Key Points
HLA reduction of primary human T cells can be achieved in a single step in combination with re-expression of defined antigen receptors.
HLA–reduced T cells maintain canonical HLA class I expression and escape NK-cell–mediated recognition in addition to T cell alloreactivity.
Visual Abstract
Adoptive cellular therapies have shown enormous potential but are complicated by personalization. Because of HLA mismatch, rejection of transferred T cells frequently occurs, compromising the T-cell graft's functionality. This obstacle has led to the development of HLA knock-out (KO) T cells as universal donor cells. Whether such editing directly affects T-cell functionality remains poorly understood. In addition, HLA KO T cells are susceptible to missing self-recognition through natural killer (NK) cells and lack of canonical HLA class I expression may represent a safety hazard. Engineering of noncanonical HLA molecules could counteract NK-cell recognition, but further complicates the generation of cell products. Here, we show that HLA KO does not alter T-cell functionality in vitro and in vivo. Although HLA KO abrogates allogeneic T-cell responses, it elicits NK-cell recognition. To circumvent this problem, we demonstrate that selective editing of individual HLA class I molecules in primary human T cells is possible. Such HLA reduction not only inhibits T-cell alloreactivity and NK-cell recognition simultaneously, but also preserves the T-cell graft's canonical HLA class I expression. In the presence of allogeneic T cells and NK cells, T cells with remaining expression of a single, matched HLA class I allele show improved functionality in vivo in comparison with conventional allogeneic T cells. Since reduction to only a few, most frequent HLA haplotypes would already be compatible with large shares of patient populations, this approach significantly extends the toolbox to generate broadly applicable cellular products.
Introduction
The adoptive transfer of T cells is a powerful treatment option for cancer, infections and autoimmune diseases.1 However, polymorphic HLA of donor T cells and recipient need to be matched to prevent rejection of transferred T cells.2-4
Generating autologous T-cell products or donor registries is time-, labor- and cost-intensive. Therefore, several approaches have been proposed to generate “universal” or at least broadly applicable allogeneic donor cells.5 This encompassed knocking out HLA class I and II through targeting of β2 microglobulin (β2M) and the transcription factor CIITA, respectively.6-8
Preliminary evidence suggests that knock-out (KO) of HLA class I has no consequence on T-cell functionality,6,8 although generally this question remains elusive,9 which is surprising given the fact that HLA class II upregulation is a marker of human T-cell activation.10
While KO of HLA prevents rejection through allogeneic T cells, NK cells can recognize such cells through “missing self.”11,12 Expression of HLA-E has been shown to counteract this problem.8 Because HLA-E would only inhibit NKG2A+ NK cells, overexpression of HLA-G has been proposed as an advantageous engineering approach13 to target KIR2DL1-4+ and ILT2+ NK cells.5 However, such additional editing steps complicate the generation process of cellular products that are intended for clinical application. An alternative strategy is, therefore, to reduce HLA diversity of transferred T cells to a minimum set of HLA alleles that match the host recipient. Such editing preserves cellular physiology. Importantly, some canonical HLA expression is, thereby, left intact, serving as a safeguard in case of viral infection or tumorigenesis.
Individual editing of HLA-A has previously been shown to be feasible.6 With the advent of CRISPR/Cas9, subsequent studies demonstrated that reduction of HLA diversity is possible through targeting individual HLA alleles in human pluripotent stem cells (iPSCs).13,14 However, editing on the level of iPSCs still requires differentiation into mature T cells for application in adoptive cell therapy. Furthermore, because of low editing efficacies and lack of surface expression for example of HLA class I in iPSCs,14 tedious selection of clones appears necessary before cellular products are ready to use. It is therefore unclear whether HLA reduction is feasible within a single editing step in primary human T cells.
Methods
T cells from peripheral blood mononuclear cells (PBMCs) and cell culture
T cells were cultured in RPMI 1640 (Gibco) supplemented with 10 % FCS, 0.025 % L-glutamine, 0.1 % HEPES, 0.001 % gentamycin and 0.002 % streptomycin (hereafter full medium) at 37 °C and 5 % CO2 unless indicated otherwise. For resting of cells, medium was supplemented with 50 IU/mL interleukin 2 (IL-2). For feeder-free expansion of cells, medium was supplemented with 180 IU/mL IL-2.
