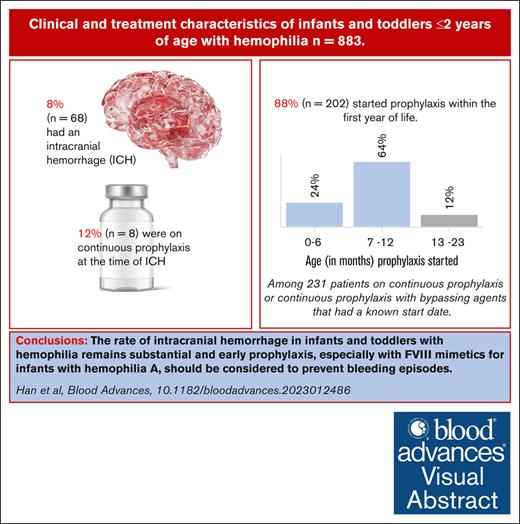
The prevalence of intracranial hemorrhage in ITs with hemophilia remains substantial.
Visual Abstract
Infants and toddlers (ITs) with hemophilia have unique bleeding features. Factor prophylaxis has been shown to decrease the risk of intracranial hemorrhage (ICH), which supports recommendations to begin at a young age. Clinical and demographic characteristics were analyzed for 883 ITs ≤2 years old with hemophilia A and B, seen at US Hemophilia Treatment Centers and enrolled in the Community Counts Registry, a surveillance program of the Centers for Disease Control and Prevention. ICH in the first 2 years of life was seen in 68 of 883 (7.7%) ITs, of whom 8 of 68 (11.8%) were on continuous prophylaxis at the time of ICH. ITs in this study usually started prophylaxis within the first year of life (mean, 10.3 months), with earlier ages of prophylaxis initiation in later birth cohorts in ITs with hemophilia A. Compared with those without a family history (FH) of hemophilia, known positive FH of hemophilia was associated with earlier age of diagnosis (P ≤ .0001) and decreased rates of vaginal delivery (P = .0006). The use of factor VIII mimetics and extended half-life clotting factor prophylaxis increased with later birth cohorts for ITs with hemophilia A and B. The study highlights that ICH rates in ITs with hemophilia remains substantial and underscores the need for further research to identify modifiable risk factors to prevent ICH by earlier diagnosis and initiating prophylaxis early, even within the first month of life.
Introduction
Congenital hemophilia is an X-linked bleeding disorder often diagnosed in infants and toddlers (ITs) ≤2 years of age.1 ITs with hemophilia have unique bleeding patterns compared with older children and adults. Intracranial hemorrhage (ICH), extracranial hemorrhage, and bleeding from the oral cavity and circumcision occur more frequently in ITs than hemarthrosis.2,3 Publications from the Universal Data Collection (UDC), a surveillance system funded by Centers for Disease Control and Prevention (CDC) and conducted at the US Hemophilia Treatment Center Network between 1998 and 2011, described the prevalence as well as bleeding and treatment characteristics in ITs with hemophilia.2,4,5
Since 2013, surveillance of ITs with hemophilia has continued through the Community Counts Public Health Surveillance of Bleeding Disorders project (CC), a collaboration between CDC, the American Thrombosis and Hemostasis Network, and the US Hemophilia Treatment Center Network.6 CC focuses on clinically relevant outcomes and emerging therapies such as the factor VIII (FVIII) mimetic (Hemlibra [emicizumab-kxwh], Genentech, Inc, Roche Group South San Francisco, CA) treatment, approved for prophylaxis in individuals of any age with hemophilia A (HA),7 and extended half-life (EHL) FVIII clotting factor concentrates (CFCs) which have been utilized in children with hemophilia.8 CC affords the opportunity for an updated evaluation of ITs with hemophilia in the evolving treatment landscape and evidence-based recommendations.
The purpose of this report was to evaluate current treatment and outcomes of ITs ≤2 years of age with hemophilia within the context of new therapeutics.
Methods
Enrollment and data collection
CC, a US-based public health registry for bleeding disorders, has been previously described.6 Briefly, CC includes data from 146 federally funded Hemophilia Treatment Centers (HTCs) throughout the United States6 and is comprised of 3 parts: the HTC Population Profile, the Registry for Bleeding Disorders Surveillance (the CC Registry), and Mortality Reporting. The data for this analysis are abstracted from the CC Registry. Patients are eligible for the CC Registry if they have an inherited bleeding disorder: hemophilia A (HA) or HB, von Willebrand disease, a rare bleeding disorder, or a platelet function disorder.
Because the CC Registry is categorized as “Public Health Practice: Surveillance,” participant informed consent was obtained only in some centers based on individual Institutional Review Board requirement. Data were collected using a combination of medical record abstraction and patient report during scheduled clinic visits. Initial visit forms, which include historic and current demographic and clinical information are completed upon enrollment. Subsequent visit forms are completed annually and include clinical information and outcomes since the previous visit.
This study included males and females of ≤2 years age born during or after 2011 enrolled in the CC Registry between 2013 and 2021 who had a diagnosis of congenital HA or HB with a clotting factor activity of <50%.
Analyzed data included age at enrollment, age at diagnosis, birth cohort (2011-2015, 2015-2018, and 2019-2021), race, ethnicity, sex, type of hemophilia (HA or HB), and hemophilia severity (severe ≤1% baseline factor activity; moderate = 1%-5% baseline factor activity; and mild ≥5% baseline factor activity), method of delivery (including the presence or absence of instrumentation), head imaging for patients diagnosed within the first 30 days of life, family history (FH) of hemophilia (+FH, ≥1 blood relative with known hemophilia; −FH, no blood relatives with known hemophilia), history of circumcision, central venous access device (CVAD) use, and bleed and treatment characteristics. HTCs were instructed to report all treatment products used since the last surveillance visit. Continuous prophylaxis was the use of any treatment product on a regular basis to prevent bleeds or to maintain tolerance to CFC or both. Data for analysis were extracted from the initial and subsequent visits over the first 2 years of life.
The study concept and design was approved by the Community Counts Publications Committee. In depth analysis of inhibitor development was not approved as part of the study plan and will be analyzed for a separate manuscript.
Data analysis
Summary statistics were used to characterize the demographic and clinical information.
The proportions of ITs with ICH and head imaging and their corresponding 95% confidence intervals were calculated for select clinical characteristics. Differences were considered statistically significant if CIs did not overlap. Differences in the distribution of other clinical characteristics and FH were assessed for statistical significance using χ2 test. All analyses were performed in SAS, version 9.4 (SAS Institute, Cary, NC).
Most institutions with HTCs regard CC as a surveillance project and require only patient authorization for participation. In a few HTCs, CC is considered research, and therefore, participants provide informed consent under institutional review board guidance.
Results
Demographics and clinical characteristics
During the study period, 883 ITs in the CC Registry met the inclusion criteria (861 males and 22 females). Seven ITs had an additional bleeding disorder and were excluded from this study. The cohort included 691 of 883 (78.3%) ITs with HA and 192 of 883 (21.7%) ITs with HB. Overall, 550 of 883 (62%) had severe hemophilia. Almost all (865/883, 97.9%) of the ITs had health insurance. The age at enrollment ranged from 0 to 23 months; however, more than half of the patients were enrolled by their first birthday. The majority were non-Hispanic White, and the distributions of race and ethnicity were similar to the US population.9Table 1 shows the demographic and clinical characteristics.
