number 156/2016BO1; ClinicalTrials.gov: NCT02692118). Informed consent was obtained from patients or legal guardians before samples were collected and processed for further analysis.
Results
Patients with ARDS have increased plasma Sema7A levels
To identify a potential change of Sema7A in pulmonary inflammation or infection, we evaluated patients undergoing major surgery with intensive care unit stay requiring mechanical ventilation and patients with ARDS who presented with severe pulmonary infection (Figure 1A). Patients without ARDS were ventilated for about 2.5 days and showed significant impairment of organ function as determined by APACHE II and SOFA scores. We measured Sema7A blood levels on the day of admission and correlated these with clinical and laboratory values. Patients with ARDS showed significantly increased Sema7A values associated with illness severity, such as APACHE II and SOFA scores (Figure 1A) . Patients with ARDS also showed a significant correlation between leukocyte counts and Sema7A levels and between Sema7A levels and oxygenation levels (Figure 1B). This points to the role of Sema7A in the inflammatory response of the lung during ARDS. Although this is, of course, only mild evidence, this leads us to further pursue a potential role of Sema7A in pulmonary inflammation.
Clinical Sema7A values correlate with leukocyte count and clinical oxygenation values in patients with ARDS. Demographic data and samples of patients undergoing elective surgery with postoperative ventilation and ICU stays and patients admitted to the ICU for ARDS with severe pulmonary inflammation who were matched with propensity score. (A) Demographic data, ICU scores, and laboratory values for both patient groups are presented as means ± standard deviations, with values compared using the Wilcoxon rank-sum test. Significant values are set in bold. (B) The correlation of various laboratory values, ventilation parameters, and oxygenation values with serum levels of Sema7A is depicted. Pearson r and the lower and upper limits of the 95% confidence interval are shown. Significant correlations are highlighted in red. BE, Base excess; ICU, intensive care unit; INR, International Normalized Ratio; LDH, Lactate dehydrogenase.
Clinical Sema7A values correlate with leukocyte count and clinical oxygenation values in patients with ARDS. Demographic data and samples of patients undergoing elective surgery with postoperative ventilation and ICU stays and patients admitted to the ICU for ARDS with severe pulmonary inflammation who were matched with propensity score. (A) Demographic data, ICU scores, and laboratory values for both patient groups are presented as means ± standard deviations, with values compared using the Wilcoxon rank-sum test. Significant values are set in bold. (B) The correlation of various laboratory values, ventilation parameters, and oxygenation values with serum levels of Sema7A is depicted. Pearson r and the lower and upper limits of the 95% confidence interval are shown. Significant correlations are highlighted in red. BE, Base excess; ICU, intensive care unit; INR, International Normalized Ratio; LDH, Lactate dehydrogenase.
Sema7A is expressed at the pulmonary immune interface and affects neutrophil migration
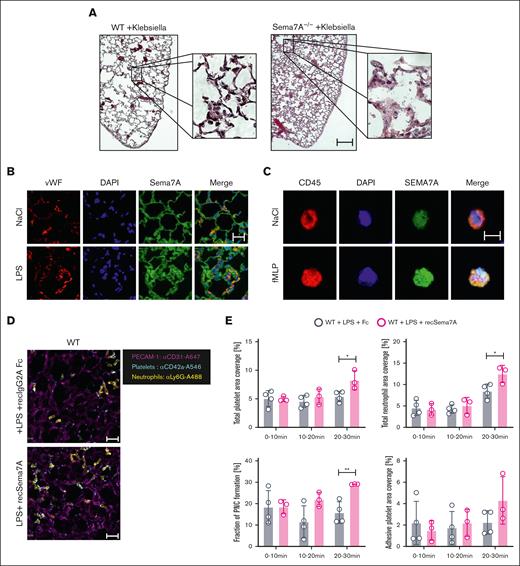
To evaluate the role of Sema7A in the lung further, we used Sema7A−/− mice and controls to establish a model of K pneumoniae–induced pneumonia and found significantly altered histological images with stronger changes in the interstitial and alveolar structures in the Sema7A−/− mice compared than controls (Figure 2A; supplemental Figure 1). The Sema7A−/− mice showed thicker interstitial spaces, more pronounced inflammation in the alveolar septa, and more pronounced tissue destruction of the lungs than controls. We then stained Sema7A in pulmonary tissue and on neutrophils, because these are the first line of defense at the pulmonary alveolar-capillary barrier during an external assault, and found that Sema7A was clearly expressed on neutrophils and the alveolar-capillary barrier (Figure 2B-C; supplemental Figure 1). We also performed WB analysis of neutrophils to show Sema7A expression on neutrophils (supplemental Figure 2). Neutrophil migration to the site of infection is another important mechanism in the defense of the pulmonary surface; therefore, we exposed Sema7A−/− mice to IV LPS injection and found significantly increased cell speed, reduced numbers of stationary cells, and reduced numbers of transmigrated neutrophils in Sema7A−/− animals compared with the controls in a cremaster model (supplemental Figure 3). Comparison of adhesion molecules on Sema7A−/− and WT PMNs showed no difference in noninflammatory conditions (supplemental Figure 4). We transferred this analysis into the pulmonary circulation after LPS inhalation with injecting recSema7A to determine whether we could visualize the effect of Sema7A within the lung. We found an increase in the area that was covered with neutrophils in the pulmonary circulation and an increase in the number of platelets interacting with neutrophils, that is, PNCs, in recSema7A–injected animals (Figure 2D-E).
Sema7A is required for neutrophil adhesion and migration during inflammation. (A) Histological cross-sections and magnifications of lung tissue from WT and Sema7A−/− mice 24 hours after the instillation of 4 × 107 cells of K pneumoniae (scale bar, 200 μm). (B) IF staining of Sema7A (green) and vWF (red) in endothelial cells of murine lung tissue and nuclear staining with DAPI (4′,6-diamidino-2-phenylindole; blue; scale bar, 20 μm). (C) IF staining showing Sema7A expression (green) on the surface of human CD45-marked PMNs, (red) treated with NaCl or fMLP for 15 minutes (scale bar, 10 μm). (D) Representative video images of PNCs in murine lungs after LPS instillation with additional recombinant Sema7A or IgG2A-Fc (controls) treatment after 30 minutes (scale bar, 30 μm). (E) Total neutrophil area coverage, total and adhesive platelet area coverage, and the fractions of PNCs formed in the lung as determined via intravital confocal microscopic analysis of the lung in WT mice instilled with 5 μg/g BW LPS, with or without additional treatment with recombinant Sema7A (the data are the mean ± SD). ∗P < .05; ∗∗P < .01; ∗∗∗P < .001 as indicated. Fc, control fragment; IF, immunofluorescence; vWF, von Willebrand Factor.