Cell culture of NK cells
For short-term culture of NK cells, cells were cultured in full medium supplemented with 100 IU/mL IL-2 at a density of 1 × 106/mL at 37 °C and 5 % CO2.
Feeder cell culture
For feeder cell–based rapid expansion, allogeneic PBMCs were mitotically inactivated by irradiation with 35 Gy. Cells were washed 2 times with full medium. Cells were adjusted to a target to feeder ratio of 1:5 and a total density of 1 × 106/mL. Medium was supplemented with 180 IU/mL IL-2 and 1 μg/mL phytohaemagglutinin. If the medium turned acidic, fresh full medium containing 50 IU/mL IL-2 was added. The feeder cell culture was renewed weekly with fresh irradiated allogeneic feeder cells.
CRISPR/Cas9 mediated KO and knock-in
Frozen PBMCs were thawed and rested overnight in full medium + 50 IU/mL IL-2. Cells were activated 2 days before electroporation using 4.8 μg aCD3/aCD28 Expamer (Juno Therapeutics), 300 IU/mL IL-2, 50 IU/mL IL-7 and 50 IU/mL IL-15 for 1 × 106 cells. Stimulus was removed by incubating in 1 mM D-Biotin (Sigma Aldrich) for 20 minutes at room temperature. Cells were electroporated (pulse code EH-110) with Cas9 RNP and 1 μg DNA template in P3 electroporation buffer (20 μL per 1 × 106 T cells; Lonza) with a 4D Nucleofector X unit (Lonza). After electroporation, cells were cultured in full medium containing 180 IU/mL IL-2.
T-cell receptor replacement for functional assays
For functional assays the endogenous T-cell receptor (TCR) was replaced with either TCR 6-2 (HLA-A∗02:01 restrictive; NLV-CMV-epitope) or with JCAR 21 (aCD19 CAR). Replacement took place via CRISPR/Cas9 mediated KO of TRAC and TRBC and use of homologous directed repair to insert construct into the endogenous TRAC locus.
Antibody staining for flow cytometry
Cells were harvested and washed 2 times in cold fluorescence-activated cell sorter (FACS) buffer (phosphate-buffered saline containing 0.5 % (w/v) bovine serum albumin, pH = 7.45). For antibody staining, cells were resuspended in cold FACS buffer containing antibodies and incubated 20 minutes on ice in the dark. Samples were then washed thrice in cold FACS buffer, filtered through a nylon mesh and analyzed on a flow cytometer. Live/dead discrimination was done by using propidiumiodide (Invitrogen).
Flow-cytometric cell sorting
For sorting, staining was performed as described above under sterile conditions. Cells were sorted in 1 mL of sterile FCS. Finally, cells were pelleted and resuspended in full medium with or without feeder cells depending on cell numbers and the following experiment.
Intracellular cytokine staining
K562 cells bearing the correct HLA were irradiated (80 Gy) and pulsed with NLV-peptide pp65495-503 (10−12 M, 10−10 M, 10−9 M, 10−8 M, 10−7 M, 10−6 M, 10−4 M) overnight at 37 °C. T cells were then coincubated with peptide pulsed K562 cells and 2 μL/mL GolgiPlug (BD Biosciences) in a 1:1 ratio for 4 hours at 37 °C. Positive control was stimulated with phorbol myristate acetate (25 ng/mL) and Ionomycin (1 μg/mL). Surface staining for CD8 (FITC, Beckman Coulter), HLA-ABC (APC, BioLegend), HLA-DR (PB, BioLegend) and mTRBC (APCFire, BioLegend) was followed by intracellular staining after permeabilization using Cytofix/Cytoperm Kit (BD Biosciences) with interferon gamma (FITC, BD Pharmingen), tumor necrosis factor α (PE-Cyanine7, eBioscience). Live/dead discrimination was done by using ethidium monoazide bromide (Invitrogen).