Demographics and clinical characteristics
| N = 883 . | . |
|---|---|
| Sex | |
| Female | 22 (2.5) |
| Male | 861 (97.5) |
| Age at enrollment | |
| <1 mo | 32 (3.6) |
| 1-6 mo | 205 (23.2) |
| 7-12 mo | 232 (26.3) |
| 13-23 mo | 414 (46.9) |
| Birth cohort | |
| 2011-2015 | 266 (30.1) |
| 2016-2018 | 405 (45.9) |
| 2019-2021 | 212 (24.0) |
| Primary insurance | |
| Commercial insurance | 419 (48.6) |
| Medicaid/Medicare/other state programs | 395 (45.8) |
| Military Health Care (TRICARE/VA/Champ-VA) | 30 (3.5) |
| Uninsured | 18 (2.1) |
| Other/unknown | 21 (-) |
| Bleeding disorder | |
| HA | 691 (78.3) |
| Mild | 105 (15.4) |
| Moderate | 120 (17.6) |
| Severe | 458 (67.1) |
| Unknown | 8 (-) |
| HB | 192 (21.7) |
| Mild | 28 (14.8) |
| Moderate | 69 (36.5) |
| Severe | 92 (48.7) |
| Unknown | 3 (-) |
| Ethnicity | |
| Hispanic | 164 (19.2) |
| Non-Hispanic | 689 (80.8) |
| Unknown | 30 (-) |
| Race | |
| White | 649 (78.6) |
| Black | 106 (12.8) |
| Asian | 25 (3.0) |
| American Indian or Alaska Native or Native Hawaiian or other Pacific Islander | 12 (1.4) |
| More than 1 race | 34 (4.1) |
| Unknown | 57 (-) |
| Family history of bleeding disorder | |
| Yes | 619 (72.4) |
| No | 236 (27.6) |
| Unknown | 28 (-) |
| Circumcision | |
| Yes | 348 (41.8) |
| No | 484 (58.2) |
| Unknown | 29 (-) |
| Not applicable | 22 (-) |
| Bleeding due to circumcision | |
| Yes | 200 (59.0) |
| No | 139 (41.0) |
| Unknown | 9 (-) |
| Not applicable | 535 (-) |
| N = 883 . | . |
|---|---|
| Sex | |
| Female | 22 (2.5) |
| Male | 861 (97.5) |
| Age at enrollment | |
| <1 mo | 32 (3.6) |
| 1-6 mo | 205 (23.2) |
| 7-12 mo | 232 (26.3) |
| 13-23 mo | 414 (46.9) |
| Birth cohort | |
| 2011-2015 | 266 (30.1) |
| 2016-2018 | 405 (45.9) |
| 2019-2021 | 212 (24.0) |
| Primary insurance | |
| Commercial insurance | 419 (48.6) |
| Medicaid/Medicare/other state programs | 395 (45.8) |
| Military Health Care (TRICARE/VA/Champ-VA) | 30 (3.5) |
| Uninsured | 18 (2.1) |
| Other/unknown | 21 (-) |
| Bleeding disorder | |
| HA | 691 (78.3) |
| Mild | 105 (15.4) |
| Moderate | 120 (17.6) |
| Severe | 458 (67.1) |
| Unknown | 8 (-) |
| HB | 192 (21.7) |
| Mild | 28 (14.8) |
| Moderate | 69 (36.5) |
| Severe | 92 (48.7) |
| Unknown | 3 (-) |
| Ethnicity | |
| Hispanic | 164 (19.2) |
| Non-Hispanic | 689 (80.8) |
| Unknown | 30 (-) |
| Race | |
| White | 649 (78.6) |
| Black | 106 (12.8) |
| Asian | 25 (3.0) |
| American Indian or Alaska Native or Native Hawaiian or other Pacific Islander | 12 (1.4) |
| More than 1 race | 34 (4.1) |
| Unknown | 57 (-) |
| Family history of bleeding disorder | |
| Yes | 619 (72.4) |
| No | 236 (27.6) |
| Unknown | 28 (-) |
| Circumcision | |
| Yes | 348 (41.8) |
| No | 484 (58.2) |
| Unknown | 29 (-) |
| Not applicable | 22 (-) |
| Bleeding due to circumcision | |
| Yes | 200 (59.0) |
| No | 139 (41.0) |
| Unknown | 9 (-) |
| Not applicable | 535 (-) |
Responses of “Unknown” are shown but not included in the denominator for percentage calculations.
Age at diagnosis by disease characteristics
The age of diagnosis was reported for 836 of 883 (94.7%) ITs. Most ITs were diagnosed within the first month of life regardless of hemophilia type, severity, or FH. Four hundred seventy-two of 584 (80.8%) ITs with a +FH of hemophilia were diagnosed within the first month of life, with another 70 of 584 (12.0%) being diagnosed between 1 and 6 months. Among those with a −FH of hemophilia, diagnosis was still most common within the first month of life, but 44 of 226 (19.5%) were not diagnosed until 7 to 12 months of age (Table 2).
Age of diagnosis by select patient characteristics
| . | Total N . | <1 mo N (%) . | 1-6 mo N (%) . | 7-12 mo N (%) . | 13-23 mo N (%) . | P value . |
|---|---|---|---|---|---|---|
| All patients∗ | 836 | 625 (74.8) | 109 (13.0) | 79 (9.5) | 23 (2.8) | |
| HA | 655 | 495 (75.6) | 83 (12.7) | 60 (9.2) | 17 (2.6) | .7738 |
| HB | 181 | 130 (71.8) | 26 (14.4) | 19 (10.5) | 6 (3.3) | |
| Mild | 123 | 85 (69.1) | 22 (17.9) | 11 (8.9) | ∗ | .0188 |
| Moderate | 179 | 132 (73.7) | 22 (12.3) | 17 (9.5) | 8 (4.5) | |
| Severe | 525 | 404 (77.0) | 61 (11.6) | 51 (9.7) | 9 (1.7) | |
| Unknown | 9 | 4 (-) | 4 (-) | ∗ | ||
| Positive FH | 584 | 472 (80.8) | 70 (12.0) | 30 (5.1) | 12 (2.1) | <.0001 |
| Negative FH | 226 | 138 (61.1) | 35 (15.5) | 44 (19.5) | 9 (4.0) | |
| Unknown FH | 26 | 15 (-) | ∗ | ∗ | ∗ |
| . | Total N . | <1 mo N (%) . | 1-6 mo N (%) . | 7-12 mo N (%) . | 13-23 mo N (%) . | P value . |
|---|---|---|---|---|---|---|
| All patients∗ | 836 | 625 (74.8) | 109 (13.0) | 79 (9.5) | 23 (2.8) | |
| HA | 655 | 495 (75.6) | 83 (12.7) | 60 (9.2) | 17 (2.6) | .7738 |
| HB | 181 | 130 (71.8) | 26 (14.4) | 19 (10.5) | 6 (3.3) | |
| Mild | 123 | 85 (69.1) | 22 (17.9) | 11 (8.9) | ∗ | .0188 |
| Moderate | 179 | 132 (73.7) | 22 (12.3) | 17 (9.5) | 8 (4.5) | |
| Severe | 525 | 404 (77.0) | 61 (11.6) | 51 (9.7) | 9 (1.7) | |
| Unknown | 9 | 4 (-) | 4 (-) | ∗ | ||
| Positive FH | 584 | 472 (80.8) | 70 (12.0) | 30 (5.1) | 12 (2.1) | <.0001 |
| Negative FH | 226 | 138 (61.1) | 35 (15.5) | 44 (19.5) | 9 (4.0) | |
| Unknown FH | 26 | 15 (-) | ∗ | ∗ | ∗ |
Responses of “Unknown” are shown but not included in the denominator for percentage calculations or in the statistical analyses.