Sema7A is required for neutrophil adhesion and migration during inflammation. (A) Histological cross-sections and magnifications of lung tissue from WT and Sema7A−/− mice 24 hours after the instillation of 4 × 107 cells of K pneumoniae (scale bar, 200 μm). (B) IF staining of Sema7A (green) and vWF (red) in endothelial cells of murine lung tissue and nuclear staining with DAPI (4′,6-diamidino-2-phenylindole; blue; scale bar, 20 μm). (C) IF staining showing Sema7A expression (green) on the surface of human CD45-marked PMNs, (red) treated with NaCl or fMLP for 15 minutes (scale bar, 10 μm). (D) Representative video images of PNCs in murine lungs after LPS instillation with additional recombinant Sema7A or IgG2A-Fc (controls) treatment after 30 minutes (scale bar, 30 μm). (E) Total neutrophil area coverage, total and adhesive platelet area coverage, and the fractions of PNCs formed in the lung as determined via intravital confocal microscopic analysis of the lung in WT mice instilled with 5 μg/g BW LPS, with or without additional treatment with recombinant Sema7A (the data are the mean ± SD). ∗P < .05; ∗∗P < .01; ∗∗∗P < .001 as indicated. Fc, control fragment; IF, immunofluorescence; vWF, von Willebrand Factor.
Sema7A binds to PlexinC1 on neutrophils
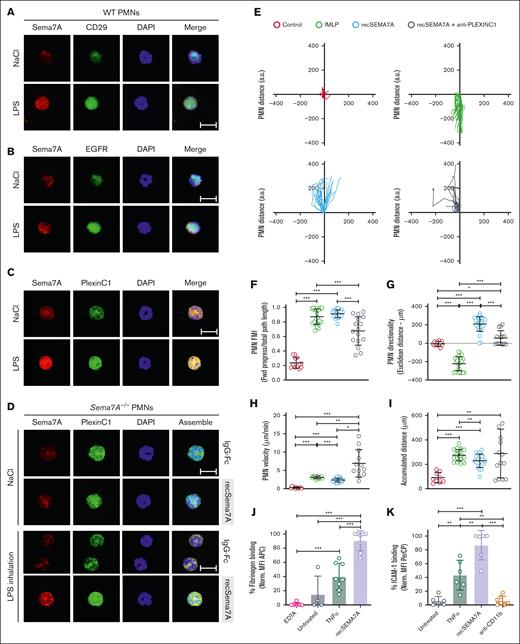
As described in “Introduction,” several receptors were described as potential Sema7A target receptors in the literature. Therefore, we aimed to identify which receptor on neutrophils binds to Sema7A. We isolated neutrophils from WT animals after NaCl or LPS inhalation and examined the localization of Sema7A during inflammatory stimulation of neutrophils. An induction of Sema7A expression during stimulation was described previously, and we were able to conform this.18 We stained for the Sema7A receptors integrin β1 (CD29), PlexinC1, and the EGF receptor, because all of these membrane proteins have been reported to be potential target receptors for Sema7A. We found a robust Sema7A signal on neutrophils after LPS inhalation, as indicated by staining for PlexinC1 and Sema7A (Figure 3C), whereas no association of Sema7A with CD29 or EGFR was observed (Figure 3A-B; supplemental Figures 5 and 6). To further validate this finding, we isolated neutrophils from Sema7A−/− mice after recSema7A injection and LPS inhalation. After this, we found robust colocalization of Sema7A with the PlexinC1 receptor on these cells, strongly suggesting a direct binding of recSema7A to PLXNC1 but not to another receptor (Figure 3D). Flowcytometry confirmed the obtained results (supplemental Figure 7).
Sema7A binds to neutrophil PlexinC1 and influences neutrophil chemotaxis. Stained neutrophils isolated from saline (NaCl)- or LPS–treated WT and Sema7A−/− mice 4 hours after incubation. (A) Expression of Sema7A (red) and CD29 (green) on PMNs harvested from WT mice treated with NaCl or LPS (scale bar, 10 μm). (B) Expression of Sema7A (red) and EGFR (green) on PMNs harvested from WT mice treated with NaCl or LPS. No protein colocalization was visible in the merged pictures in either condition (scale bar, 10μm). (C) Expression of Sema7A (red) and PlexinC1 (green) on PMNs harvested from WT mice treated with NaCl or LPS. Sema7A expression is highly increased in LPS–treated mice, and the merged pictures show a strong interaction between Sema7A and PlexinC1 (scale bar, 10 μm). (D) Surface PMN expression of Sema7A (red) and PlexinC1 (green) in Sema7A−/− mice after the injection of exogenous recombinant Sema7A or IgG-Fc (control) after LPS or NaCl (controls) inhalation. Strong binding of exogenous Sema7A to Sema7A−/− PMNs was observed in LPS inhalation group. Multiple acquisitions of stained cells were analyzed from independently performed triplicate experiments (scale bar, 10 μm). (E) Human PMNs were subjected to different stimuli in bidirectional chemotactic chambers. Acquired time lapse videos over a 3-hour period were analyzed. Representative plots of PMN chemotactic tracks toward NaCl (control; red), fMLP (green), recombinant human SEMA7A (recSema7A; blue), or recSema7A together with antibodies against human PlexinC1 (anti-PLEXINC1; gray). (F-I) Comparison of the chemotaxis parameters forward migration index (FMI), Euclidean distance under the aspect of the direction, PMN velocity, and accumulated PMN distance. (J) PMN binding affinity was indicated by APC−labeled fibrinogen on the surface of Ly6G-positive PMNs, as analyzed by FACS. The EDTA group was the internal negative control to measure the baseline autofluorescence, the untreated group was the fibrinogen-negative control, TNF-α was used as a potent PMN stimulator, and treatment of PMNs with recombinant SEMA7A before APC−labeled fibrinogen represented the fibrinogen binding target group of interest. The fibrinogen-APC MFI was normalized and is displayed as a percentage. (K) PMN binding affinity was indicated by PerCP-labeled ICAM-1 on the surface of Ly6G-positive PMNs by FACS. The untreated group was the ICAM-1–negative control, TNF-α was used as a potent PMN stimulator, and treatment of PMNs with recombinant SEMA7A before PerCP-labeled ICAM-1 represented the ICAM-1 binding target group of interest. CD11b antibody treatment was used as a control for the inactivation of ICAM-1 binding. The ICAM-1 PerCP MFI was normalized and is displayed as a percentage. In (F-K), all group comparisons were performed by unpaired 2-tailed Student t tests (the data are the mean ± SD); ∗P < .05; ∗∗P < .01; ∗∗∗P < .001 as indicated. MFI, Mean Fluorescence Intensity.
