Key Points
The GPIbα cytoplasmic tail is required for platelet general activation and thrombus formation beyond the GPIbα–VWF axis.
The GPIbα cytoplasmic tail promotes platelet activation by activating PKC through sequestering 14-3-3 from PKC.
Visual Abstract
Glycoprotein Ibα (GPIbα), the ligand-binding subunit of platelet GPIb-IX complex, interacts with von Willebrand factor (VWF) exposed at the injured vessel wall, initiating platelet adhesion, activation, hemostasis, and thrombus formation. The cytoplasmic tail of GPIbα interacts with 14-3-3ζ, regulating the VWF-GPIbα–elicited signal transduction and VWF binding function of GPIbα. However, we unexpectedly found that the GPIbα–14-3-3ζ association, beyond VWF-dependent function, is essential for general platelet activation. We found that the myristoylated peptide of GPIbα C-terminus MPαC, a potential GPIbα inhibitor, by itself induced platelet aggregation, integrin αIIbβ3 activation, granule secretion, and phosphatidylserine (PS) exposure. Conversely, the deletion of the cytoplasmic tail of GPIbα in mouse platelets (10aa−/−) decreased platelet aggregation, integrin αIIbβ3 activation, granule secretion, and PS exposure induced by various physiological agonists. Phosphoproteome-based kinase activity profiling revealed significantly upregulated protein kinase C (PKC) activity in MPαC-treated platelets. MPαC-induced platelet activation was abolished by the pan-PKC inhibitor and PKCα deletion. Decreased PKC activity was observed in both resting and agonist-stimulated 10aa−/− platelets. GPIbα regulates PKCα activity by sequestering 14-3-3ζ from PKCα. In vivo, the deletion of the GPIbα cytoplasmic tail impaired mouse hemostasis and thrombus formation and protected against platelet-dependent pulmonary thromboembolism. Therefore, our findings demonstrate an essential role for the GPIbα cytoplasmic tail in regulating platelet general activation and thrombus formation beyond the VWF–GPIbα axis.
Introduction
Glycoprotein Ibα (GPIbα), a type I transmembrane protein, is the ligand-binding subunit of the GPIb-IX complex. The N-terminal 45-kDa domain in the extracellular part of GPIbα contains binding sites for the ligand von Willebrand factor (VWF).1,2 The interaction of GPIbα with VWF exposed to the damaged vessel wall initiates platelet adhesion, transduces inward signaling, and activates integrin αIIbβ3, leading to stable platelet adhesion, hemostasis, and thrombus formation.3,4
The cytoplasmic domain of GPIbα contains binding sites for filamin A and 14-3-3ζ. Filamin A, which interacts with GPIbα at amino acids within the 557-579 sequence, attaches the GPIb-IX complex to the membrane skeleton, and is important for GPIb-IX surface expression.5-7 Three binding sites for 14-3-3ζ in the cytoplasmic domain of GPIbα have been reported by our group, and other researchers, at residues Ser606-Leu610, Leu580-Ser590, and Ala551-Arg564.8-10 The 606-610 sequence is the first identified 14-3-3ζ binding domain.8 The phosphorylation at Ser609 regulates the interaction of GPIbα with 14-3-3ζ.11 The association of 14-3-3ζ with the GPIbα 606-610 sequence is important in VWF-GPIbα-mediated signaling, including integrin αIIbβ3 activation, cytosolic calcium mobilization, Src family kinase activation, and cell apoptosis, and is critical in regulating the VWF binding function of GPIbα.12-15
In addition to VWF-dependent functions, the GPIbα C-terminal 14-3-3 binding domain regulates cell functions in the absence of VWF. For example, deletion of the GPIbα cytoplasmic tail (605-610) altered thrombopoietin-mediated megakaryocyte proliferation, polyploidization, and the expression of apoptotic markers in maturing megakaryocytes.16 Reconstitution of full-length GPIbα but not truncated GPIbα (at amino acid 591), inhibited Chinese hamster ovary (CHO) cell proliferation in the absence of VWF.17 Moreover, GPIbα regulates platelet procoagulant activity in response to various agonists that do not act through GPIbα, independent of its extracellular domain.18 These studies have suggested a possible regulatory effect of the GPIbα cytoplasmic tail on non–GPIbα-VWF interaction-initiated cell functions. However, the role of this region in non–GPIbα-VWF interaction-initiated platelet activation remains unclear.
In this study, we show that the GPIbα cytoplasmic tail regulates general platelet activation and thrombosis formation beyond the VWF-GPIbα axis. The enhancement or deletion of the GPIbα cytoplasmic tail disrupts 14-3-3 homeostasis, activating or inhibiting protein kinase C (PKC) and platelet function. Our study presents a new role of GPIbα in regulating platelet activation.
Methods
Human studies
All experiments using human participants were performed in accordance with the Declaration of Helsinki and approved by the ethics committee of The First Affiliated Hospital of Soochow University. Written informed consent was obtained from participants before inclusion in the study.
Animals
All animal experiments complied with the regulatory standards and were approved by the ethics committee of The First Affiliated Hospital of Soochow University. The 10aa−/− mice lacking 10 amino acids at the C-terminus of GPIbα (deletion of amino acids 709-718, corresponding to the deletion of human GPIbα 601-610 based on a high degree of similarity between the human and mouse GPIbα cytoplasmic domains16) were constructed through CRISPR/CRISPR-associated protein 9 (Cas9)–mediated genome editing. The CRISPR/Cas9–dual guide RNA system (guide RNA1: 5′-AGGACCTATTGGGCACAGTGG-3′; guide RNA2: 5′-ACTTCACTGGACTCTACTAGG-3′) was constructed by deleting a 30–base pair (bp) nucleotide sequence at the C-terminus of murine Gp1ba gene Exon 2, which resulted in the deletion of 10 amino acids. Gp1ba-Mut-F/Gp1ba-wild-type-R primers were used for genotyping by polymerase chain reaction. Targeted region sequences were F: 5′- ACACCACATAGTCTGGACATGG -3′ and R: 5′- TAGCCTGACAATGTTCTGAGTG -3′, and the product sizes were 513 bp for wild-type (WT) mice, and 460 bp for 10aa−/− mice. The mutation had been backcrossed onto the C57BL/6J background for at least 10 generations before this study. Mice were 6 to 12 weeks old, and experiments included balanced groups of male and female mice, unless otherwise stated.
B6/JGpt-Prkcaem1Cd5428/Gpt mice (T027858) were purchased from GemPharmatech Co, Ltd (Nanjing, China). C57BL/6J WT mice were purchased from JOINN Laboratories (Suzhou, China).
Statistics analysis
Statistical analysis was performed using GraphPad Prism 8 software. Shapiro-Wilk test and Brown-Forsythe test were done for normality and variance, respectively. Numeric data were analyzed using 1-way (for a single variant) or 2-way (for multiple variants) analysis of variance. Two groups were compared by Mann-Whitney or 2-tailed Student t test. Different levels of significance were indicated as ∗P < .05, ∗∗P < .01, and ∗∗∗P < .001. All animal experiments were subject to randomization based on litter. No animals or samples were excluded from the study. The sample size was predetermined based on the variability observed in prior experiments and preliminary data.