P values result from χ2 test for age of diagnosis distribution across the respective patient characteristics.
Forty-seven patients with unknown age of diagnosis were excluded from the table.
For known values (except in instances where complementary suppression is necessary), data are not shown for fields with N ≤ 5 to protect patient confidentiality. Additional cells may be suppressed to prevent derivation of these counts by subtraction.
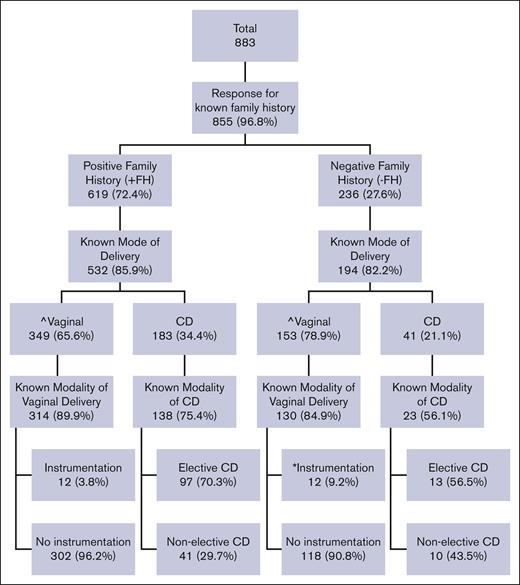
Delivery characteristics
Among ITs with data regarding FH and mode of delivery, 224 of 726 (30.9%) were delivered by cesarean delivery (CD) and 502 of 726 (69.1%) by vaginal route. A +FH of hemophilia was associated with a significantly lower proportion of vaginal deliveries compared with those with a −FH (349/532, 65.6% vs 153/194, 78.9%; P = .0015) and less instrumentation with vaginal delivery compared with −FH (12/314, 3.8% vs 12/130, 9.2%; P = .0208) (Figure 1).
Distribution of mode of delivery and instrumentation by family history (FH) of hemophilia. ˆKnown FH is associated with lower percentage of vaginal delivery compared with negative FH (65.6% vs 78.9%; P = .0015). ∗Known FH is associated with less instrumentation with vaginal delivery compared with negative FH (3.8% vs 9.2%; P = .0208). P values result from χ2 test evaluating FH and the use of vaginal birth and instrumentation. CD, cesarean delivery.
Distribution of mode of delivery and instrumentation by family history (FH) of hemophilia. ˆKnown FH is associated with lower percentage of vaginal delivery compared with negative FH (65.6% vs 78.9%; P = .0015). ∗Known FH is associated with less instrumentation with vaginal delivery compared with negative FH (3.8% vs 9.2%; P = .0208). P values result from χ2 test evaluating FH and the use of vaginal birth and instrumentation. CD, cesarean delivery.
Head imaging and ICH
Two hundred forty-two of 883 (27.4%) ITs underwent head imaging within the first month of life. Of those with known data, 220 of 236 (93.2%) were diagnosed with hemophilia within the first month of life, 172 of 242 (71.7%) had severe hemophilia, 169 of 240 (71.6%) had a +FH of hemophilia, and 34 of 238 (14.3%) had a history of ICH (Table 3). Reasons for head imaging and specific results were not collected.
Frequencies, proportions, and rates of patients who underwent head imaging within the first month of life and those who had ICH by select patient characteristics
| . | Total . | Head imaging within first 30 d of life . | ICH ever . | ICH within first 30 d of life . | |||
|---|---|---|---|---|---|---|---|
| # (% of total) . | # (% with head imaging within first 30 d of life) . | Rate per 100 ITs (95% CI) . | # (% of ICH) . | Rate per 100 ITs (95% CI) . | # (% with ICH within first 30 d of life) . | Rate per 100 ITs (95% CI) . | |
| All | 883 (100%) | 242 (27.4%) | 27.4 (24.5-30.3) | 68 (7.7%) | 7.7 (5.9-9.5) | 24 (2.7%) | 2.7 (1.6-3.8) |
| HA | 691 (78.3) | 201 (83.1%) | 29.1 (25.7-32.5) | 57 (83.8%) | 8.2 (6.2-10.3) | ∗ | ∗ |
| HB | 192 (21.7) | 41 (16.9%) | 21.4 (15.6-27.2) | 11 (16.2%) | 5.7 (2.4-9.0) | ∗ | ∗ |
| Age of diagnosis | |||||||
| <1 mo | 625 (74.8%) | 220 (93.2%) | 35.2 (31.5-38.9) | 53 (79.1%) | 8.5 (6.3-10.7) | 22 (91.7%) | 3.5 (2.1-5.0) |
| ≥1 mo | 211 (25.2%) | 16 (6.8%) | 7.6 (4.0-11.2) | 14 (20.9%) | 6.6 (3.3-10.0) | ∗ | 0.9 (0 to 2.3) |
| Unknown | 47 (-) | 6 (-) | 1 (-) | ∗ | |||
| Severity | |||||||
| Severe | 550 (63.1%) | 172 (71.7%) | 31.3 (27.4-35.1) | 53 (77.9%) | 9.6 (7.2-12.1) | 17 (70.8%) | 3.1 (1.6-4.5) |
| Moderate/Mild | 322 (36.9%) | 68 (28.1) | 21.1 (16.7-25.6) | 15 (22.1%) | 4.7 (2.4-7.0) | 7 (29.2%) | 2.2 (0.6-3.8) |
| Moderate | 189 (21.7%) | 46 (19.2%) | 24.3 (18.2-30.5) | ||||
| Mild | 133 (15.3%) | 22 (9.2%) | 16.5 (10.2-22.9) | ||||
| Unknown | 11 (-) | 2 (-) | 4 (-) | ||||
| FH | |||||||
| No | 236 (27.6%) | 67 (28.4%) | 28.4 (22.6-34.1) | 25 (36.8%) | 10.6 (6.7-14.5) | 8 (33.3%) | 3.4 (1.1-5.7) |
| Yes | 619 (72.4%) | 169 (71.6%) | 27.3 (23.8-30.8) | 43 (63.2%) | 6.9 (4.9-8.9) | 16 (66.7%) | 2.6 (1.3-3.8) |
| Unknown | 28 (-) | 6 (-) | 0 (-) | 0 (-) | |||
| Method of delivery | |||||||
| Cesarean delivery | 226 (30.4%) | 19 (10.8%) | 8.4 (4.8-12.0) | 12 (21.4%) | 5.3 (2.4-8.2) | ∗ | ∗ |
| Vaginal delivery | 517 (69.6%) | 157 (89.2%) | 30.4 (26.4-34.3) | 44 (78.6%) | 8.5 (6.1-10.9) | 17 (77.3%) | 3.3 (1.8-4.8) |
| Unknown | 140 (-) | 66 (-) | 12 (-) | ∗ | |||
| ICH history | |||||||
| No | 810 (92.3%) | 204 (85.7%) | 25.2 (22.2-28.2) | N/A | N/A | N/A | N/A |
| Yes | 68 (7.7%) | 34 (14.3%) | 50.0 (38.1-61.9) | 68 (100.0%) | N/A | 24 (100.0%) | 35.3 (23.9-46.7) |
| Unknown | 5 (-) | 4 (-) | N/A | N/A | N/A | N/A | |
| Age of first ICH | |||||||
| <1 mo | 24 (35.3%) | 23 (67.6%) | 95.8 (87.8-100.0) | 24 (35.3%) | N/A | 24 (100.0%) | N/A |
| ≥1 mo | 44 (64.7%) | 11 (32.3%) | 25.0 (12.2-37.8) | 44 (64.7%) | N/A | N/A | N/A |