NK-cell assay
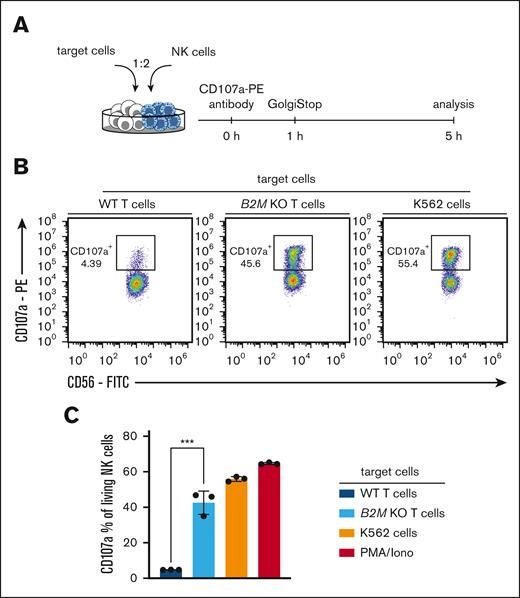
One day before the analysis, PBMCs were isolated from fresh blood and sorted for CD3− CD8− CD56+ cells. The next day, NK cells were coincubated with T cells in a 1:2 ratio for 5 hours in the presence of CD107a PE (BioLegend) antibody. After 1 hour, 6 μg/mL GolgiStop (BD Biosciences) was added. Cells were then washed and additional antibody staining for flow cytometry was performed.
xCELLigence killing assay
HepG2 cells were loaded with NLV-peptide pp65495-503 (10−6 M) for 2 hours at room temperature. 8 × 104 peptide pulsed HepG2 cells were seeded per well onto an E-Plate (OLS) and placed in an xCELLigence RTCA System (ACEA Bio). T cells were added when curve hits saturation no less than 24 hours later. As positive control, 100 μL of full medium containing 2 % Triton-X was added; and HepG2 cells cultured alone served as negative control. xCELLigence RTCA Software Pro (ACEA) and Prism 9 (GraphPad) were used to analyze the data.
Mixed lymphocytes reaction assay
Fresh blood was collected from 2 donors. PBMCs were isolated and half of the cells were mitotically inactivated through irradiation (35 Gy). Cells from the donor used for target-cell generation were then cocultured with irradiated cells from the same donor (auto priming) or from the second donor (allo priming) for 7 days with 10 IU/mL IL-2 at 37°C. After priming of the effector cells, cells were labeled with the eBioscience Cell Proliferation Dye eFluorTM 450 Kit (Thermo Fisher) according to manufacturer’s protocol. Target cells were labeled with CFSE Cell Division Tracker Kit (Biolegend) according to manufacturer’s protocol. Target and effector cells were then cocultured for 48 hours. Cells were harvested, washed, and additional antibody staining for flow cytometry was performed.
In vivo transfer in syngeneic infection mouse model
Before T-cell transfer NSG-HLA-A2/HHD mice (Jackson Laboratories) were irradiated with 2 Gy to create a niche for the transferred cells. CD8+ TCR-transgenic T cells with and without B2M KO were then injected intraperitoneally. The endogenous TCR was orthotopically replaced with a CMV-specific TCR (TCR 6-2). The next day, mice were infected intraperitoneally with 5 × 103 PFU mCMV-NLV (virus provided by Luka Cicin-Sain). On day 7 after infection, the mice were euthanized and the liver was processed for flow cytometry analysis. Lymphocytes were isolated with Percoll (GE Healthcare) and red blood cell lysis was performed with ACT buffer (10% v/v 0.17 M Tris-HCl pH = 7.5, 90% v/v 0.17 M NH4Cl). Cells were stained with hCD8 PE (Invitrogen), mTRBC APCFire780 (BioLegend), β2m APC (BioLegend) and hTCR FITC (BioLegend) antibodies.
In vivo transfer in humanized mouse model
Female 4-week-old NSG-SGM3 mice (Jackson Laboratories) were humanized with human CD34+ cells following irradiation with 1 Gy. Human immune system reconstitution took place over 12 weeks. Blood was analyzed via flow cytometry to identify different immune-cell populations. PBMCs used as effector cells all received an aCD19-CAR (JCAR 21) knock-in into the endogenous TRAC gene locus and were (in the case of HLA reduction) simultaneously edited for all HLA class I alleles except for HLA-A∗02:01. Finally, the cells were administered directly after electroporation without prior sorting via the intravenous route into recipient mice.