Results
The myristoylated peptide of GPIbα C-terminus (MPαC) induces the activation of washed human platelets
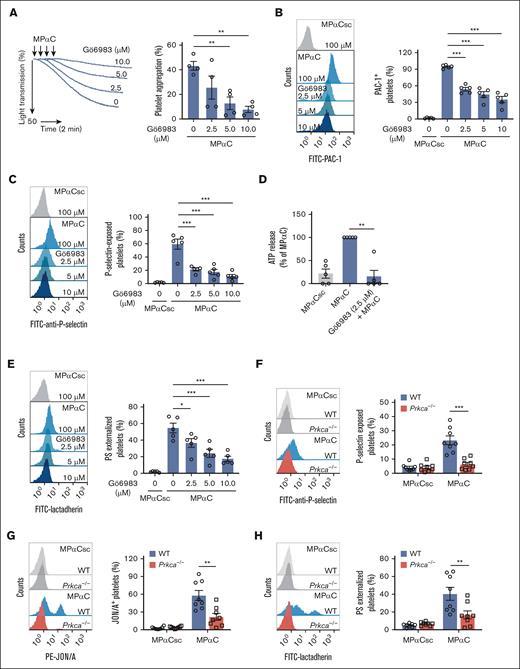
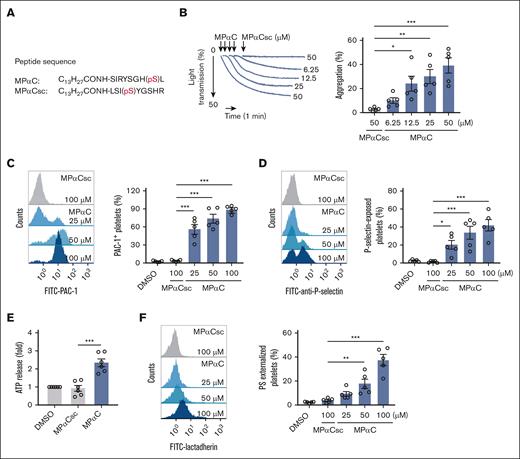
MPαC is a GPIbα C-terminal sequence (GPIbα 602-610) peptide with Ser609 phosphorylated (Figure 1A).15 The peptide is known to inhibit VWF-mediated platelet aggregation by regulating VWF-GPIbα interaction through sequestering 14-3-3ζ from GPIbα.15 To investigate the role of the GPIbα cytoplasmic tail in non–VWF-GPIbα interaction-initiated platelet function, washed human platelets were incubated with the peptide MPαC in the absence of VWF or any other platelet agonists. Surprisingly, MPαC by itself was sufficient to induce washed human platelet aggregation but not the scrambled control peptide MPαCsc (Figure 1B). Examination of platelet PAC-1 binding demonstrated that MPαC dose-dependently activated integrin αIIbβ3 but not MPαCsc or the vehicle control dimethyl sulfoxide (Figure 1C). MPαC by itself also induced platelet α-granule and dense granule secretion, as determined by platelet surface P-selectin exposure and adenosine 5'-triphosphate (ATP) release, respectively (Figure 1D-E).
MPαC induces washed human platelet activation. (A) Sequences of the myristoylated peptide MPαC containing the C-terminal 9 amino acid residues of human GPIbα and scrambled control peptide MPαCsc. (B) Representative traces of MPαC-induced washed human platelet aggregation (left) and the quantification of maximal aggregation rate (right; n = 5 independent experiments). (C-D) MPαC-induced PAC-1 binding (C) and P-selectin exposure (D) on washed human platelets (n = 5 independent experiments). (E) MPαC-induced ATP release in washed human platelets (n = 6 independent experiments). (F) MPαC-induced PS externalization on washed human platelets (n = 5 independent experiments). One-way analysis of variance (ANOVA) followed by Dunnett post hoc test (compared with MPαCsc) in panels B-F. Data are shown as mean ± standard error of the mean (SEM) in panels B-F. ∗P < .05, ∗∗P < .01, and ∗∗∗P < .001.
MPαC induces washed human platelet activation. (A) Sequences of the myristoylated peptide MPαC containing the C-terminal 9 amino acid residues of human GPIbα and scrambled control peptide MPαCsc. (B) Representative traces of MPαC-induced washed human platelet aggregation (left) and the quantification of maximal aggregation rate (right; n = 5 independent experiments). (C-D) MPαC-induced PAC-1 binding (C) and P-selectin exposure (D) on washed human platelets (n = 5 independent experiments). (E) MPαC-induced ATP release in washed human platelets (n = 6 independent experiments). (F) MPαC-induced PS externalization on washed human platelets (n = 5 independent experiments). One-way analysis of variance (ANOVA) followed by Dunnett post hoc test (compared with MPαCsc) in panels B-F. Data are shown as mean ± standard error of the mean (SEM) in panels B-F. ∗P < .05, ∗∗P < .01, and ∗∗∗P < .001.
It has been shown that GPIbα regulates platelet procoagulant activity independent of the extracellular domain.18 Platelet phosphatidylserine (PS) externalization in response to agonists is an essential component of platelet procoagulant activity. To determine the role of the GPIbα cytoplasmic tail in platelet procoagulant activity, PS externalization was measured in MPαC-treated platelets by detecting fluorescein isothiocyanate–conjugated lactadherin binding. PS externalization was dose dependently increased in MPαC-treated platelets (Figure 1F).
MPαC was further verified to dissociate 14-3-3ζ from GPIbα in human platelets (supplemental Figure 1A), as previously reported,15 and bind purified recombinant 14-3-3ζ in vitro (supplemental Figure 1B).
Moreover, the discrepancy between current and previous data was explained.15,19 We first repeated the experiments and showed that MPαC indeed abolished ristocetin (allows soluble VWF binding to GPIbα-induced platelet aggregation in human platelet-rich plasma [PRP]; supplemental Figure 2A). In contrast to its effect on washed platelets (Figure 1B), even 100 μM MPαC could not induce platelet aggregation in human PRP (supplemental Figure 2B). Furthermore, MPαC-induced washed platelet aggregation was dose dependently inhibited by human plasma (supplemental Figure 3), which appeared to cover the platelet-activating property of MPαC. In agreement with this notion, MPαC enhanced adenosine 5′-diphosphate (ADP)-induced aggregation in washed platelets but not in PRP (supplemental Figure 4). Thus, the substances in plasma may explain the discrepancy between current and previous data.15,19
Taken together, these data demonstrate that the exogenous addition of the GPIbα cytoplasmic tail peptide induces the activation of washed human platelets.