| ICH associated with trauma | |||||||
| No | 26 (52.0%) | 17 (65.4%) | 65.4 (47.1-83.7) | 26 (52.0%) | N/A | 11 (57.9%) | 42.3 (23.3-61.3) |
| Yes | 24 (48.0%) | 9 (34.6%) | 37.5 (18.1-56.9) | 24 (48.0%) | N/A | 8 (42.1%) | 33.3 (14.5-52.2) |
| Unknown | 18 (-) | 8 (-) | 18 (-) | 5 (-) | ∗ | ||
| Continuous prophylaxis when ICH occurred | |||||||
| No | 57 (87.7%) | ∗ | ∗ | 57 (87.7%) | N/A | ∗ | ∗ |
| Yes | 8 (12.3%) | ∗ | ∗ | 8 (12.3%) | N/A | ∗ | ∗ |
| Unknown | 3 (-) | 3 (-) | |||||
| . | Total . | Head imaging within first 30 d of life . | ICH ever . | ICH within first 30 d of life . | |||
|---|---|---|---|---|---|---|---|
| # (% of total) . | # (% with head imaging within first 30 d of life) . | Rate per 100 ITs (95% CI) . | # (% of ICH) . | Rate per 100 ITs (95% CI) . | # (% with ICH within first 30 d of life) . | Rate per 100 ITs (95% CI) . | |
| All | 883 (100%) | 242 (27.4%) | 27.4 (24.5-30.3) | 68 (7.7%) | 7.7 (5.9-9.5) | 24 (2.7%) | 2.7 (1.6-3.8) |
| HA | 691 (78.3) | 201 (83.1%) | 29.1 (25.7-32.5) | 57 (83.8%) | 8.2 (6.2-10.3) | ∗ | ∗ |
| HB | 192 (21.7) | 41 (16.9%) | 21.4 (15.6-27.2) | 11 (16.2%) | 5.7 (2.4-9.0) | ∗ | ∗ |
| Age of diagnosis | |||||||
| <1 mo | 625 (74.8%) | 220 (93.2%) | 35.2 (31.5-38.9) | 53 (79.1%) | 8.5 (6.3-10.7) | 22 (91.7%) | 3.5 (2.1-5.0) |
| ≥1 mo | 211 (25.2%) | 16 (6.8%) | 7.6 (4.0-11.2) | 14 (20.9%) | 6.6 (3.3-10.0) | ∗ | 0.9 (0 to 2.3) |
| Unknown | 47 (-) | 6 (-) | 1 (-) | ∗ | |||
| Severity | |||||||
| Severe | 550 (63.1%) | 172 (71.7%) | 31.3 (27.4-35.1) | 53 (77.9%) | 9.6 (7.2-12.1) | 17 (70.8%) | 3.1 (1.6-4.5) |
| Moderate/Mild | 322 (36.9%) | 68 (28.1) | 21.1 (16.7-25.6) | 15 (22.1%) | 4.7 (2.4-7.0) | 7 (29.2%) | 2.2 (0.6-3.8) |
| Moderate | 189 (21.7%) | 46 (19.2%) | 24.3 (18.2-30.5) | ||||
| Mild | 133 (15.3%) | 22 (9.2%) | 16.5 (10.2-22.9) | ||||
| Unknown | 11 (-) | 2 (-) | 4 (-) | ||||
| FH | |||||||
| No | 236 (27.6%) | 67 (28.4%) | 28.4 (22.6-34.1) | 25 (36.8%) | 10.6 (6.7-14.5) | 8 (33.3%) | 3.4 (1.1-5.7) |
| Yes | 619 (72.4%) | 169 (71.6%) | 27.3 (23.8-30.8) | 43 (63.2%) | 6.9 (4.9-8.9) | 16 (66.7%) | 2.6 (1.3-3.8) |
| Unknown | 28 (-) | 6 (-) | 0 (-) | 0 (-) | |||
| Method of delivery | |||||||
| Cesarean delivery | 226 (30.4%) | 19 (10.8%) | 8.4 (4.8-12.0) | 12 (21.4%) | 5.3 (2.4-8.2) | ∗ | ∗ |
| Vaginal delivery | 517 (69.6%) | 157 (89.2%) | 30.4 (26.4-34.3) | 44 (78.6%) | 8.5 (6.1-10.9) | 17 (77.3%) | 3.3 (1.8-4.8) |
| Unknown | 140 (-) | 66 (-) | 12 (-) | ∗ | |||
| ICH history | |||||||
| No | 810 (92.3%) | 204 (85.7%) | 25.2 (22.2-28.2) | N/A | N/A | N/A | N/A |
| Yes | 68 (7.7%) | 34 (14.3%) | 50.0 (38.1-61.9) | 68 (100.0%) | N/A | 24 (100.0%) | 35.3 (23.9-46.7) |
| Unknown | 5 (-) | 4 (-) | N/A | N/A | N/A | N/A | |
| Age of first ICH | |||||||
| <1 mo | 24 (35.3%) | 23 (67.6%) | 95.8 (87.8-100.0) | 24 (35.3%) | N/A | 24 (100.0%) | N/A |
| ≥1 mo | 44 (64.7%) | 11 (32.3%) | 25.0 (12.2-37.8) | 44 (64.7%) | N/A | N/A | N/A |
| ICH associated with trauma | |||||||
| No | 26 (52.0%) | 17 (65.4%) | 65.4 (47.1-83.7) | 26 (52.0%) | N/A | 11 (57.9%) | 42.3 (23.3-61.3) |
| Yes | 24 (48.0%) | 9 (34.6%) | 37.5 (18.1-56.9) | 24 (48.0%) | N/A | 8 (42.1%) | 33.3 (14.5-52.2) |
| Unknown | 18 (-) | 8 (-) | 18 (-) | 5 (-) | ∗ | ||
| Continuous prophylaxis when ICH occurred | |||||||
| No | 57 (87.7%) | ∗ | ∗ | 57 (87.7%) | N/A | ∗ | ∗ |
| Yes | 8 (12.3%) | ∗ | ∗ | 8 (12.3%) | N/A | ∗ | ∗ |
| Unknown | 3 (-) | 3 (-) | |||||
Categorical data are presented as n (%).
N/A, not applicable; 95% CI, confidence interval.
Known fields with N of greater than 0 but less than 5 have been suppressed to protect patient confidentiality. Additional cells may be suppressed to prevent derivation of these counts by subtraction.
Sixty-eight of 883 (7.7%) ITs had an ICH reported during the first 2 years of life, with most (44/68, 64.7%) of the ICHs occurring at 1 month of age or older (25/68, 36.8% at 1-6 months; 11/68, 16.2% at 7-12 months; 8/68, 11.8% at 13-23 months). ITs with severe hemophilia had a significantly higher rate of ICH compared with those with mild or moderate hemophilia (9.6 vs 4.7 per 100 ITs, respectively) (Table 3). There were no significant differences in ICH occurrence between ITs with HA or HB (Table 3).
Of the ITs that had an ICH, 8 of 68 (11.8%) were on continuous prophylaxis at the time of ICH, and 24 of 68 (35.3%) ICHs were preceded by an episode of head trauma (Table 3). Data on the type of head trauma, sites of ICH, long-term sequelae and consequences of ICH, and gestational age of affected ITs were not collected.
Twenty-four of 68 (35.3%) ITs with ICH experienced the bleeding event within the first month of life. Seventeen of 24 (70.8%) with ICH in the first month of life were delivered by vaginal route, and 8 of 24 (33.3%) had reported trauma. Data on the type of head trauma and its association with birth were not collected.