Flow cytometry
Samples were acquired on a Cytoflex (S) flow cytometer (Beckman Coulter). Flow sorting was carried out on a MoFlo Astrios EQ (Beckman Coulter).
Data analysis
All flow cytometry data were analyzed with FlowJo v10 and GraphPad PRISM 9 software. Genomic KO was scored using Synthego’s ICE. xCELLigence RTCA Software Pro (ACEA) was used for analysis of xCELLigence assays.
Written informed consent was obtained from blood donors, and use of the blood samples was approved according to national law by the local institutional review board (Ethikkommission der Medizinischen Fakultät der Technischen Universität München).
Results
Generation of HLA KO primary human T cells with reduced allogeneic recognition
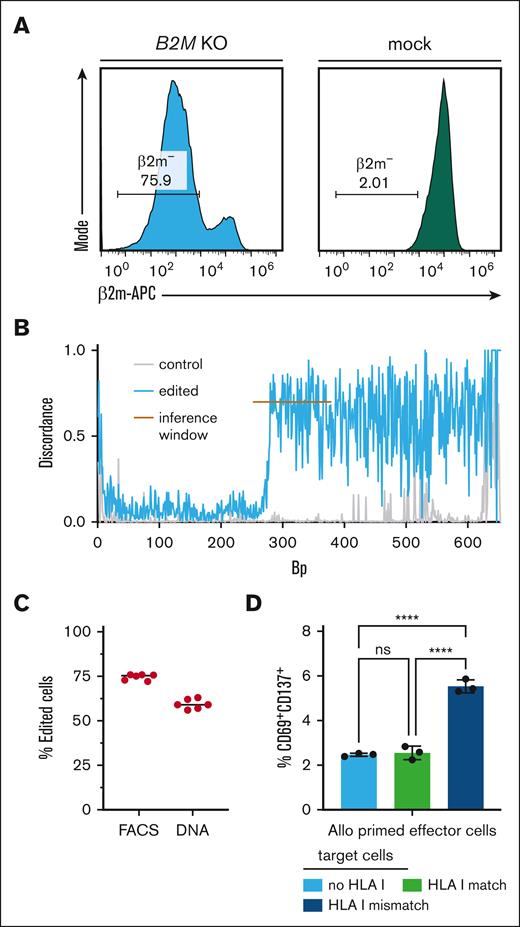
To replicate HLA KO as a strategy to circumvent T-cell alloreactivity, we knocked out the B2M or CIITA gene through specific gRNAs in primary human T cells using electroporation of CRISPR/Cas9 gRNA ribonucleoproteins15 (Figure 1A-C; supplemental Figure 1A-B).
Generation of HLA KO primary human T cells with reduced allogeneic recognition. (A) Flow-cytometric analysis of β2m– T cells (gated on living lymphocytes) from B2M gRNA edited (left) and unedited (right) PBMCs of a healthy donor. (B) Discordance plot as generated by ICE analysis of a β2m-edited sample (blue) in comparison with mock-edited cells (gray, control). Discordance refers to the extent of disagreement between the wild type and edited sample at each base within a defined inference window (orange line). (C) Percentage of successful HLA class I KO as detected by flow cytometry (FACS) and in ICE analysis after sequencing (DNA) (n = 6 technical replicates). (D) Percentage of CD69+CD137+ CD8+ effector cells after 48 hours of coculture with indicated target cells (n = 3 technical replicates). Effector cells were peripheral mononuclear blood cells from donor A, cocultured for 7 days together with PBMCs from donor B (allo priming). Target cells were autologous unedited cells from donor A (HLA I match), allogeneic B2M KO cells (no HLA I), and allogeneic unedited cells (HLA I mismatch) from donor B. Target cells were sorted for CD3+ and successful KO, if applicable. Statistical testing by ordinary two-way ANOVA and Tukey's multiple comparison test, mean with SD; ns, not significant; ∗∗∗∗ p < .0001.