Sema7A binds to neutrophil PlexinC1 and influences neutrophil chemotaxis. Stained neutrophils isolated from saline (NaCl)- or LPS–treated WT and Sema7A−/− mice 4 hours after incubation. (A) Expression of Sema7A (red) and CD29 (green) on PMNs harvested from WT mice treated with NaCl or LPS (scale bar, 10 μm). (B) Expression of Sema7A (red) and EGFR (green) on PMNs harvested from WT mice treated with NaCl or LPS. No protein colocalization was visible in the merged pictures in either condition (scale bar, 10μm). (C) Expression of Sema7A (red) and PlexinC1 (green) on PMNs harvested from WT mice treated with NaCl or LPS. Sema7A expression is highly increased in LPS–treated mice, and the merged pictures show a strong interaction between Sema7A and PlexinC1 (scale bar, 10 μm). (D) Surface PMN expression of Sema7A (red) and PlexinC1 (green) in Sema7A−/− mice after the injection of exogenous recombinant Sema7A or IgG-Fc (control) after LPS or NaCl (controls) inhalation. Strong binding of exogenous Sema7A to Sema7A−/− PMNs was observed in LPS inhalation group. Multiple acquisitions of stained cells were analyzed from independently performed triplicate experiments (scale bar, 10 μm). (E) Human PMNs were subjected to different stimuli in bidirectional chemotactic chambers. Acquired time lapse videos over a 3-hour period were analyzed. Representative plots of PMN chemotactic tracks toward NaCl (control; red), fMLP (green), recombinant human SEMA7A (recSema7A; blue), or recSema7A together with antibodies against human PlexinC1 (anti-PLEXINC1; gray). (F-I) Comparison of the chemotaxis parameters forward migration index (FMI), Euclidean distance under the aspect of the direction, PMN velocity, and accumulated PMN distance. (J) PMN binding affinity was indicated by APC−labeled fibrinogen on the surface of Ly6G-positive PMNs, as analyzed by FACS. The EDTA group was the internal negative control to measure the baseline autofluorescence, the untreated group was the fibrinogen-negative control, TNF-α was used as a potent PMN stimulator, and treatment of PMNs with recombinant SEMA7A before APC−labeled fibrinogen represented the fibrinogen binding target group of interest. The fibrinogen-APC MFI was normalized and is displayed as a percentage. (K) PMN binding affinity was indicated by PerCP-labeled ICAM-1 on the surface of Ly6G-positive PMNs by FACS. The untreated group was the ICAM-1–negative control, TNF-α was used as a potent PMN stimulator, and treatment of PMNs with recombinant SEMA7A before PerCP-labeled ICAM-1 represented the ICAM-1 binding target group of interest. CD11b antibody treatment was used as a control for the inactivation of ICAM-1 binding. The ICAM-1 PerCP MFI was normalized and is displayed as a percentage. In (F-K), all group comparisons were performed by unpaired 2-tailed Student t tests (the data are the mean ± SD); ∗P < .05; ∗∗P < .01; ∗∗∗P < .001 as indicated. MFI, Mean Fluorescence Intensity.
Sema7A induces neutrophil migration through PlexinC1
Chemotaxis is an essential step by which neutrophils migrate to the site of infection and/or inflammation. We used an in vitro cell migration assay with neutrophils exposed to fMLP or Sema7A or neutrophils preincubated with anti-PLXNC1 antibody before Sema7A exposure. Treatment with fMLP was used to simulate bacterial exposure of neutrophils and resulted in the attraction of neutrophils toward the highest concentration, with profound migratory velocity and distance (Figure 3E-I). In contrast, recSEMA7A resulted in the repulsion of neutrophils, with similar velocities and distances as the effect of fMLP. This effect could be reduced when neutrophils were preincubated with anti-PLEXINC1 antibodies before the experiment (Figure 3E-I). This finding showed that neutrophil migration moved away from Sema7A, and the inhibition of PlexinC1 alters this chemorepulsive effect. Next, we examined whether Sema7A influenced the activation of MAC-1 by measuring fibrinogen binding. Sema7A induced the binding of fibrinogen to MAC-1, suggesting the significant influence of Sema7A on integrin activation (Figure 3J). In addition, we also performed an ICAM-1 binding assay and found that Sema7A significantly increased the binding of ICAM-1 to neutrophils stimulated with Sema7A. This finding suggests that Sema7A induces a functional upregulation of key integrins involved in neutrophil migration (Figure 3K) and thereby significantly promotes this process.
Human neutrophil proteomics analysis confirms the influence of Sema7A on integrin expression
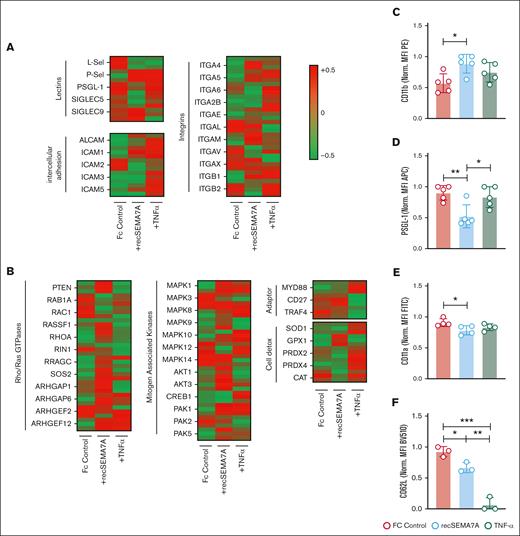
To confirm the functional data and gain a better understanding of exactly how neutrophils react to Sema7A, we decided to stimulate neutrophils directly with recSEMA7A (Fc control respectively) for 15 minutes. We also exposed neutrophils to TNF-α (100 pg/mL), because TNF-α is a cardinal cytokine in the alveolar space during the early phase of ARDS and the attraction of neutrophils. We found a significant increase of the expression of PSGL-1 and ICAM-1 through Sema7A (Figure 4A). This was the opposite of the effect of TNF-α and resulted in a decrease in L-selectin and an increase in ICAM-1. Phosphoproteomics analysis showed that the potential pathways involved in this effect were the MAP kinase and PTEN pathways (Figure 4B; supplemental Figure 8). To confirm the obtained results, we examined some of the proteins on neutrophils through flow cytometry and found that the expression of CD11b was increased after recSEMA7A stimulation. In addition, we were able to confirm that PSGL-1 and L-selectin were reduced, and this was dependent on the Plexin C1 receptor (Figure 4C-F; supplemental Figure 9).