Eleven of 68 (16.2%) ITs with ICH experienced recurrence; all 11 of 68 had severe hemophilia, and 8 of 68 were not on prophylaxis at the time of recurrence (data not shown). Five of 68 ITs had ICH recur within 30 days of initial ICH (the median time between first ICH and recurrence was 101 days).
Circumcision
Circumcision data were available for 832 of 861 male ITs, 348 of 832 (41.8%) of whom were circumcised (Table 1). Hemophilia severity was known for 346 of 348 circumcised ITs, 225 of 346 severe, 72 of 346 moderate, and 49 of 346 mild. Two hundred of 348 (57.5%) circumcised ITs experienced bleeding due to circumcision (Table 1), including 143 of 225 (63.6%), 32 of 72 (44.4%), and 25 of 49 (51.0%) with severe, moderate, and mild hemophilia, respectively.
Three hundred thirty-five of 807 ITs who were circumcised had information about FH, of whom 176 of 355 (52.5%) had a +FH of hemophilia, and 159 of 355 (47.5%) had a −FH of hemophilia. In comparison, among the ITs who were uncircumcised with information about FH, 402 of 472 (85.2%) had a +FH and 70 of 472 (14.8%) had a −FH of hemophilia.
Overall, ITs with +FH of hemophilia were less often circumcised compared with those with −FH of hemophilia to be circumcised (30.4% vs 69.4%; P ≤ .0001). Among those that were circumcised, bleeding was more common in ITs with a −FH of hemophilia than those with a +FH of hemophilia (79.4% vs 38.4%; P ≤ .0001), likely due to prophylactic treatment of infants with known hemophilia.
Treatment characteristics
Six hundred seventy patients had at least 1 treatment product reported. CFCs were the most commonly reported product used by 408 of 670 ITs. The distribution of product type is shown in Table 4.
Distribution of product usage since birth by product type and severity of hemophilia among ITs (n = 670)
| Type of product . | HA . | HB . | ||||
|---|---|---|---|---|---|---|
| 537 had at least 1 product type prescribed . | 133 had at least 1 product type prescribed . | |||||
| Mild . | Moderate . | Severe . | Mild . | Moderate . | Severe . | |
| n = 60 (ITs in this disease category with any product use) . | n = 82 (ITs in this disease category with any product use) . | n = 395 (ITs in this disease category with any product use) . | n = 13 (ITs in this disease category with any product use) . | n = 47 (ITs in this disease category with any product use) . | n = 73 (ITs in this disease category with any product use) . | |
| Clotting factor (recombinant and pd) | 34 (56.7%) | 53 (64.6%) | 226 (57.2%) | 9 (69.2%) | 38 (80.9%) | 48 (65.8%) |
| Emicizumab | 0 (0.0%) | ∗ | 28 (7.1%) | N/A | N/A | N/A |
| Other (including bypassing agent, aminocaproic acid) or multiple products | 26 (43.3%) | ∗ | 141 (35.7%) | ∗ | 9 (19.1%) | 25 (34.2%) |
| Type of product . | HA . | HB . | ||||
|---|---|---|---|---|---|---|
| 537 had at least 1 product type prescribed . | 133 had at least 1 product type prescribed . | |||||
| Mild . | Moderate . | Severe . | Mild . | Moderate . | Severe . | |
| n = 60 (ITs in this disease category with any product use) . | n = 82 (ITs in this disease category with any product use) . | n = 395 (ITs in this disease category with any product use) . | n = 13 (ITs in this disease category with any product use) . | n = 47 (ITs in this disease category with any product use) . | n = 73 (ITs in this disease category with any product use) . | |
| Clotting factor (recombinant and pd) | 34 (56.7%) | 53 (64.6%) | 226 (57.2%) | 9 (69.2%) | 38 (80.9%) | 48 (65.8%) |
| Emicizumab | 0 (0.0%) | ∗ | 28 (7.1%) | N/A | N/A | N/A |
| Other (including bypassing agent, aminocaproic acid) or multiple products | 26 (43.3%) | ∗ | 141 (35.7%) | ∗ | 9 (19.1%) | 25 (34.2%) |
Categorical data are presented as n (%).
N/A, not applicable.
Known fields with N of greater than 0 but less than 5 have been suppressed to protect patient confidentiality.
Regarding treatment regimen at the most recent visit, 252 of 691 (36.5%) ITs with HA were on continuous prophylaxis or continuous prophylaxis with bypassing agents, of whom 229 of 252 (90.9%) had severe hemophilia. Among ITs with HB, 38 of 192 (19.8%) were on continuous prophylaxis, and 33 of 38 (86.8%) had severe disease (Table 5).
Distribution of current treatment products used for prophylaxis and age of prophylaxis initiation between birth cohorts 2011 to 2021
| . | Total . | 2011-2015 . | 2016-2018 . | 2019-2021 . | P value . |
|---|---|---|---|---|---|
| # (%) . | # (%) . | # (%) . | # (%) . | ||
| Hemophilia A | 691 | 203 | 325 | 163 | |
| Continuous prophylaxis (including continuous prophylaxis with bypassing agents plus ITI) | 252 (36.5%) | 71 (35.0%) | 119 (36.6%) | 62 (38.0%) | |
| Severe hemophilia | 229 (90.9%) | 64 (90.1%) | 107 (89.9%) | 58 (93.5%) | |
| Age at initiation of prophylaxis mean (mo) | |||||
| Mean (SD) | 10.1 ± 6.2 | 10.8 ± 6.8 | 11.1 ± 5.6 | 7.1 ± 5.6 | |
| 0-6 mo | 50 (25.4%) | 17 (24.6%) | 15 (17.6%) | 18 (41.9%) | <.0001 |
| 7-23 mo | 147 (74.6%) | 52 (75.4%) | 70 (82.4%) | 25 (58.1%) | |
| 7-12 mo | 123 (62.4%) | 41 (59.4%) | 58 (68.2%) | ∗ | |
| 13-23 mo | 24 (12.2%) | 11 (15.9%) | 12 (14.1%) | ∗ | |
| N/A or unknown | 55 (-) | 2 (-) | 34 (-) | 19 (-) | |
| Current product type | |||||
| Recombinant, SHL clotting factor | ∗ | 44 (62.0) | 32 (26.9) | ∗ | <.0001 |
| Recombinant, EHL clotting factor | ∗ | 10 (14.1) | 18 (15.1) | ∗ | |