PKC is activated in MPαC-treated platelets
Platelet activation is closely related to protein phosphorylation and kinase activation.20-22 To explore the mechanism for MPαC-induced platelet activation, we aimed to identify the major kinases activated by MPαC. The regulatory state of a kinase is reflected through alterations in the phosphorylation levels of its substrate sites. Thus, we assessed protein phosphorylation profiles in platelets stimulated with MPαC in comparison to those stimulated with MPαCsc, and changes in kinase activity were predicted based on known kinases and their substrates and phosphosites. To measure protein phosphorylation events largely attributed to MPαC activation alone, platelets were incubated with peptides in the presence of apyrase, aspirin, and tirofiban to inhibit feedback signals from ADP release, thromboxane generation, and integrin αIIbβ3 activation. In total, 4472 phosphosites, 4989 phosphopeptides, and 1805 phosphoproteins were identified (supplemental Data Set 1). Using a threshold (a fold change of >1.2 or <0.83, and P < .05), 351 upregulated and 881 downregulated phosphopeptides were identified in MPαC-treated platelets in comparison with MPαCsc-treated platelets (Figure 2A; supplemental Data Set 1). Based on the known kinases and their substrates in the database, 171 kinases of 903 phosphosites were annotated and 654 kinase–substrate interactions were predicted (supplemental Data Set 2). The activities of the 171 kinases were estimated based on the phosphorylation levels of their substrates (supplemental Data Set 3). The kinases predicted to be significantly activated and inhibited (P < .05) are shown in Figure 2B. Among the 22 kinases with upregulated activity, protein kinase Cα (PKCα) topped the list, with a z score of 3.96 (P = .000037; Figure 2B; supplemental Data Set 3). Besides PKCα, the activities of PKCι, PKCβ, and PKCζ were also estimated to increase (Figure 2B; supplemental Data Set 3). To confirm PKC activation, the phosphorylation of the PKC substrate pleckstrin was determined in MPαC-treated platelets by western blot. MPαC dose dependently increased the phosphorylation level of pleckstrin but not MPαCsc or dimethyl sulfoxide (Figure 2C). Collectively, PKC is activated in MPαC-treated platelets.
MPαC activates PKC in platelets. (A) Volcano plot showing the distribution of the significance and fold change of identified phosphopeptides between MPαC- and MPαCsc-treated human platelets (n = 3). Red and blue dots indicate the significantly increased (351) and decreased (818) phosphopeptides in MPαC- vs MPαCsc-treated samples (fold change >1.2 or <0.83; P < .05 by 2-tailed Student t test). (B) Kinases predicted to be activated and inhibited. The x-axis represents the score of kinase activity, and the y-axis represents the kinases predicted to be significantly regulated (P < .05). Red represents the activation status, and blue represents the inhibition status. (C) Representative blot of MPαC-induced PKC substrate (pleckstrin) phosphorylation in washed human platelets and quantification of the densitometry in the blots (n = 5 independent experiments). One-way ANOVA followed by Dunnett post hoc test (compared with vehicle control dimethyl sulfoxide [DMSO]) in panel C. Data are shown as mean ± SEM for panel C. ∗P < .05 and ∗∗∗P < .001.
MPαC activates PKC in platelets. (A) Volcano plot showing the distribution of the significance and fold change of identified phosphopeptides between MPαC- and MPαCsc-treated human platelets (n = 3). Red and blue dots indicate the significantly increased (351) and decreased (818) phosphopeptides in MPαC- vs MPαCsc-treated samples (fold change >1.2 or <0.83; P < .05 by 2-tailed Student t test). (B) Kinases predicted to be activated and inhibited. The x-axis represents the score of kinase activity, and the y-axis represents the kinases predicted to be significantly regulated (P < .05). Red represents the activation status, and blue represents the inhibition status. (C) Representative blot of MPαC-induced PKC substrate (pleckstrin) phosphorylation in washed human platelets and quantification of the densitometry in the blots (n = 5 independent experiments). One-way ANOVA followed by Dunnett post hoc test (compared with vehicle control dimethyl sulfoxide [DMSO]) in panel C. Data are shown as mean ± SEM for panel C. ∗P < .05 and ∗∗∗P < .001.
MPαC-induced platelet activation depends on PKC activation
Phosphoproteome-based kinase activity profiling revealed that the activities of PKCα, PKCι, PKCβ, and PKCζ increased with MPαC treatment. PKCα, PKCβ, and PKCζ are expressed in human platelets.23 To investigate the role of PKC in MPαC-induced platelet activation, the effect of the pan-PKC inhibitor Gö6983 was first tested. Gö6983 significantly inhibited MPαC-induced human platelet aggregation and integrin αIIbβ3 activation (Figure 3A-B), granule secretion (Figure 3C-D), and PS exposure (Figure 3E). Moreover, MPαC-induced platelet activation was examined in PKCα-deleted mouse platelets. MPαC-induced αIIbβ3 activation, P-selectin externalization, and PS exposure were markedly reduced in Prkca−/− platelets (Figure 3F-H). Treatment of human washed platelets with the PKC activator phorbol 12-myristate 13-acetate (PMA), dose dependently induced platelet integrin αIIbβ3 activation, P-selectin exposure, and PS externalization (supplemental Figure 5), confirming the relationship between PKC activation and platelet activation. Collectively, MPαC-induced platelet activation depends on PKC activation.
MPαC-induced platelet activation depends on PKC. (A-E) The effect of the pan-PKC inhibitor Gö6983 on (A) MPαC-induced washed human platelet aggregation (n = 4 independent experiments), (B) PAC-1 binding, (C) P-selectin exposure, (D) ATP release, (E) and PS externalization (n = 5 independent experiments). (F) MPαC-induced P-selectin exposure, (G) JON/A (a monoclonal antibody against activated mouse integrin αIIbβ3) binding, and (H) PS externalization on washed WT and Prkca−/− platelets (n = 8 mice per genotype). One-way ANOVA followed by Dunnett post hoc test in panels A-E; 2-tailed Student t test in panels F-H. Data are shown as mean ± SEM for panels A-H. ∗P < .05, ∗∗P < .01, and ∗∗∗P < .001.
MPαC-induced platelet activation depends on PKC. (A-E) The effect of the pan-PKC inhibitor Gö6983 on (A) MPαC-induced washed human platelet aggregation (n = 4 independent experiments), (B) PAC-1 binding, (C) P-selectin exposure, (D) ATP release, (E) and PS externalization (n = 5 independent experiments). (F) MPαC-induced P-selectin exposure, (G) JON/A (a monoclonal antibody against activated mouse integrin αIIbβ3) binding, and (H) PS externalization on washed WT and Prkca−/− platelets (n = 8 mice per genotype). One-way ANOVA followed by Dunnett post hoc test in panels A-E; 2-tailed Student t test in panels F-H. Data are shown as mean ± SEM for panels A-H. ∗P < .05, ∗∗P < .01, and ∗∗∗P < .001.