Essential neutrophil integrins are influenced by SEMA7A. Human PMNs were incubated with NaCl, 10 ng/mL TNF-α, or 2 μg/mL recSEMA7A for 15 minutes before proteomics analysis. The acquired raw data were analyzed after normalization. To analyze the samples, a 1-Factorial linear model was fitted with LIMMA, resulting in a 2-sided t test or F test based on moderated statistics. All presented P values were adjusted for multiple analyses by controlling the false discovery rate according to Benjamini and Hochberg. Proteins were defined as differential when |logFC| >.5 and an adjusted P value <.05 from triplicate experiments. (A) Expression of neutrophil surface lectin proteins and membrane integrin proteins from harvested samples. (B) Expression of intracellular neutrophil Rho/Ras GTPases, mitogen-associated kinases, adapter proteins, and detoxifying enzymes. (C) PMN surface expression of CD11b after 15 minutes of incubation with recSEMA7A or TNF-α. Measurement was performed by FACS, and the MFI (PE) was normalized to the highest measured value. (D) PMN surface expression of PSGL-1 after 15 minutes of incubation with recSEMA7A or TNF-α. (E) PMN surface expression of CD11a after 15 minutes of incubation with recSEMA7A or TNF-α. (F) PMN surface expression of CD62L after 15 minutes of incubation with recSEMA7A or TNF-α. Measurement was performed by FACS, and the MFI (BV510) was normalized to the highest measured value. Multiple cells were analyzed from independently performed experiments in triplicate. Group comparisons were performed by unpaired 2-tailed Student t tests (the data are the mean ± SD). ∗P < .05; ∗∗P < .01; ∗∗∗P < .001 as indicated. MFI, Mean Fluorescence Intensity; PE, Phycoerythrin.
Essential neutrophil integrins are influenced by SEMA7A. Human PMNs were incubated with NaCl, 10 ng/mL TNF-α, or 2 μg/mL recSEMA7A for 15 minutes before proteomics analysis. The acquired raw data were analyzed after normalization. To analyze the samples, a 1-Factorial linear model was fitted with LIMMA, resulting in a 2-sided t test or F test based on moderated statistics. All presented P values were adjusted for multiple analyses by controlling the false discovery rate according to Benjamini and Hochberg. Proteins were defined as differential when |logFC| >.5 and an adjusted P value <.05 from triplicate experiments. (A) Expression of neutrophil surface lectin proteins and membrane integrin proteins from harvested samples. (B) Expression of intracellular neutrophil Rho/Ras GTPases, mitogen-associated kinases, adapter proteins, and detoxifying enzymes. (C) PMN surface expression of CD11b after 15 minutes of incubation with recSEMA7A or TNF-α. Measurement was performed by FACS, and the MFI (PE) was normalized to the highest measured value. (D) PMN surface expression of PSGL-1 after 15 minutes of incubation with recSEMA7A or TNF-α. (E) PMN surface expression of CD11a after 15 minutes of incubation with recSEMA7A or TNF-α. (F) PMN surface expression of CD62L after 15 minutes of incubation with recSEMA7A or TNF-α. Measurement was performed by FACS, and the MFI (BV510) was normalized to the highest measured value. Multiple cells were analyzed from independently performed experiments in triplicate. Group comparisons were performed by unpaired 2-tailed Student t tests (the data are the mean ± SD). ∗P < .05; ∗∗P < .01; ∗∗∗P < .001 as indicated. MFI, Mean Fluorescence Intensity; PE, Phycoerythrin.
Neutrophil- and platelet-derived Sema7A expression alters leukocyte migration during inflammation
In the next step, we sought to identify the source of Sema7A mediating the observed in vivo effects. We pursued this in the intravital cremaster model because the pulmonary imaging model is very complex and labor intensive and it would be almost impossible to image all the needed animals in this model.
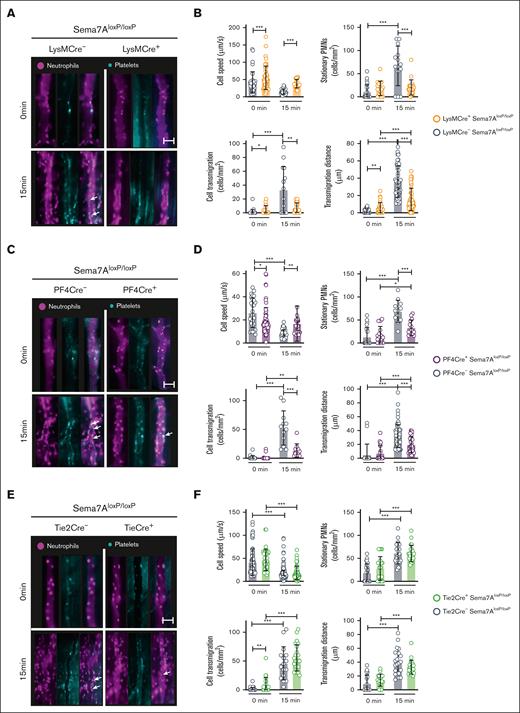
We have previously shown that red blood cell–derived Sema7A is important during sterile inflammation induced by myocardial reperfusion injury. We did not find an involvement of red blood cell–derived Sema7A in neutrophil migration by intravital microscopy. Next, we used LysMCre+Sema7AloxP/loxP animals. These mice showed significantly reduced numbers of adherent and transmigrated cells and significant differences in cell speed compared with those of littermate controls (Figure 5A-B). We found similar results when we examined PF4Cre+Sema7AloxP/loxP mice in this model. These animals also showed reduced transmigration and adhesion properties in the microcirculation after LPS challenge (Figure 5C-D). The possibility remained that endothelial-expressed Sema7A could bind to neutrophils and cause the observed results. When examining Tie2Cre+Sema7AloxP/loxP animals, to our surprise, we found no contribution of endothelial Sema7A to neutrophil attachment or transmigration (Figure 5E-F; supplemental Figure 10).