| Plasma derived clotting factor | ∗ | 11 (15.5) | 24 (20.2) | ∗ | |
| Bypassing agents | ∗ | 6 (8.5) | ∗ | ∗ | |
| Emicizumab | ∗ | N/A | 16 (13.4) | 40 (64.5) | |
| Unknown | ∗ | 0 (-) | ∗ | ∗ | |
| CVAD | |||||
| No | 130 (52.2%) | 30 (42.3%) | 53 (44.9%) | 47 (78.3%) | <.0001 |
| Yes | 119 (47.8%) | 41 (57.7%) | 65 (55.1%) | 13 (21.7%) | |
| Current product type | |||||
| Recombinant, SHL clotting factor | 47 (39.5%) | ∗ | ∗ | ∗ | |
| Recombinant, EHL clotting factor | 18 (15.1%) | ∗ | ∗ | ∗ | |
| Plasma derived clotting factor | 28 (23.5%) | ∗ | ∗ | ∗ | |
| Bypassing agents | 7 (5.9%) | ∗ | ∗ | ∗ | |
| Emicizumab | 13 (10.9%) | ∗ | ∗ | ∗ | |
| Unknown | 6 (-) | ∗ | ∗ | ∗ | |
| ICH | |||||
| No | 209 (83.6%) | 56 (80.0%) | 102 (86.4%) | 51 (82.3%) | .4873 |
| Yes | 41 (16.4%) | 14 (20.0%) | 16 (13.6%) | 11 (17.7%) | |
| Current product type | |||||
| Recombinant, SHL clotting factor | 13 (31.7%) | ∗ | ∗ | ∗ | |
| Recombinant, EHL clotting factor | ∗ | ∗ | ∗ | ∗ | |
| Plasma derived clotting factor | 7 (17.1%) | ∗ | ∗ | ∗ | |
| Bypassing agents | ∗ | ∗ | ∗ | ∗ | |
| Emicizumab | 11 (26.8%) | ∗ | ∗ | ∗ | |
| Hemophilia B | 192 | 63 | 80 | 49 | |
| Continuous prophylaxis (including continuous prophylaxis with bypassing agents plus ITI) | 38 (19.8%) | 12 (19.0%) | 16 (20.0%) | 10 (20.4%) | |
| Severe hemophilia | 33 (86.8%) | 11 (91.7%) | 13 (81.3%) | 9 (90.0%) | |
| Age at initiation of prophylaxis mean (mo) | |||||
| Mean (SD) | 11.6 ± 5.7 | 12.6 ± 6.4 | 10.5 ± 4.5 | 12.0 ± 6.9 | |
| 0-6 mo | ∗ | ∗ | ∗ | ∗ | |
| 7-23 mo | 29 (76.3%) | 10 (0.0%) | 13 (0.0%) | 6 (0.0%) | |
| Current product type | |||||
| Recombinant, SHL clotting factor | ∗ | 8 (66.7) | ∗ | ∗ | .059 |
| Recombinant, EHL clotting factor | ∗ | ∗ | 11 (68.8) | ∗ | |
| CVAD | |||||
| No | 13 (35.1%) | ∗ | ∗ | ∗ | .047 |
| Yes | 24 (64.9%) | 9 (75.0%) | 10 (66.7%) | ∗ | |
| Current product type | |||||
| Recombinant, SHL clotting factor | 10 (41.7%) | ∗ | ∗ | ∗ | |
| Recombinant, EHL clotting factor | 13 (54.2%) | ∗ | ∗ | ∗ | |
| Unknown/other | 1 (-) | ∗ | ∗ | ∗ | |
| ICH | |||||
| No | 31 (81.6%) | ∗ | ∗ | ∗ | |
| Yes | 7 (18.4%) | ∗ | ∗ | ∗ |
| . | Total . | 2011-2015 . | 2016-2018 . | 2019-2021 . | P value . |
|---|---|---|---|---|---|
| # (%) . | # (%) . | # (%) . | # (%) . | ||
| Hemophilia A | 691 | 203 | 325 | 163 | |
| Continuous prophylaxis (including continuous prophylaxis with bypassing agents plus ITI) | 252 (36.5%) | 71 (35.0%) | 119 (36.6%) | 62 (38.0%) | |
| Severe hemophilia | 229 (90.9%) | 64 (90.1%) | 107 (89.9%) | 58 (93.5%) | |
| Age at initiation of prophylaxis mean (mo) | |||||
| Mean (SD) | 10.1 ± 6.2 | 10.8 ± 6.8 | 11.1 ± 5.6 | 7.1 ± 5.6 | |
| 0-6 mo | 50 (25.4%) | 17 (24.6%) | 15 (17.6%) | 18 (41.9%) | <.0001 |
| 7-23 mo | 147 (74.6%) | 52 (75.4%) | 70 (82.4%) | 25 (58.1%) | |
| 7-12 mo | 123 (62.4%) | 41 (59.4%) | 58 (68.2%) | ∗ | |
| 13-23 mo | 24 (12.2%) | 11 (15.9%) | 12 (14.1%) | ∗ | |
| N/A or unknown | 55 (-) | 2 (-) | 34 (-) | 19 (-) | |
| Current product type | |||||
| Recombinant, SHL clotting factor | ∗ | 44 (62.0) | 32 (26.9) | ∗ | <.0001 |
| Recombinant, EHL clotting factor | ∗ | 10 (14.1) | 18 (15.1) | ∗ | |
| Plasma derived clotting factor | ∗ | 11 (15.5) | 24 (20.2) | ∗ | |
| Bypassing agents | ∗ | 6 (8.5) | ∗ | ∗ | |
| Emicizumab | ∗ | N/A | 16 (13.4) | 40 (64.5) | |
| Unknown | ∗ | 0 (-) | ∗ | ∗ | |
| CVAD | |||||
| No | 130 (52.2%) | 30 (42.3%) | 53 (44.9%) | 47 (78.3%) | <.0001 |
| Yes | 119 (47.8%) | 41 (57.7%) | 65 (55.1%) | 13 (21.7%) | |
| Current product type | |||||
| Recombinant, SHL clotting factor | 47 (39.5%) | ∗ | ∗ | ∗ | |
| Recombinant, EHL clotting factor | 18 (15.1%) | ∗ | ∗ | ∗ | |
| Plasma derived clotting factor | 28 (23.5%) | ∗ | ∗ | ∗ | |
| Bypassing agents | 7 (5.9%) | ∗ | ∗ | ∗ | |
| Emicizumab | 13 (10.9%) | ∗ | ∗ | ∗ | |
| Unknown | 6 (-) | ∗ | ∗ | ∗ | |
| ICH | |||||
| No | 209 (83.6%) | 56 (80.0%) | 102 (86.4%) | 51 (82.3%) | .4873 |
| Yes | 41 (16.4%) | 14 (20.0%) | 16 (13.6%) | 11 (17.7%) | |
| Current product type | |||||
| Recombinant, SHL clotting factor | 13 (31.7%) | ∗ | ∗ | ∗ | |
| Recombinant, EHL clotting factor | ∗ | ∗ | ∗ | ∗ | |
| Plasma derived clotting factor | 7 (17.1%) | ∗ | ∗ | ∗ | |
| Bypassing agents | ∗ | ∗ | ∗ | ∗ | |
| Emicizumab | 11 (26.8%) | ∗ | ∗ | ∗ | |
| Hemophilia B | 192 | 63 | 80 | 49 | |
| Continuous prophylaxis (including continuous prophylaxis with bypassing agents plus ITI) | 38 (19.8%) | 12 (19.0%) | 16 (20.0%) | 10 (20.4%) | |
| Severe hemophilia | 33 (86.8%) | 11 (91.7%) | 13 (81.3%) | 9 (90.0%) | |
| Age at initiation of prophylaxis mean (mo) | |||||
| Mean (SD) | 11.6 ± 5.7 | 12.6 ± 6.4 | 10.5 ± 4.5 | 12.0 ± 6.9 | |
| 0-6 mo | ∗ | ∗ | ∗ | ∗ | |
| 7-23 mo | 29 (76.3%) | 10 (0.0%) | 13 (0.0%) | 6 (0.0%) | |
| Current product type | |||||
| Recombinant, SHL clotting factor | ∗ | 8 (66.7) | ∗ | ∗ | .059 |
| Recombinant, EHL clotting factor | ∗ | ∗ | 11 (68.8) | ∗ | |
| CVAD | |||||
| No | 13 (35.1%) | ∗ | ∗ | ∗ | .047 |
| Yes | 24 (64.9%) | 9 (75.0%) | 10 (66.7%) | ∗ | |
| Current product type | |||||
| Recombinant, SHL clotting factor | 10 (41.7%) | ∗ | ∗ | ∗ | |
| Recombinant, EHL clotting factor | 13 (54.2%) | ∗ | ∗ | ∗ | |
| Unknown/other | 1 (-) | ∗ | ∗ | ∗ | |
| ICH | |||||
| No | 31 (81.6%) | ∗ | ∗ | ∗ | |
| Yes | 7 (18.4%) | ∗ | ∗ | ∗ |
Categorical are presented as n (%).