Deletion of the GPIbα cytoplasmic tail reduces various agonist-induced platelet aggregation, αIIbβ3 activation, granule secretion, and PS exposure
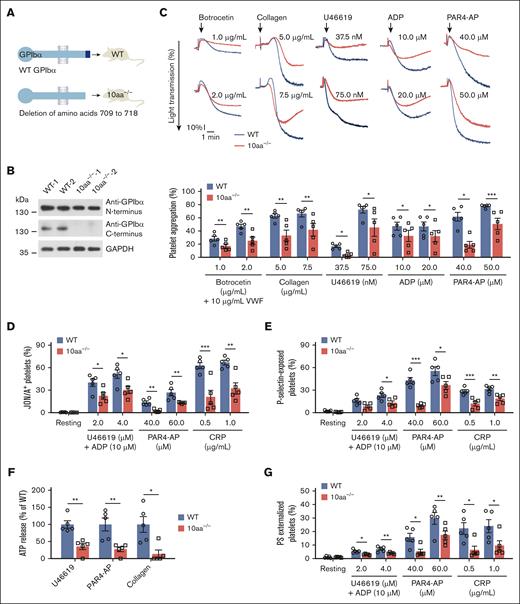
To further ascertain the role of the GPIbα cytoplasmic tail in platelet function, a GPIbα cytoplasmic 10-residue–deleted mouse model was generated using CRISPR/Cas9 technology (Figure 4A-B; supplemental Figure 6A-B). The deletion of the C-terminal 10 residues diminished the association of 14-3-3ζ with GPIbα in 10aa−/− mouse platelets (supplemental Figure 6C). The platelet count was slightly lower and the size was slightly larger in 10aa−/− mice (supplemental Figure 6D-E), but the morphology of the platelets was normal (supplemental Figure 6F).
The deletion of GPIbα C-terminal 10 residues reduces various agonist-induced platelet aggregation, αIIbβ3 activation, granule secretion, and PS exposure. (A) Schematic depicting the construction of 10aa−/− mice. (B) Western blot analysis of GPIbα in WT and 10aa−/− platelet lysates with anti-GPIbα N- and C-terminal antibodies. Protein concentrations have been adjusted to the same level in platelet lysates. Blots are representative of 6 mice per genotype. The lack of C-terminal amino acids was also verified by mass spectrometry (data not shown). (C) Representative agonist-induced platelet aggregation traces in washed WT and 10aa−/− platelets (upper) and quantification of maximal aggregation rate (lower) (n = 5 independent experiments). (D) Agonist-induced JON/A binding, (E) P-selectin exposure, (F) ATP release, and (G) PS externalization in washed WT and 10aa−/− platelets (n = 5 mice per genotype). Two-tailed Student t test in panels C,F. Two-way ANOVA followed by Bonferroni post hoc test in panels D-E,G. Data are shown as mean ± SEM for panels C-G. ∗P < .05, ∗∗P < .01, and ∗∗∗P < .001.
The deletion of GPIbα C-terminal 10 residues reduces various agonist-induced platelet aggregation, αIIbβ3 activation, granule secretion, and PS exposure. (A) Schematic depicting the construction of 10aa−/− mice. (B) Western blot analysis of GPIbα in WT and 10aa−/− platelet lysates with anti-GPIbα N- and C-terminal antibodies. Protein concentrations have been adjusted to the same level in platelet lysates. Blots are representative of 6 mice per genotype. The lack of C-terminal amino acids was also verified by mass spectrometry (data not shown). (C) Representative agonist-induced platelet aggregation traces in washed WT and 10aa−/− platelets (upper) and quantification of maximal aggregation rate (lower) (n = 5 independent experiments). (D) Agonist-induced JON/A binding, (E) P-selectin exposure, (F) ATP release, and (G) PS externalization in washed WT and 10aa−/− platelets (n = 5 mice per genotype). Two-tailed Student t test in panels C,F. Two-way ANOVA followed by Bonferroni post hoc test in panels D-E,G. Data are shown as mean ± SEM for panels C-G. ∗P < .05, ∗∗P < .01, and ∗∗∗P < .001.
To investigate the effect of GPIbα cytoplasmic tail truncation on platelet activation, various agonist-induced platelet aggregations were compared between WT and 10aa−/− washed platelets. VWF-GPIbα–dependent platelet aggregation was induced by VWF plus botrocetin, which allows soluble VWF binding to mouse GPIbα. The 10aa−/− platelets displayed decreased aggregation in response to VWF/botrocetin (Figure 4C), which is not due to the decreased binding of VWF to 10aa−/− platelets because the binding of VWF to WT and 10aa−/− platelets were comparable (supplemental Figure 6G). This is consistent with the requirement of GPIbα–14-3-3ζ association for VWF-GPIbα interaction-induced signaling.12,14 Collagen-induced aggregation was also decreased in 10aa−/− platelets (Figure 4C), which seems understandable because the intracellular regions of GPIbα and GPVI have physical and functional associations.24-26 Interestingly, 10aa−/− platelets showed reduced aggregation in response to ADP and U46619 (Figure 4C), suggesting a role of the GPIbα cytoplasmic tail in purinergic P2Y and thromboxane A2 (TP) receptor-mediated signaling. Because thrombin binds to protease-activated receptors (PARs) and GPIbα simultaneously and there is cooperation between GPIbα and PAR signaling in thrombin stimulation,27 PAR4-activating peptide (PAR4-AP) was used to examine the effect of the GPIbα cytoplasmic tail deletion on PAR signaling instead of thrombin. Platelet aggregation was also reduced in 10aa−/− platelets in response to PAR4-AP (Figure 4C). The reduced aggregation did not result from the reduction of the corresponding receptors because the protein levels of the receptors were comparable between 10aa−/− and WT platelets (supplemental Figure 6H-I).
Next, we examined the effect of the GPIbα C-terminal 10-residue deletion on platelet function in more detail. Integrin αIIbβ3 activation was reduced in U46619 plus ADP, PAR4-AP, and collagen-related polypeptide (CRP)-stimulated 10aa−/− platelets compared with WT platelets (Figure 4D). Investigation of P-selectin exposure and ATP release showed impairment of α-granule and dense granule secretion in 10aa−/− platelets (Figure 4E-F). Total P-selectin and ATP contents were comparable between WT and 10aa−/− platelets (supplemental Figure 6I-J). PS externalization was also reduced in agonist-stimulated 10aa−/− platelets (Figure 4G). The difference in PS exposure was not due to the varying PS contents because the calcium ionophore A23187 induced equal PS externalization in WT and 10aa−/− platelets (supplemental Figure 6K). These data show that deletion of the GPIbα cytoplasmic tail impaired various agonist-induced platelet aggregation, integrin αIIbβ3 activation, granule secretion, and PS exposure.