Tissue-specific expression of Sema7A controls neutrophil migration in response to inflammation. Intravital microscopic analysis of murine cremaster tissue after IV. LPS stimulation shows the role of Sema7A expression in different cells during inflammation. (A) Representative video images of the microvasculature of LysMCre+Sema7AloxP/loxP mice and littermate controls exposed to LPS for 15 minutes compared with the baseline control (0 min; scale bar, 50 μm). (B) Cell speed, transmigration, transmigration distance, and stationary PMNs in LysMCre+Sema7AloxP/loxP and littermate controls were analyzed by intravital microscopy after exposure to LPS for 15 minutes and compared with the baseline control (0 min). (C) Representative video images of the microvasculature of PF4Cre+Sema7AloxP/loxP mice and littermate controls exposed to LPS for 15 minutes compared with the baseline control (0 min; scale bar, 50 μm). (D) Cell speed, transmigration, transmigration distance, and stationary PMNs of PF4Cre+Sema7AloxP/loxP mice and littermate controls were analyzed by intravital microscopy after exposure to LPS for 15 minutes and compared with the baseline control (0 min; scale bar, 50 μm). (E) Representative video images of the microvasculature of Tie2Cre+Sema7AloxP/loxP mice and littermate controls exposed to LPS for 15 minutes and compared with the baseline control (0 min). (F) Cell speed, transmigration, transmigration distance, and stationary PMNs in Tie2Cre+Sema7AloxP/loxP and littermate controls were analyzed by intravital microscopy after exposure to LPS for 15 minutes and compared with the baseline control (0 min). Triplicate experiments were performed, and multiple cells were tracked for 15 to 20 minutes after LPS incubation over periods of 10 seconds at 90 fps. From the acquired videos, cells were tracked manually, and relevant group comparisons were performed by unpaired 2-tailed Student t tests (the data are the mean ± SD). ∗P < .05; ∗∗P < .01; ∗∗∗P < .001 as indicated, (arrows mark PNCs).
Tissue-specific expression of Sema7A controls neutrophil migration in response to inflammation. Intravital microscopic analysis of murine cremaster tissue after IV. LPS stimulation shows the role of Sema7A expression in different cells during inflammation. (A) Representative video images of the microvasculature of LysMCre+Sema7AloxP/loxP mice and littermate controls exposed to LPS for 15 minutes compared with the baseline control (0 min; scale bar, 50 μm). (B) Cell speed, transmigration, transmigration distance, and stationary PMNs in LysMCre+Sema7AloxP/loxP and littermate controls were analyzed by intravital microscopy after exposure to LPS for 15 minutes and compared with the baseline control (0 min). (C) Representative video images of the microvasculature of PF4Cre+Sema7AloxP/loxP mice and littermate controls exposed to LPS for 15 minutes compared with the baseline control (0 min; scale bar, 50 μm). (D) Cell speed, transmigration, transmigration distance, and stationary PMNs of PF4Cre+Sema7AloxP/loxP mice and littermate controls were analyzed by intravital microscopy after exposure to LPS for 15 minutes and compared with the baseline control (0 min; scale bar, 50 μm). (E) Representative video images of the microvasculature of Tie2Cre+Sema7AloxP/loxP mice and littermate controls exposed to LPS for 15 minutes and compared with the baseline control (0 min). (F) Cell speed, transmigration, transmigration distance, and stationary PMNs in Tie2Cre+Sema7AloxP/loxP and littermate controls were analyzed by intravital microscopy after exposure to LPS for 15 minutes and compared with the baseline control (0 min). Triplicate experiments were performed, and multiple cells were tracked for 15 to 20 minutes after LPS incubation over periods of 10 seconds at 90 fps. From the acquired videos, cells were tracked manually, and relevant group comparisons were performed by unpaired 2-tailed Student t tests (the data are the mean ± SD). ∗P < .05; ∗∗P < .01; ∗∗∗P < .001 as indicated, (arrows mark PNCs).
PNC formation is significantly increased by Sema7A
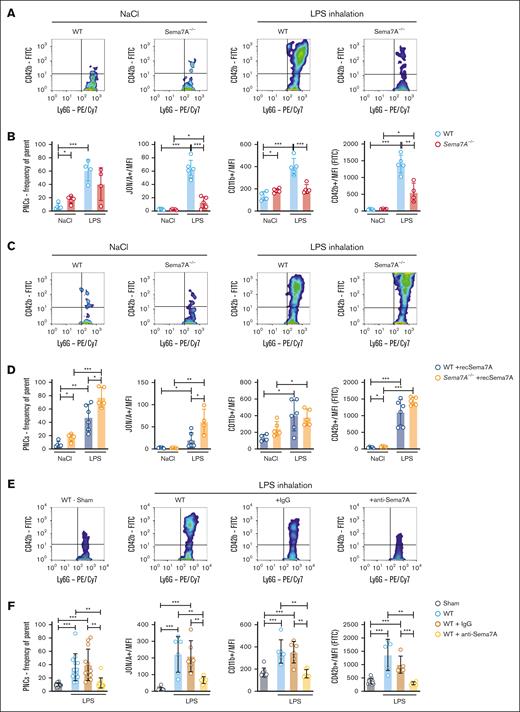
PNCs are significant effectors of the immune response during the early phase of inflammation. Because we have shown that Sema7A promotes integrin activation on neutrophils, we next performed flow cytometric analysis of WT and Sema7A−/− animals 5 minutes after LPS inhalation. We found reduced numbers of PNCs in the blood of Sema7A−/− animals and reduced expression of platelet or neutrophil activation markers (Figure 6A-B; for gating strategy, see supplemental Figure 11). We next injected recSema7A into Sema7A−/− animals and found that after injection of 1 μg per mouse recSema7A, the formation of PNCs in the blood was restored in Sema7A−/− animals (Figure 6C-D). To test whether this effect could be counteracted, we injected anti-Sema7A antibodies at the start of LPS inhalation. Anti-Sema7A antibodies clearly reduced the number of PNCs in the vasculature of experimental animals compared with control immunoglobulin G–treated animals (Figure 6E-F; supplemental Figure 12). In contrast, the phagocytosis rate and superoxide production of PMNs were not directly affected by freely available Sema7A or Plexin C1 blockade (supplemental Figure 13). These data clearly demonstrate the PNC-inducing properties of Sema7A, which are largely mediated by integrin activation on neutrophils through Sema7A.