Responses of “Unknown” are not shown or included in the statistical analyses.
P values result from χ2 test for birth cohort across the respective patient characteristics.
ITI, immune tolerance induction; N/A, not applicable.
Known fields with N of greater than 0 but less than 5 have been suppressed to protect patient confidentiality. Additional cells may be suppressed to prevent derivation of these counts by subtraction.
Most ITs started prophylaxis between 7 and 12 months of age regardless of birth cohort (median 12 months of age), and though not significantly different between cohorts (P = .06), prophylaxis initiation at 0 to 6 months of age was more common among those born between 2019 and 2021. Additionally, ITs with HA born after 2018 started prophylaxis sooner than those born in earlier years (P ≤ .0001) (Table 5).
Patients born between 2011 and 2015 were most often treated with standard half-life (SHL) CFCs. The use of SHL CFCs was most common in those with HA born between 2016 and 2018, but some also used plasma-derived (pd) CFCs, and/or EHL CFCs throughout the study period. On the other hand, among those with HA born between 2019 and 2021, emicizumab was the most commonly used current prophylaxis product (Table 5).
Emicizumab prophylaxis was reported as the current treatment product in 56 patients with HA. Most, 40 of 56 (71%), of these ITs were born between 2019 and 2021 (Table 5). Among the 40 patients born between 2019 and 2021 and reporting emicizumab as their primary treatment product, 38 had severe HA (data not shown).
Among ITs with HB, the sample size was too small to meaningfully assess treatment product usage.
CVAD presence
One hundred eighty-six of 883 (21.1%) ITs had a CVAD within the first 2 years of life, including 143 (119 with HA and 24 with HB) who were currently treated with continuous prophylaxis or continuous prophylaxis with bypassing agents. Of the ITs with HA on prophylaxis who had CVADs, 47 of 119 (39.5%) were treated with recombinant SHL CFCs, 28 of 119 (23.5%) with plasma-derived CFCs, 18 of 119 (15.1%) with recombinant EHL CFCs, and 13 of 119 (10.9%) with emicizumab. The timing of CVAD placement relative to treatment initiation was not collected. Looking across birth cohorts, the rate of CVAD among those with HA that were treated with prophylaxis decreased significantly from 41 of 71 (57.7%) in those born between 2011 and 2015 to 13 of 62 (21.0%) among those born between 2019 and 2021 (P ≤ .0001) (Table 5).
Among the ITs with HB that had a CVAD, 13 of 24 (54.2%) were on EHL factor prophylaxis and 10 of 24 (41.7%) were on SHL factor for prophylaxis (Table 5). The use of CVAD decreased from 75.0% in ITs with HB born between 2011 and 2015 to almost 50.0% among those born between 2019 and 2021 (P = .047).
Discussion
This report describes characteristics and complications experienced by ITs with hemophilia ≤2 years of age who participated in the CDC Public Health Surveillance Project for Bleeding Disorders called Community Counts. Our sample represents a large cohort of babies seen at HTCs across the United States during the study years 2013 to 2021. The current study found that compared with historical data, babies in the United States are being diagnosed with hemophilia and started on prophylaxis at a younger age.2,10 Despite these changes, there are still substantial bleeding complications including ICH, particularly in the first month of life, and the median age of prophylaxis initiation (12 months) is above the age at which children are the highest risk for ICH and typically before prophylaxis initiation. In fact, the majority were not on prophylaxis at the time of ICH. FVIII prophylaxis has been shown to decrease the risk of ICH by ∼50% in children and adults with severe hemophilia,11,12 and initiation of prophylaxis as soon as possible after diagnosis may be an effective strategy to reduce the risk of ICH in ITs. Treatment product type evolved over time with increasing use of EHL CFC and emicizumab which can allow for earlier initiation of prophylaxis due to less frequent dosing and subcutaneous administration respectively.
ICH
ICH contributes to substantial morbidity and mortality,13,14 and ICH has been associated with long-term neurological sequelae and death and requires immediate treatment for improved outcomes. ICH in ITs with hemophilia occurs up to 44 to 60 times more often than in the nonhemophilia population.14 In our study, the overall 8% prevalence of ICH in our study of ITs born between 2011 and 2021, is unfortunately just as high as in a previous study of ITs born between 1998 and 2011,2,4 and 1.7% experiencing 2 or more ICHs during the study period. In a retrospective study of children with severe hemophilia born between 2006 and 2015, Andersson et al demonstrated that 9 out of 450 neonates (first 28 days of life) with severe hemophilia had ICH, with incidence of ∼2%.14 In our analysis, the prevalence of ICH in babies <1 month of age was similar at 2.7%.
Strategies to reduce the rate of ICH in the first month of life include avoiding birth trauma in those with a +FH of hemophilia. Birth trauma may result from mode of delivery and instrumentation. Whether CS should be recommended for all potentially affected deliveries remains controversial. Davies and Kadir15 reported that newborns the odds of ICH was significantly lower with CS compared with unassisted CS (odds ratio [OR], 0.34; 95% confidence interval, 0.14-0.83; P = .018). Alternatively, in published data from the UDC,2,4 ICH was more common with vaginal compared with CS delivery, but the results were not statistically significant. Additionally, in the PedNet (Pediatric Network on haemophilia management) multicenter study of over 1000 infants with moderate or severe hemophilia, there was no significant difference in ICH between vaginal delivery and CS.16 In our study a higher percentage of ITs delivered by vaginal delivery had ICH within the first 30 days of life compared with those delivered by CS. With inconclusive data regarding mode of delivery, it is important to implement shared decision-making between provider and families.
On the other hand, there are clear recommendations to avoid instrumentation given the higher risk of ICH with assisted vaginal delivery (eg, using vacuum extraction, forceps, fetal scalp monitoring) is a risk factor for ICH in infants with hemophilia.16,17
Even though +FH history was associated with a significantly lower proportion of vaginal deliveries, less instrumentation with vaginal delivery, and a lower incidence of ICH, the rate or 2.6 per 100 IT is still substantial, and the current strategies to reduce ICH based on +FH are insufficient to prevent all ICH in the neonatal period suggesting missed opportunities to utilize FH for safe delivery management, earlier diagnosis, and earlier treatment intervention.
Even without optimal risk mitigation strategies, it is critical to identify all women with a +FH of hemophilia who may deliver a potentially affected fetus to coordinate multidisciplinary care for the safety of the mother and infant and make a diagnosis as soon as possible.18
Circumcision
Neonates with hemophilia commonly experience bleeding complications from surgeries or procedures, such as circumcision and injections.2,4 In our study, ∼40% of babies were circumcised, which is lower than the national circumcision rate of ∼58% and attributed to recommendations to defer circumcision in males with hemophilia.19 In those males with a +FH hemophilia who had circumcision, the rates of bleeding were overall lower than in those with a −FH. This is attributed to peri-procedure hemostatic management, however, the rate of 40% still experiencing bleeding is substantial. This highlights that though known +FH is important to prevent bleeding, it is not enough to prevent all circumcision-related bleeding, and methods to further reduce the rate of circumcision-related bleeding need to be revisited.