PKC activity is decreased in 10aa−/− platelets
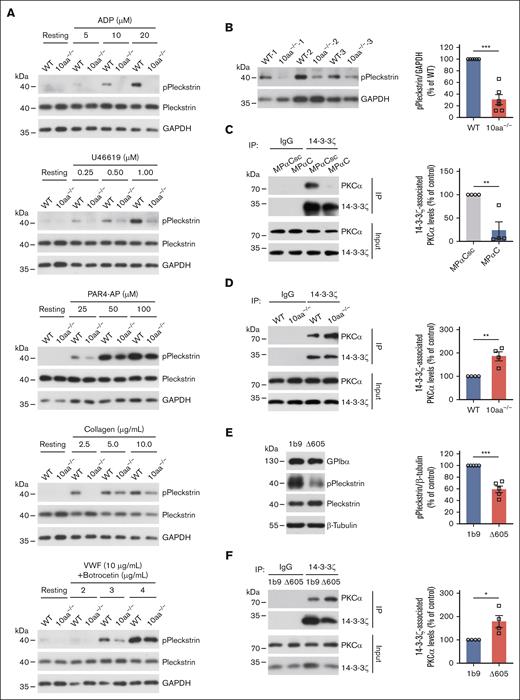
PKC was activated by MPαC treatment; thus we hypothesized that 10aa−/− platelets have decreased PKC activity. The phosphorylation of the PKC substrate dose dependently increased when WT platelets were stimulated with ADP, U46619, PAR4-AP, collagen, and VWF plus botrocetin (Figure 5A), consistent with the fact that PKC is activated in P2Y12-, TP-, PAR4-, GPVI-, and GPIbα-mediated signaling pathways.28 The 10aa−/− platelets showed less pleckstrin phosphorylation in all agonist stimulations (Figure 5A; supplemental Figure 7), suggesting that PKC is less activated in 10aa−/− platelets. Then, basal PKC activity was compared between resting WT and 10aa−/− platelets. Relatively long exposure times were used to show basal pleckstrin phosphorylation (Figure 5B). Resting 10aa−/− platelets showed a 70% decrease in pleckstrin phosphorylation (Figure 5B). In summary, deletion of the GPIbα cytoplasmic tail reduced PKC activity in resting and activated platelets.
PKC activity is decreased in 10aa−/− platelets, and GPIbα cytoplasmic tail regulates PKC activity by sequestering 14-3-3. (A) Representative blots of ADP-, U46619-, PAR4-AP-, collagen- and botrocetin/VWF-induced phosphorylation of PKC substrate (pleckstrin) in WT and 10aa−/− platelets (n = 3 independent experiments). (B) Representative blot of PKC substrate (pleckstrin) phosphorylation in resting WT and 10aa−/− platelets and quantification of densitometry in the blots (n = 6 mice per genotype). (C) Representative blots and quantification of immunoprecipitation of PKCα with 14-3-3ζ in human platelets treated with MPαC and MPαCsc (n = 4 independent experiments). (D) Representative blots and quantification of immunoprecipitation of PKCα with 14-3-3ζ in resting WT and 10aa−/− platelets (n = 4 independent experiments). (E) Representative blot and quantification of PKC substrate (pleckstrin) phosphorylation in 1b9 and Δ605 cells (n = 5 independent experiments). (F) Representative blots and quantification of immunoprecipitation of PKCα with 14-3-3ζ in 1b9 and Δ605 cells (n = 4 independent experiments). Two-tailed Student t test in panels B-F. Data are shown as mean ± SEM for panels B-F. ∗P < .05, ∗∗P < .01, and ∗∗∗P < .001.
PKC activity is decreased in 10aa−/− platelets, and GPIbα cytoplasmic tail regulates PKC activity by sequestering 14-3-3. (A) Representative blots of ADP-, U46619-, PAR4-AP-, collagen- and botrocetin/VWF-induced phosphorylation of PKC substrate (pleckstrin) in WT and 10aa−/− platelets (n = 3 independent experiments). (B) Representative blot of PKC substrate (pleckstrin) phosphorylation in resting WT and 10aa−/− platelets and quantification of densitometry in the blots (n = 6 mice per genotype). (C) Representative blots and quantification of immunoprecipitation of PKCα with 14-3-3ζ in human platelets treated with MPαC and MPαCsc (n = 4 independent experiments). (D) Representative blots and quantification of immunoprecipitation of PKCα with 14-3-3ζ in resting WT and 10aa−/− platelets (n = 4 independent experiments). (E) Representative blot and quantification of PKC substrate (pleckstrin) phosphorylation in 1b9 and Δ605 cells (n = 5 independent experiments). (F) Representative blots and quantification of immunoprecipitation of PKCα with 14-3-3ζ in 1b9 and Δ605 cells (n = 4 independent experiments). Two-tailed Student t test in panels B-F. Data are shown as mean ± SEM for panels B-F. ∗P < .05, ∗∗P < .01, and ∗∗∗P < .001.
The GPIbα cytoplasmic tail regulates PKC activity by sequestering 14-3-3
The dissociation of 14-3-3, an endogenous inhibitor of PKC, from PKC results in PKC activation.29,30 Thus, it is likely that GPIbα regulates PKC activity by sequestering 14-3-3 protein. Because PKCα was estimated to be most activated by MPαC (Figure 2B), the association of 14-3-3ζ with PKCα was investigated in different scenarios. We found that MPαC treatment of human platelets reduced the association of 14-3-3ζ with PKCα compared with MPαCsc treatment (Figure 5C). Conversely, without the C-terminal 14-3-3ζ binding site in GPIbα in 10aa−/− platelets, the association of 14-3-3ζ with PKCα was enhanced (Figure 5D). Furthermore, CHO cells expressing full-length human GPIbα (1b9) or truncated GPIbα lacking the C-terminal 6 residues (Δ605)15 were used to verify the finding. 1b9 cells had a higher level of pleckstrin phosphorylation than Δ605 cells (Figure 5E). Consistently, the lack of GPIbα-14-3-3ζ interaction enhanced the association of PKCα with 14-3-3ζ in Δ605 cells, compared with that in 1b9 cells (Figure 5F). These data demonstrate that the GPIbα cytoplasmic tail regulates PKCα activity by sequestering 14-3-3ζ.