Activation of neutrophils and PNC formation is Sema7A dependent. Murine blood was collected from WT and Sema7A−/− mice after LPS inhalation and analyzed by flow cytometry. (A) Representative color dot blots of PNCs (Ly6G+/CD42b+ events) in WT and Sema7A−/− blood from NaCl (control) or LPS–inhaled mice. (B) PNC formation, platelet effector glycoprotein 2b/3a (GP2b/3a) expression (antibody clone JON/A MFI), PMN activity marker CD11b (MFI) expression, and platelet activity marker CD42b (MFI) expression were assessed by flow cytometry in the mice described in panel A. (C) Representative dot blots of PNCs (Ly6G+/CD42b+ events) in the blood of WT and Sema7A−/− mice treated with recombinant Sema7A (recSema7A) after NaCl (control) or LPS inhalation. (D) PNC formation, platelet effector GPIIb/IIIa expression (antibody clone JON/A MFI), PMN activity marker CD11b (MFI) expression, and platelet activity marker CD42b (MFI) expression were assessed by flow cytometry in the mice described in panel C. (E) Representative dot blots of PNCs (Ly6G+/CD42b+ events) in the blood of WT mice that were untreated or treated with IgG or the Sema7A-blocking antibody (anti-Sema7A) after LPS inhalation compared with mice without conditioning (Sham). (F) PNC formation, platelet effector GPIIb/IIIa expression (antibody clone JON/A MFI), PMN activity marker CD11b (MFI) expression, and platelet activity marker CD42b (MFI) expression were assessed by flow cytometry in the mice described in panel E. Group comparisons were performed by unpaired 2-tailed Student t tests (the data are the mean ± SD). ∗P < .05; ∗∗P < .01; ∗∗∗P < .001 as indicated. IgG, immunoglobulin G; MFI, Mean Fluorescence Intensity.
Activation of neutrophils and PNC formation is Sema7A dependent. Murine blood was collected from WT and Sema7A−/− mice after LPS inhalation and analyzed by flow cytometry. (A) Representative color dot blots of PNCs (Ly6G+/CD42b+ events) in WT and Sema7A−/− blood from NaCl (control) or LPS–inhaled mice. (B) PNC formation, platelet effector glycoprotein 2b/3a (GP2b/3a) expression (antibody clone JON/A MFI), PMN activity marker CD11b (MFI) expression, and platelet activity marker CD42b (MFI) expression were assessed by flow cytometry in the mice described in panel A. (C) Representative dot blots of PNCs (Ly6G+/CD42b+ events) in the blood of WT and Sema7A−/− mice treated with recombinant Sema7A (recSema7A) after NaCl (control) or LPS inhalation. (D) PNC formation, platelet effector GPIIb/IIIa expression (antibody clone JON/A MFI), PMN activity marker CD11b (MFI) expression, and platelet activity marker CD42b (MFI) expression were assessed by flow cytometry in the mice described in panel C. (E) Representative dot blots of PNCs (Ly6G+/CD42b+ events) in the blood of WT mice that were untreated or treated with IgG or the Sema7A-blocking antibody (anti-Sema7A) after LPS inhalation compared with mice without conditioning (Sham). (F) PNC formation, platelet effector GPIIb/IIIa expression (antibody clone JON/A MFI), PMN activity marker CD11b (MFI) expression, and platelet activity marker CD42b (MFI) expression were assessed by flow cytometry in the mice described in panel E. Group comparisons were performed by unpaired 2-tailed Student t tests (the data are the mean ± SD). ∗P < .05; ∗∗P < .01; ∗∗∗P < .001 as indicated. IgG, immunoglobulin G; MFI, Mean Fluorescence Intensity.
Sema7A−/− animals show altered pulmonary defense and reduced survival in a model of K pneumoniae infection
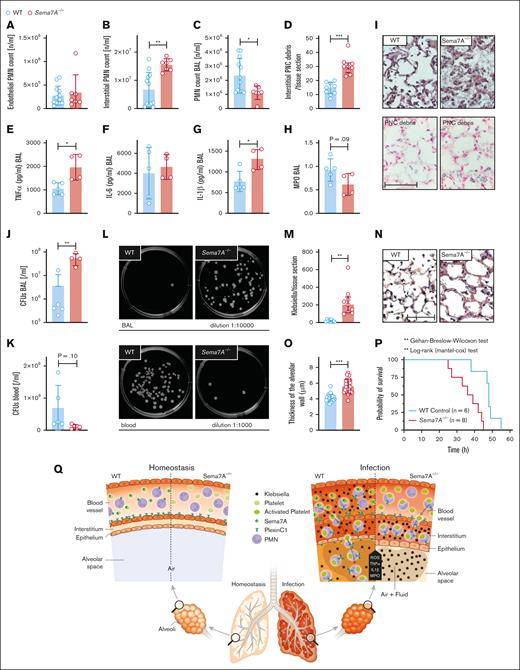
Bacterial invasion in the lung, the development of pneumonia, and intrapulmonary inflammation are essential mechanisms during the defense against invading pathogens at the external pulmonary surface. We next examined whether Sema7A plays a role in this process. To do so, we used control and Sema7A−/− animals to establish a model of K pneumoniae–induced pneumonia and evaluated pulmonary inflammation 24 hours after the initiation of the experiment as well as the overall survival. Sema7A did not result in altered oxidative burst or phagocytosis capacity of neutrophils (supplemental Figure 13). Next, we assessed the sequential recruitment of neutrophils into pulmonary tissue. We found that neutrophils were significantly more present in the interstitial space and significantly less present in the BALF of Sema7A−/− animals than in that of controls (Figure 7A-C, I). We found a higher number of PNCs in the interstitial of the lungs of Sema7A−/− animals (Figure 7D). The inflammatory cytokines interleukin-1β and TNF-α were increased in Sema7A−/− animals, whereas interleukin-6 levels were unchanged (Figure 7E-H). There were more colony forming units of K pneumoniae present in the lung and fewer in the blood of Sema7A−/− animals than in control animals, showing the intra-alveolar defense is impaired in the Sema7A−/− animals (Figure 7J-M). When we determined the vascular permeability and the edema formed within the interstitial space, we found increased edema formation in the Sema7A−/− animals (Figure 7N-O). All these findings translated into worse outcomes in the survival of Sema7A−/− animals than that of controls (Figure 7P). The thickening and enlargement of the alveolar surface, the vessel walls, and thus the difficulty in penetrating cell layers in the direction of the blood flow delayed the successful migration of bacteria into the blood. The infection caused by K pneumoniae remained confined to the specific site of the lung without spreading to the bloodstream.