Prophylaxis type and age of initiation
In our study, prophylaxis was initiated at a median age of 12 months (interquartile range [IQR], 11.0-12.0), and a mean of 10.3 months, a little earlier than previous reports of prophylaxis initiation at a median age of 15.6 months (IQR, 10.8-22.8).10 ITs with HA born in earlier birth cohorts were most often treated with recombinant SHL products. It is worthwhile to note that there was also an increase in both recombinant EHL and pd clotting factor use across birth cohorts until 2019 to 2021, when emicizumab became the most commonly used current prophylaxis product. The use of pd-CFCs in our study could reflect data from a randomized clinical trial, Survey of Inhibitors in Plasma-Product Exposed Toddlers (SIPPET), which was published in 2016 and showed an increased risk of FVIII inhibitor development when exposed to recombinant FVIII CFCs compared with pd-FVIII CFCs in previously untreated patients (PUPs).20 Although, pd-FVIII CFCs were not universally implemented based on the study, some providers recommended early treatment with pd-FVIII to reduce inhibitor risk.20
Our study provides surveillance data on 56 ITs with HA between the ages of 2 and 23 months old, born between 2016 and 2021, on emicizumab prophylaxis, which was approved for use by the US Food and Drug Administration in 2017 to prevent bleeding in patients of all ages with HA. There are several case studies demonstrating clinical efficacy and safety of emicizumab in the neonatal period,10,21-23 and there is growing interest in the use of emicizumab for infants as the subcutaneous mode of administration overcomes the challenge of frequent venipuncture and can allow for earlier initiation as a neonate. Earlier initiation of prophylaxis has the potential to prevent ICH and avoid hemarthroses which may occur prior to prophylaxis initiation.10 Moreover, the primary analysis from HAVEN 7 trial, a phase 3 multicenter, open-label study evaluating the efficacy, safety, and pharmacokinetics and pharmacodynamics of emicizumab prophylaxis in infants ≤12 months of age with severe HA without inhibitors, demonstrated clinical efficacy and safety, further supporting the benefit of initiating emicizumab from birth.24 The study was not powered to evaluate the efficacy of emicizumab for prevention of ICH, but no participants on emicizumab had ICH, which is lower than expected based on the number enrolled and previously discussed ICH rates. We hypothesize that earlier initiation of emicizumab prophylaxis may protect from ICH during the first few months of life when the risk of ICH is highest.21 This is consistent with the National Bleeding Disorders Foundation Medical and Scientific Advisory Council recommendation for prophylaxis with emicizumab in neonates with HA at any time after birth due the increased risk of ICH.21 Moreover, the number of ITs on emicizumab prophylaxis increased over the study period, likely reflecting utilization of new treatment options at HTCs in the US, easier mode of administration, and national guidelines supporting early initiation of prophylaxis to prevent ICH. Awareness and knowledge about emicizumab prophylaxis in infants with HA and its ability to prevent life-threatening bleeding, should be discussed with patients and their families in the initial conversations after diagnosis.
CVAD use
CVAD placement in ITs with hemophilia has been used to facilitate frequency venous infusions given the challenges of venous access in this population.25 Unfortunately, complications of CVADs include infection, bleeding, and thrombosis.2 In our study, ∼21% of infants with hemophilia receiving prophylaxis had a CVAD, which was slightly less than those infants in the previous UDC study.2,4 We expect that the rates will continue to decrease with treatment protocols which do not depend on very frequent venous access, including CFCs dosed once or less per week and non-factor products which are administered subcutaneously.
Study limitations
The CC Registry is a public health monitoring program which is run through US HTCs with funding from the CDC. Based on available funding and program goals, it is not feasible to collect as granular data (eg, all dates, treatment details) as with an industry-funded clinical trial. In addition, the registry was designed in 2012 before the approval of EHL CFCs and emicizumab and publication of the SIPPET study. Regarding FH, it is not known whether +FH of hemophilia only included mothers with hemophilia or carrier status or also included any family members with a history of hemophilia. Though this report included 18 (2%) females with hemophilia, due to small sample size, this analysis did not stratify by sex to observe for any differences in our findings. Several sources have recommended testing at risk females for hemophilia as soon as feasible to allow appropriate management for procedures, bleeding episodes, trauma, and life planning.26-28 It would be prudent to continue studying females with hemophilia on a larger scale, develop evidence-based guidelines, training of health care professional and families, and developing further research for females with hemophilia so that diagnosis is not delayed. Whether prophylaxis was started in relation to a bleeding complication vs following diagnosis was not recorded. Regarding ICH, it was unknown whether ITs who experienced ICH episodes were receiving on-demand therapy or prophylaxis prior, whether all ICH cases in the newborn period occurred because of birth trauma only, and type of imaging modality to diagnose ICH. Beyond the newborn period, data on the cause of ICH was also not collected. Similarly, data on the circumstances surrounding the delivery, including whether some of the CS deliveries were unplanned or occurred following the onset of labor, were not collected. More comprehensive data on birth and delivery is, therefore, needed in further studies. Dates of CVAD placement and the number of days the CVAD was in place were not recorded such that the correlation between CVAD use and type of hemophilia treatment could not be determined. This report did not include evaluation of inhibitors and will be addressed in a separate analysis. Although there were limitations to this analysis, these results provided important information about the ongoing high rate of ICH, impact of FH of hemophilia, and emicizumab prophylaxis in ITs in ≤2 years old with hemophilia.
Conclusions
Despite improvement in age of diagnosis and initiation of prophylaxis, the ICH prevalence has not decreased since 2011 and remains substantial. Additional studies are needed to identify measures to reduce ICH as well. Opportunities exist to prevent ICH by earlier diagnosis and initiating prophylaxis even within the first month of life, including emicizumab for infants with HA. Use of prophylaxis which is not dependent on CVAD may reduce CVAD placement and complications.
Acknowledgments
The authors thank the patients and their families, providers, and staff, of the federally funded Hemophilia Treatment Centers in the United States. J.H.H. thanks her mentor C.T. who guided her career development, as well as all the co-authors who supported this collaboration. The authors appreciate the critical review by J. Michael Soucie.
The results and conclusions of this study are those of the author(s) and do not necessarily represent the official stance of the Centers for Disease Control and Prevention.
Authorship
Contribution: C.T. led the study; J.H.H., B.D., A.M., and C.T. analyzed the data; J.H.H. wrote the manuscript; and B.D., A.M., R.K., M.M.-J., and C.T critically reviewed the manuscript.
Conflict-of-interest disclosure: R.K. is a consultant for BioMarin, CSL Behring, Novo Nordisk, Pfizer, and Sanofi Genzyme and received honorarium, and reports institutional research funding from Novo Nordisk and Sanofi Genzyme. C.T. is a consultant for CSL Behring, Genentech, Pfizer, Regeneron, Sanofi Genzyme, and Spark Therapeutics and received honorarium and reports research funding from Novo Nordisk. A.M. is a consultant for Genentech, Alexion, Spark, and Kedrion and received honorarium. The remaining authors declare no competing financial interests.
Correspondence: Jennifer H. Han, Indiana Hemophilia & Thrombosis Center, 8326 Naab Rd, Indianapolis, IN 46260; email: jhan@ihtc.org.
References
Author notes
Presented orally in abstract form at the 64th annual meeting of the American Society of Hematology, New Orleans, LA, 10 December 2022.
Data are available upon reasonable request from author Brandi Dupervil (brandidupervil@cdc.gov). The data will be made available according to Centers for Disease Control and Prevention data sharing policies.