MPαC rescues the functional defects of 10aa−/− platelets
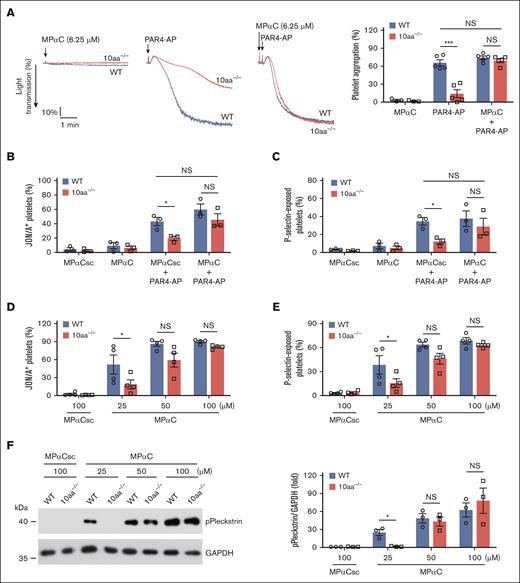
To further ascertain the role of the GPIbα cytoplasmic tail in platelet activation, the hypothesis that the addition of MPαC compensates for GPIbα 10-residue deletion and restores platelet activity in 10aa−/− platelets was tested. Low-dose MPαC (6.25 μM) was insufficient to induce washed mouse platelet aggregation but restored PAR4-AP–induced 10aa−/− platelet aggregation to the level of WT platelets (Figure 6A). Low-dose MPαC also restored PAR4-AP–induced 10aa−/− platelet integrin αIIbβ3 activation (Figure 6B) and P-selectin exposure (Figure 6C). Relatively high-dose MPαC-induced platelet activation in WT and 10aa−/− platelets was also examined. MPαC (>25 μM) by itself could induce integrin αIIbβ3 activation and P-selectin exposure in washed WT mouse platelets (Figure 6D-E). Decreased integrin αIIbβ3 activation and P-selectin exposure were observed in 10aa−/− platelets treated with 25 μM MPαC (Figure 6D-E). Comparable levels of activation markers were exhibited in WT and 10aa−/− platelets with increasing peptide concentrations (Figure 6D-E). MPαC-induced PKC activation was consistent with the platelet activation markers in WT and 10aa−/− platelets (Figure 6F). These data further confirm the role of the GPIbα cytoplasmic tail in regulating platelet function.
MPαC rescues the functional defects of 10aa−/− platelets. (A-C) The effect of (A) low-dose MPαC- (6.25 μM) on PAR4-AP– (40 μM) induced washed WT and 10aa−/− platelet aggregation (n = 5 independent experiments), (B) JON/A binding (n = 3 independent experiments), and (C) P-selectin exposure (n = 3 independent experiments). (D) High-dose MPαC only–induced washed WT and 10aa−/− platelet JON/A binding (n = 4 independent experiments), (E) P-selectin exposure (n = 4 independent experiments), (F) PKC substrate (pleckstrin) phosphorylation (n = 3 independent experiments). Two-way ANOVA followed by Bonferroni post hoc test in panels A-F. Data are shown as mean ± SEM for panels A-F. ∗P < .05 and ∗∗∗P < .001; NS, not significant.
MPαC rescues the functional defects of 10aa−/− platelets. (A-C) The effect of (A) low-dose MPαC- (6.25 μM) on PAR4-AP– (40 μM) induced washed WT and 10aa−/− platelet aggregation (n = 5 independent experiments), (B) JON/A binding (n = 3 independent experiments), and (C) P-selectin exposure (n = 3 independent experiments). (D) High-dose MPαC only–induced washed WT and 10aa−/− platelet JON/A binding (n = 4 independent experiments), (E) P-selectin exposure (n = 4 independent experiments), (F) PKC substrate (pleckstrin) phosphorylation (n = 3 independent experiments). Two-way ANOVA followed by Bonferroni post hoc test in panels A-F. Data are shown as mean ± SEM for panels A-F. ∗P < .05 and ∗∗∗P < .001; NS, not significant.
Deletion of the GPIbα cytoplasmic tail impairs thrombosis in vivo
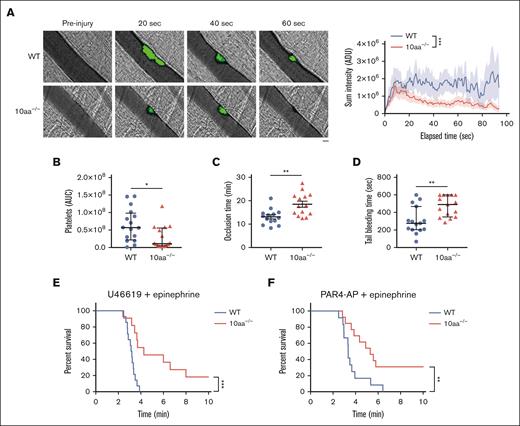
The physiological relevance of the GPIbα cytoplasmic tail in vivo was examined. We first determine its role in thrombosis by performing laser-induced injuries in the cremasteric artery, and FeCl3-induced injuries in the mesenteric artery. As observed, platelet accumulation in laser-induced injuries in the cremasteric artery was decreased in 10aa−/− mice compared with in WT mice (Figure 7A-B). FeCl3-induced arterial occlusion was delayed in 10aa−/− mice compared with that in WT mice (18.46 ± 4.84 minutes vs 13.09 ± 3.30 minutes; Figure 7C). Determination of hemostasis by tail bleeding assay showed that the tail bleeding time was extended in 10aa−/− mice compared with that in WT mice (473 ± 117 seconds vs 309 ± 155 seconds; Figure 7D). These data suggest the role of the GPIbα cytoplasmic tail in hemostasis and thrombosis.
Deletion of the GPIbα cytoplasmic tail impairs thrombosis in vivo. (A) Representative fluorescence images of platelets (green) with bright field images obtained from WT and 10aa−/− mice (n = 5 mice per genotype) at the indicated times after laser-induced injury of endothelium (original magnification, ×200). Sum platelet fluorescent intensities after laser injury were calculated and plotted at 0.84-second intervals (n = 16 thrombi from 5 mice per genotype). Scale bar, 10 μm. (B) The area under curve (AUC) of fluorescent signal corresponding to platelet accumulation was calculated and plotted for each thrombus formed in WT and 10aa−/− mice (n = 16 thrombi from 5 mice per genotype). (C) FeCl3-induced mesenteric arteriole occlusion time in WT and 10aa−/− mice (n = 14 mice per genotype). (D) Tail bleeding times in WT and 10aa−/− mice (n = 15 mice per genotype). (E-F) Survival curve of WT and 10aa−/− mice after intravenous injection of a mixture of U46619 and epinephrine (E, n = 14 mice per genotype) and PAR4-AP and epinephrine (F, n = 12 mice per genotype). Mann-Whitney U test in panels A-B,D; 2-tailed Student t test in panel C; log-rank (Mantel-Cox) test in panels E-F. Data are shown as mean ± SEM for panels A,C and median ± interquartile range (IQR) for panels B,D. ∗P < .05, ∗∗P < .01, and ∗∗∗P < .001.
Deletion of the GPIbα cytoplasmic tail impairs thrombosis in vivo. (A) Representative fluorescence images of platelets (green) with bright field images obtained from WT and 10aa−/− mice (n = 5 mice per genotype) at the indicated times after laser-induced injury of endothelium (original magnification, ×200). Sum platelet fluorescent intensities after laser injury were calculated and plotted at 0.84-second intervals (n = 16 thrombi from 5 mice per genotype). Scale bar, 10 μm. (B) The area under curve (AUC) of fluorescent signal corresponding to platelet accumulation was calculated and plotted for each thrombus formed in WT and 10aa−/− mice (n = 16 thrombi from 5 mice per genotype). (C) FeCl3-induced mesenteric arteriole occlusion time in WT and 10aa−/− mice (n = 14 mice per genotype). (D) Tail bleeding times in WT and 10aa−/− mice (n = 15 mice per genotype). (E-F) Survival curve of WT and 10aa−/− mice after intravenous injection of a mixture of U46619 and epinephrine (E, n = 14 mice per genotype) and PAR4-AP and epinephrine (F, n = 12 mice per genotype). Mann-Whitney U test in panels A-B,D; 2-tailed Student t test in panel C; log-rank (Mantel-Cox) test in panels E-F. Data are shown as mean ± SEM for panels A,C and median ± interquartile range (IQR) for panels B,D. ∗P < .05, ∗∗P < .01, and ∗∗∗P < .001.