Generation of HLA KO primary human T cells with reduced allogeneic recognition. (A) Flow-cytometric analysis of β2m– T cells (gated on living lymphocytes) from B2M gRNA edited (left) and unedited (right) PBMCs of a healthy donor. (B) Discordance plot as generated by ICE analysis of a β2m-edited sample (blue) in comparison with mock-edited cells (gray, control). Discordance refers to the extent of disagreement between the wild type and edited sample at each base within a defined inference window (orange line). (C) Percentage of successful HLA class I KO as detected by flow cytometry (FACS) and in ICE analysis after sequencing (DNA) (n = 6 technical replicates). (D) Percentage of CD69+CD137+ CD8+ effector cells after 48 hours of coculture with indicated target cells (n = 3 technical replicates). Effector cells were peripheral mononuclear blood cells from donor A, cocultured for 7 days together with PBMCs from donor B (allo priming). Target cells were autologous unedited cells from donor A (HLA I match), allogeneic B2M KO cells (no HLA I), and allogeneic unedited cells (HLA I mismatch) from donor B. Target cells were sorted for CD3+ and successful KO, if applicable. Statistical testing by ordinary two-way ANOVA and Tukey's multiple comparison test, mean with SD; ns, not significant; ∗∗∗∗ p < .0001.
Next, we induced alloreactivity through coincubation of effector T cells from one healthy donor for 7 days with allogeneic target T cells from another healthy donor with complete HLA mismatch, followed by 48 hours of restimulation. This led to preferential activation of HLA mismatched target cells (Figure 1D). Recognition of allogeneic T cells was significantly inhibited when β2M− T cells after B2M KO (supplemental Figure 1C-D) were used as target cells (Figure 1D).
Intrinsic in vitro and in vivo functionality of HLA class I and II–deficient primary human T cells
Given the potential of HLA KO to generate T-cell products that can escape allogeneic T-cell recognition, we next studied whether loss of HLA expression is associated with changes in T-cell functionality.6,8,9 We equipped T cells with a TCR specific for the HLA-A∗02:01-restricted cytomegalovirus (CMV) epitope pp65495-503 via orthotopic TCR replacement.15,16 Simultaneous to TCR knock-in (TCR KI) into the endogenous TCR Alpha Constant (TRAC) gene locus and KO of TCR Beta Constant (TRBC), we performed no additional editing (“TCR KI only”), knocked out endogenous B2M (“B2M KO”) or CIITA (“CIITA KO”), followed by purity sorting on CD8+ hTCR− mTRBC+ cells (supplemental Figure 2). We then coincubated these effector cells with peptide-loaded K562 cells and checked for release of interferon gamma and tumor necrosis factor α (Figure 2A). HLA-edited cells showed no change in their sensitivity of target-cell recognition compared with unedited cells (Figure 2B). We also investigated killing of peptide-loaded HepG2 target cells using a live-cell imaging system. Again, neither CIITA KO (Figure 2C) nor B2M KO (Figure 2D) affected effector function of TCR-transgenic T cells.
Intrinsic in vitro functionality of HLA class I and II–deficient primary human T cells. (A) Representative flow-cytometric intracellular cytokine staining of interferon gamma (IFN-γ) and tumor necrosis factor α (TNFα) in response to no antigen, increasing amounts of antigen or phorbol 12-myristate 13-acetate (PMA) and ionomycin; human PBMCs as effector cells underwent orthotopic TCR replacement with CMV NLV–specific TCR 6-2 (TCR KI) and were simultaneously edited with B2M gRNA (B2M KO) or CIITA gRNA (CIITA KO); K562 cells loaded with NLV-peptide pp65495-503 as antigen were used for stimulation. (B) Quantification of data from panel A (n = 2-3 technical replicates, mean ± standard deviation [SD]), x-axis showing logarithmic molar peptide concentration; neg, negative control. (C) Killing of target cells (HepG2 cells pulsed with NLV-peptide pp65495-503) by HLA class II KO T cells as measured through changes in cell index over time (left panel) in an xCELLigence assay and quantified as the area under the curve (right panel) calculated over the entire time period shown; addition of effector cells indicated by dashed line; positive control of target-cell lysis achieved through addition of detergent Triton-X; negative control (neg. ctrl.) shows uninhibited target-cell growth through absence of effector cells; mock-edited effector cells were used as control to show nonspecific effect on target-cell growth through superseding; CD8+ T cells from panels A and B were used as effector cells and sorted for successful editing (CD8+ hTCR− mTRBC+ β2m−/CIITA−); statistical testing by ordinary 1-way analysis of variance (ANOVA) and Tukey multiple comparisons test), n = 3 technical replicates, mean ± SD; ns, not significant; ∗∗∗∗P < .0001. (D) As in panel C, with HLA class I KO T cells as investigated effector cells.