Sema7A is crucial for pulmonary defense against Klebsiella-induced pneumonia. In a murine model of bacterial-induced lung injury, 4 × 107 gram-negative K pneumoniae was administered by intratracheal instillation directly into the lungs of WT and Sema7A−/− mice. Measurements of PMN counts on the endothelial surface (A), in the interstitial space (B), in the BAL (C), and PNC numbers (D) per tissue section (magnification, 1000×) 24 hours after Klebsiella instillation in histological lung sections of WT and Sema7A−/− mice (n = 3 per group on 3 different layers). The proinflammatory cytokines TNF-α (E), IL-6 (F), IL-1β (G), and myeloperoxidase activity (H) within the BAL of WT and Sema7A−/− mice. (I) Histological sections demonstrating the quantity of Klebsiella, the alveolar inflammation (H&E staining), and PNC debris (PNC-specific staining) 24 hours after instillation (scale bar, 50 μm; magnification, 1000×). Colony forming units in BALF (J) and blood (K) taken 24 hours after Klebsiella instillation and incubated on nutrient agar plates for 24 h. (L) Representative images of cultured bacteria and (M) counts per tissue sections of K pneumoniae. (N) Representative images of H&E stained sections focusing on lung tissue injury 24 hours after Klebsiella instillation. (scale bar, 50 μm; magnification, 1000×). (O) Thickness of alveolar wall in tissue sections of WT and Sema7A−/− mice 24 hours after K pneumoniae instillation (n = 3 per group; 10 random fields of view per mouse). (P) Survival curves of WT and Sema7A−/− animals after the instillation of 4 × 107 cells of K pneumoniae (n ≥ 6 per group). Group comparisons were performed by unpaired 2-tailed Student t tests; the data are the mean ± SD. For statistical comparisons of survival, the Gehan-Breslow-Wilcoxon test and the log-rank (Mantel-Cox) test were performed. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001 as indicated. (Q) Schematic drawing of the role of Sema7a in pulmonary infection and defense. In pulmonary hemostasis, Sema7A is expressed on neutrophils and other tissues (left). During pulmonary infection Sema7A gains pathophysiological importance. Sema7A binds to Plexin C1, activates neutrophils, and increases the expression of integrins and L-selectin on their surface. This is important for a coordinated immunological response and the defense of the lung. BALF, bronchoalveolar fluid; H&E, hematoxylin-eosin; IL-6, interleukin-6.
Sema7A is crucial for pulmonary defense against Klebsiella-induced pneumonia. In a murine model of bacterial-induced lung injury, 4 × 107 gram-negative K pneumoniae was administered by intratracheal instillation directly into the lungs of WT and Sema7A−/− mice. Measurements of PMN counts on the endothelial surface (A), in the interstitial space (B), in the BAL (C), and PNC numbers (D) per tissue section (magnification, 1000×) 24 hours after Klebsiella instillation in histological lung sections of WT and Sema7A−/− mice (n = 3 per group on 3 different layers). The proinflammatory cytokines TNF-α (E), IL-6 (F), IL-1β (G), and myeloperoxidase activity (H) within the BAL of WT and Sema7A−/− mice. (I) Histological sections demonstrating the quantity of Klebsiella, the alveolar inflammation (H&E staining), and PNC debris (PNC-specific staining) 24 hours after instillation (scale bar, 50 μm; magnification, 1000×). Colony forming units in BALF (J) and blood (K) taken 24 hours after Klebsiella instillation and incubated on nutrient agar plates for 24 h. (L) Representative images of cultured bacteria and (M) counts per tissue sections of K pneumoniae. (N) Representative images of H&E stained sections focusing on lung tissue injury 24 hours after Klebsiella instillation. (scale bar, 50 μm; magnification, 1000×). (O) Thickness of alveolar wall in tissue sections of WT and Sema7A−/− mice 24 hours after K pneumoniae instillation (n = 3 per group; 10 random fields of view per mouse). (P) Survival curves of WT and Sema7A−/− animals after the instillation of 4 × 107 cells of K pneumoniae (n ≥ 6 per group). Group comparisons were performed by unpaired 2-tailed Student t tests; the data are the mean ± SD. For statistical comparisons of survival, the Gehan-Breslow-Wilcoxon test and the log-rank (Mantel-Cox) test were performed. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001 as indicated. (Q) Schematic drawing of the role of Sema7a in pulmonary infection and defense. In pulmonary hemostasis, Sema7A is expressed on neutrophils and other tissues (left). During pulmonary infection Sema7A gains pathophysiological importance. Sema7A binds to Plexin C1, activates neutrophils, and increases the expression of integrins and L-selectin on their surface. This is important for a coordinated immunological response and the defense of the lung. BALF, bronchoalveolar fluid; H&E, hematoxylin-eosin; IL-6, interleukin-6.
Discussion
Defense against invading pathogens is an essential function of neutrophils that maintains the integrity and functionality of the lung. This function is important in preventing the infiltration of pathogens into the lung and eventually human circulation. Neutrophil arrest on the endothelium and migration into the alveolar space are important early mechanisms of a multistep process. We report here that Sema7A is an important regulator of neutrophil migration to the alveolar space, and the repression of Sema7A results in altered adhesion and delayed neutrophil migration. Sema7A activates integrins on neutrophils through the PlexinC1 receptor and influences the chemotactic behavior of these cells. In vivo, this translates into a reduced immune response within the lung. As a result, Sema7A influences the early phase of inflammation, which is relevant to the outcome in murine experimental sepsis induced by pneumonia (Figure 7Q).