We also examined the role of the GPIbα cytoplasmic tail in an agonist/epinephrine-induced pulmonary thromboembolism assay (a thrombosis model in which platelet activation is the most prominent feature).31 Consistent with ex vivo data, 10aa−/− mice were protected from death due to U46619/epinephrine- and PAR4-AP/epinephrine-induced pulmonary thromboembolism (Figure 7E-F).
Next, the compensatory effect of low-dose MPαC for the GPIbα C-terminal 10-residue deletion was tested in in vivo models. The delay of FeCl3-induced arterial occlusion by 10aa−/− platelets was restored by MPαC (supplemental Figure 8A). In addition, the administration of low-dose MPαC to 10aa−/− mice decreased the tail bleeding time and the survival from PAR4-AP/epinephrine–induced pulmonary thromboembolism to the levels of WT mice (supplemental Figure 8B-C). These results further validated the role of the GPIbα cytoplasmic tail in hemostasis and thrombosis.
Taken together, deletion of the GPIbα cytoplasmic tail impairs thrombosis in vivo.
Discussion
In this study, our data demonstrate that the GPIbα cytoplasmic tail regulates general platelet activation. To the best of our knowledge, we have found, for the first time, that the GPIbα cytoplasmic tail promotes the general platelet activation by sequestering 14-3-3 from PKC to potentiate PKC activity. It also regulates mouse hemostasis, thrombosis, and platelet activation–dependent pulmonary thromboembolism in vivo.
The GPIbα cytoplasmic tail peptide MPαC was designed to dissociate 14-3-3 from GPIbα and has been shown to abolish ristocetin/VWF-induced platelet aggregation by reducing VWF–GPIbα interaction without affecting platelet aggregations induced by other agonists (U46619, ADP, and collagen).11,15,19 MPαC also inhibited GPIbα ligand binding domain–dependent platelet adhesion to lipopolysaccharide-stimulated endothelial cells, alleviating mouse thrombosis and mortality in lipopolysaccharide challenge.19 A recent study showed that MPαC inhibited calcium mobilization and platelet aggregation induced by thrombin (another GPIbα ligand) by inhibiting GPIbα downstream signaling without affecting thrombin–GPIbα interaction.27 Thus, MPαC is a promising GPIbα inhibitor of the VWF–GPIbα signaling axis (or other GPIbα ligand signaling axes) and a useful tool to explore the pathophysiological relevance of GPIbα ligand binding–elicited signaling. However, its role as “overexpression” of the GPIbα cytoplasmic tail has not been investigated. In this study, in order to examine the role of the GPIbα cytoplasmic tail in non-VWF–initiated (or other GPIbα ligand–initiated) platelet activation, MPαC itself was incubated with washed platelets without plasma or any other agonist. Surprisingly, MPαC induced washed platelet aggregation and expression of platelet activation markers. Thus, besides inhibiting GPIbα-dependent function,15,19,27 MPαC also activates platelets. To draw this conclusion carefully, the inhibition experiments in the literature were repeated in this experimental setup. Indeed, MPαC abolished ristocetin-induced platelet aggregation in human PRP. The inhibitory effect of MPαC on thrombin-induced washed platelet aggregation27 was also reproduced in our experimental setup (supplemental Figure 9). Thus, MPαC has dual effects on washed platelets. In the presence of GPIbα ligands, MPαC inhibits GPIbα ligand–induced platelet activation according to the literature27 but, at the same time, activates platelets; in the absence of GPIbα ligands, MPαC only activates platelets. The platelet-activating effect of MPαC suggests the previously unidentified role of the GPIbα cytoplasmic tail.
Notably, unlike in washed platelets, even 100 μM MPαC did not induce platelet aggregation in human PRP. Our results further showed that plasma prevented MPαC-induced platelet aggregation. Thus, it is reasonable to see that MPαC did not enhance U46619-, ADP-, or collagen-induced platelet aggregation in PRP in the previous reports.15,19 In accordance with this, MPαC enhanced ADP-induced platelet aggregation in washed platelets but not in PRP. Albumin, haptoglobin, and lipoproteins in the plasma inhibit agonist-induced washed platelet aggregation32-34 and might have an inhibitory effect against MPαC-induced platelet aggregation. Future studies should be performed to validate the associated mechanism. Although MPαC cannot induce platelet activation in PRP in vitro, the in vivo administration of low-dose MPαC to compensate for the deletion of the GPIbα cytoplasmic tail in 10aa−/− mice rescued their defects in hemostasis and thrombosis, suggesting that the in vivo data of 10aa−/− mice are at least partially irrelevant to the inhibitory effect of the plasma in the in vitro experiments.
Moreover, 10aa−/− mice further demonstrated the role of the GPIbα cytoplasmic tail in promoting platelet activation. The deletion of the GPIbα C-terminal 10 residues not only impaired VWF- but also collagen-, ADP-, U46619-, and PAR4-AP–induced platelet aggregation. ADP-, U46619-, PAR4-AP–, and CRP-induced integrin αIIbβ3 activation, granule secretion, and PS exposure were also reduced in 10aa−/− platelets. Although the interaction with 14-3-3 occurs at the C-terminus of GPIbα, the regulatory effect is not limited to GPIbα but is extended to other receptor-related platelet functions. Therefore, in the absence of the 14-3-3ζ binding domain of GPIbα in 10aa−/− platelets, in vivo and in vitro platelet functions were reduced. These data are supported by the findings that CRP-induced platelet P-selectin exposure and integrin αIIbβ3 activation were reduced in platelets deficient in the last 24 residues of GPIbα.24 Moreover, artery thrombus formation was impaired in GPIbα C-terminal 6-residue–deficient mice.35 Unlike this observation, normal hemostasis was observed in the GPIbα C-terminal 6-residue– and 24-residue–deficient mice,16,24 and CRP-, thrombin-, and ADP-induced platelet aggregations and normal arterial thrombus formation were observed in the 24-residue–deficient mice.24 To our knowledge, no detailed study of platelet activation for the GPIbα C-terminal 6-residue–deficient mice has been reported in the literature.16 Currently, we do not know the reasons for the different phenotypes among the 3 mouse models. Nevertheless, to the best of our knowledge, we, for the first time, demonstrate that the deletion of the GPIbα cytoplasmic tail impairs ADP-, U46619-, PAR4-AP–induced platelet activation and protects pulmonary thromboembolism–induced mouse death.
14-3-3 proteins are active cofactors in regulating cell functions by interacting with their targets.36,37 Platelets express 6 14-3-3 isoforms, including 14-3-3ζ, 14-3-3β, 14-3-3γ, 14-3-3ε, 14-3-3η, and 14-3-3θ.38 All 6 isoforms interact with GPIbα.38 Thus, the GPIbα-related distribution of 14-3-3 should be critical to platelet function. This concept is supported by the observation that the reconstitution of full-length GPIbα in CHO cells impaired cell stress fiber formation by sequestering 14-3-3ζ from the cytoskeleton regulatory protein Rac but not the truncated GPIbα, which lacks the 14-3-3 binding site.39 In this study, we demonstrate, for the first time, that the GPIbα cytoplasmic tail regulates platelet activation by regulating PKC activity through sequestering 14-3-3 from PKC. The exogenous addition of the GPIbα cytoplasmic tail (MPαC) to platelets substantially sequesters 14-3-3 from PKC to induce PKC activation (Figures 2C and 5C) and PKC-dependent platelet activation (Figure 3). Conversely, deletion of the GPIbα cytoplasmic tail allows more 14-3-3 to interact with PKC (Figure 5D), inhibiting basal PKC activity, leaving it less activated by agonist-evoked signaling (Figure 5A-B),28 and reducing platelet activation (Figure 4). The binding site for the GPIbα cytoplasmic tail is located in the 14-3-3ζ helix I region (residues 202-231),40 which is included in the putative C-terminal PKC-inhibitory domain (124-245) of 14-3-3ζ,30,41 suggesting possible competitive interactions of GPIbα and PKC with 14-3-3. However, the precise binding information needs to be further determined. In addition, the 14-3-3ζ–deficient platelets did not present the same phenotype as that of the platelets treated with MPαC, which sequestered 14-3-3ζ in this study.42 Given that all 6 14-3-3 isoforms can bind to the cytoplasmic tail of GPIbα38 and PKC activity could be suppressed by any 14-3-3 isoform,29 14-3-3ζ deficiency in the platelets might be compensated by other 14-3-3 isoforms. In light of this, except for 14-3-3ζ, other 14-3-3 isoforms in platelets may have the same function as that of 14-3-3ζ described in these observations. Because 14-3-3 has numerous target proteins,36 GPIbα and the GPIbα cytoplasmic tail might play more unidentified roles in platelets, megakaryocytes, and even some hematological and solid tumor cells expressing GPIbα,43,44 which warrants further investigation.
In conclusion, our study identifies the GPIbα cytoplasmic tail as a regulator of general platelet activation, helping to understand the mechanism of GPIbα in regulating multiple cell functions and implying the role of GPIbα in more physiological and pathological processes. This finding provides a conceptual advance in understanding the physiological relevance of platelet GPIbα.
Acknowledgments
The authors thank Junling Liu (Shanghai Jiao Tong University, Shanghai, China) for providing botrocetin. The authors thank Jianlin Qiao (Xuzhou Medical University, Xuzhou, China) for providing collagen-related polypeptide. The visual abstract was created with BioRender.com.
This work was supported by grants from the Key Program of the National Natural Science Foundation of China (81130008 [K.D.]), the Key International Cooperation Projects of the National Natural Science Foundation of China (81820108003 [K.D.]), National Natural Science Foundation of China (81770117 [K.D.] and 82070121 [R.Y.]), the Priority Academic Program Development of Jiangsu Higher Education Institutions; Jiangsu Province’s Key Medical Center (ZX201102), Jiangsu Province’s Outstanding Medical Academic Leader Program (K.D.), and Gusu Health Talent (GSWS2022008 [R.Y.]).
Authorship
Contribution: R.Y., Y.X., K.Z., and J.L. designed research and analyzed data; R.Y., Y.X., K.Z., J.L., Y.S., C.H., X.G., M.Y., C.S., L.Y., S.L., B.Y., F.M., and L.C. performed the experiments and analyzed the results; C.R. provided advice and technical and material support; R.Y. and K.D. wrote the manuscript; K.D. conceived the idea, designed, initiated, and supervised the project; and all authors reviewed and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Kesheng Dai, Jiangsu Institute of Hematology, Cyrus Tang Medical Institute, The First Affiliated Hospital of Soochow University, Key Laboratory of Thrombosis and Hemostasis, Ministry of Health, National Clinical Research Center for Hematological Diseases, Suzhou 215006, China; email: kdai@suda.edu.cn.
References
Author notes
R.Y., Y.X., K.Z., and J.L. contributed equally to this study.
The mass spectrometry data have been deposited to the ProteomeXchange Consortium via the iProX partner repository (https://www.iprox.cn) under Project ID IPX0008514000.
For original data, please contact the corresponding author, Kesheng Dai (kdai@suda.edu.cn).
The full-text version of this article contains a data supplement.

![MPαC activates PKC in platelets. (A) Volcano plot showing the distribution of the significance and fold change of identified phosphopeptides between MPαC- and MPαCsc-treated human platelets (n = 3). Red and blue dots indicate the significantly increased (351) and decreased (818) phosphopeptides in MPαC- vs MPαCsc-treated samples (fold change >1.2 or <0.83; P < .05 by 2-tailed Student t test). (B) Kinases predicted to be activated and inhibited. The x-axis represents the score of kinase activity, and the y-axis represents the kinases predicted to be significantly regulated (P < .05). Red represents the activation status, and blue represents the inhibition status. (C) Representative blot of MPαC-induced PKC substrate (pleckstrin) phosphorylation in washed human platelets and quantification of the densitometry in the blots (n = 5 independent experiments). One-way ANOVA followed by Dunnett post hoc test (compared with vehicle control dimethyl sulfoxide [DMSO]) in panel C. Data are shown as mean ± SEM for panel C. ∗P < .05 and ∗∗∗P < .001.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/8/13/10.1182_bloodadvances.2023012308/2/m_blooda_adv-2023-012308-gr2.jpeg?Expires=1765892135&Signature=AVP5tdQLaLdA2GXkFVJnOKkXrlbLD~kUyk0aNb2A~Nq53OmQoBWzUpgaDaZxIbEgot4E46LCXQ8OHB~juiDbJYSA0ROEjyoLn3bExD7qATvRwWzbgqMi40NYO-zMkKpUa060CxaLKC3-OLjOJN-NQhMh2DlJN5i2CEUyDEyF~bX2InqBOf91znIGRdPUdkxT6RK-MKw6ZNJ3BEAAqQquzhb1hELlMj8AB~TmxD2etS7S5TBq7~HrvmMYPK7ysKZr3jaJSihBlDHB1VBbPOXCJboonon5Qr2piZnVdjVfAJS6ufzCVnuvIXPnA-FY4tOHcwlIz~O3I-hXVVB73gr59w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)