The activation of neutrophils is essential for the migration of these cells into the alveolar space, in which their main task is to limit the assault on the lung from external invaders. Several adhesion receptors of the integrin class have previously been described to be important for this process, notably CD11b/CD18 (MAC-1) and other adhesive membrane proteins such as PSGL-1 or L-selectin.19 The activation of L-selectin was shown to be essential for the migration of neutrophils into the lung to limit the extent of pulmonary sepsis, and lack of functional L-selectin resulted in susceptibility to pulmonary infection.7 Similar effects were described for CD11b and its activation.20 A complex interplay between CD11b, WASP, and CD42c regulates the polarization of neutrophils and attachment to microtubules on the endothelial surface.21 This interplay is then necessary for the meaningful and coordinated migration of neutrophils to the site of inflammation or infection. In addition, PSGL-1 is also an essential mediator of neutrophil attachment to the endothelium and is involved into the formation of PNCs.22,23 This formation of PNCs can also result in obstruction of the microvasculature of the lung and thereby reduce oxygenation.24 We have demonstrated that Sema7A triggers the functional upregulation of CD11b and downregulation of L-selectin and PSGL-1 on neutrophils and thereby modulates their chemotactic migration. As a result, neutrophils are activated, which essentially influences neutrophil chemotactic migration. When Sema7A is present, neutrophils are activated in a synchronistic manner and migrate across the alveolar-capillary barrier to reach the alveolar space and combat invading pathogens. In the absence of Sema7A, this coordinated induction does not occur, and neutrophils migrate in an uncoordinated fashion. We observed that neutrophils in Sema7A−/− animals showed altered adhesion and transmigration in response to inflammatory stimuli, which translated into an altered migration pattern during bacterial infection in the lung and neutrophil arrest in the interstitial space, in which they cannot be sufficiently activated against invading bacteria. Thus, the animals showed impaired host defense and died earlier than animals with physiological Sema7A expression. This is in line with our previous results showing that Sema7A aggravates pulmonary inflammation.16 In this previous study, we showed the effect of Sema7A on pulmonary endothelial and epithelial cells and that Sema7A enhanced cytokine production in these cells. We extended this work now and identified the specific action of Sema7A on neutrophils and PNC formation. We also showed that Sema7A correlated with increased pulmonary inflammation through its action on myeloid derived cells.16 In line with this, in this study, we show an increase of Sema7A in patients with severe ARDS. This increased Sema7A is likely shed from neutrophils or pulmonary tissue through the activation of caspases or released from platelets that contain Sema7A in sufficient amount as one of their proteins.25,26
To our knowledge, PlexinC1-dependent integrin activation has not been described before in neutrophils. The role of Plexin C1 during inflammation and pulmonary inflammation was evaluated previously and showed a significant effect of PlexinC1 expression during mechanical ventilation.27,28 However, the fact that Sema7A has a significant effect on CD11b, L-selectin, and other adhesion receptors has not been previously shown. We were also able to demonstrate that the pathways controlling the activation of these integrins are influenced by Sema7A binding to PlexinC1. Sema7A was shown in the past to induce cytokine storm in T-cells though a mechanism that was dependent on α1β1 integrin receptor in T-cells.11 However, we could not confirm the binding of Sema7A to β1 integrin on neutrophils and showed that Sema7A binds to the PlexinC1 receptor instead. Previous work has demonstrated that PlexinC1 is likely involved in the migration of neutrophils and other cells, which was confirmed in models using the genetic deletion of PlexinC1.28,29 We now show here that this occurs by the binding of Sema7A to PlexinC1. In addition, whether the soluble form of Sema7A mediates the activation of integrins, which are essential for neutrophil migration during inflammation and infection, is unclear. Recent work has demonstrated that Sema7A is essential for a coordinated sequence of events during inflammation but also for the resolution of inflammation.30 In accordance with this work, we also showed that Sema7A expression is important for survival during bacterial infection. Here, we used a model of K pneumoniae, whereas Korner et al30 used a model of cecal ligation and puncture, and both showed a survival benefit in animals expressing Sema7A. However, one must keep in mind that the expression of Sema7A and Sema7A target receptors is organ specific; therefore, organ–specific immune responses to inflammatory or infectious stimuli are possible in response to this protein. We have previously shown that neutrophil migration is altered through Sema7A during myocardial infarction and in hypoxic tissue inflammation.12,13 During hypoxia, the induction of endothelial Sema7A resulted in increased transendothelial neutrophil migration. However, these mechanisms are not involved in the results described here. We describe a novel mechanism of integrin activation in neutrophils through direct signaling mediated by Sema7A engaging with Plexin C1.
In summary, we have shown that Sema7A directly triggers the functional upregulation of integrins in neutrophils and thereby modulates their adhesion and migration, which to a significant extent determines outcomes during pulmonary infection. Data in human patients with ARDS corroborate this mechanism of Sema7A-mediated promotion of neutrophil migration.
Acknowledgments
The authors thank Michaela Hoch-Gutbrod for technical assistance and support.
This work was supported by Deutsche Forschungsgemeinschaft (DFG) CRC/TR 240 “Platelets – Molecular, cellular and systemic functions in health and disease” (Project number 374031971) TP B07 (P.R. and B.N.) and DFG-RO 3671/14-1 (P.R.).
Authorship
Contribution: T.G., D.K., L.T., K.H., K.G.H., P.B., T.B., and A.Z. performed experiments, analyzed data, and wrote parts of the manuscript; C.E., K.-L.H.-S., M. Koeppen, S.G., M. Bamberg, H.M., M. Blaha, F.K., K.N., A.F., M. Keller, and A.M.B. performed experiments and analyzed data; H.A.H. collected patient samples; B.N. designed research, analyzed data, and wrote parts of the manuscript; and P.R. designed study and overall research plan and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Peter Rosenberger, Department of Anesthesiology and Intensive Care Medicine, Universitätsklinikum Tübingen, Hoppe-Seyler Straße 3, 72076 Tübingen, Germany; email: peter.rosenberger@medizin.uni-tuebingen.de.
References
Author notes
T.G., D.K., and L.T. contributed equally to this study.
Data are available on request from the corresponding author, Peter Rosenberger (peter.rosenberger@medizin.uni-tuebingen.de).
The full-text version of this article contains a data supplement.