Intrinsic in vitro functionality of HLA class I and II–deficient primary human T cells. (A) Representative flow-cytometric intracellular cytokine staining of interferon gamma (IFN-γ) and tumor necrosis factor α (TNFα) in response to no antigen, increasing amounts of antigen or phorbol 12-myristate 13-acetate (PMA) and ionomycin; human PBMCs as effector cells underwent orthotopic TCR replacement with CMV NLV–specific TCR 6-2 (TCR KI) and were simultaneously edited with B2M gRNA (B2M KO) or CIITA gRNA (CIITA KO); K562 cells loaded with NLV-peptide pp65495-503 as antigen were used for stimulation. (B) Quantification of data from panel A (n = 2-3 technical replicates, mean ± standard deviation [SD]), x-axis showing logarithmic molar peptide concentration; neg, negative control. (C) Killing of target cells (HepG2 cells pulsed with NLV-peptide pp65495-503) by HLA class II KO T cells as measured through changes in cell index over time (left panel) in an xCELLigence assay and quantified as the area under the curve (right panel) calculated over the entire time period shown; addition of effector cells indicated by dashed line; positive control of target-cell lysis achieved through addition of detergent Triton-X; negative control (neg. ctrl.) shows uninhibited target-cell growth through absence of effector cells; mock-edited effector cells were used as control to show nonspecific effect on target-cell growth through superseding; CD8+ T cells from panels A and B were used as effector cells and sorted for successful editing (CD8+ hTCR− mTRBC+ β2m−/CIITA−); statistical testing by ordinary 1-way analysis of variance (ANOVA) and Tukey multiple comparisons test), n = 3 technical replicates, mean ± SD; ns, not significant; ∗∗∗∗P < .0001. (D) As in panel C, with HLA class I KO T cells as investigated effector cells.
Next, we studied whether HLA editing would affect T-cell functionality in vivo. We adoptively transferred TCR KI cells with or without B2M KO into irradiated NSG/HHD HLA-A∗02:01-transgenic recipient mice,17 which were subsequently infected with human NLV-peptide–expressing murine CMV (mCMV)16 (supplemental Figure 3A). Human T cells could be recovered from livers on day 8 (supplemental Figure 3B). The frequency of β2M− T cells was completely preserved compared with the infusion product (supplemental Figure 3C). This indicates that β2M− T cells are neither positively nor negatively selected in vivo and confirms that HLA class I editing does not change intrinsic T-cell functionality.
NK-cell recognition of HLA class I deficient primary human T cells
NK cells may recognize HLA-negative cells through a “missing self” mechanism.11,12 We therefore coincubated NK cells with unedited T cells or B2M KO T cells and analyzed NK-cell activation (Figure 3A). Sort-purified B2M KO T cells induced NK-cell activation significantly more than WT T cells, almost to the same degree as positive controls did (Figure 3B-C). These data confirm that HLA KO T cells are susceptible to rejection through NK cells.
NK-cell recognition of HLA class I–deficient primary human T cells. (A) Experimental setup for NK-cell recognition assay. (B) Representative flow-cytometric analysis of CD107a+ NK cells (percentage of living CD56+ CD8− lymphocytes) after 5 hours of coincubation with indicated target T cells, K562 cells or PMA/Iono; cells were sorted for successful editing. (C) Quantification of data shown in panel B; n = 3 technical replicates; statistical testing was done using an unpaired 2-tailed t test and results are only shown for comparison of WT and B2M KO T cells; mean ± SD; ∗∗∗P < .001.
NK-cell recognition of HLA class I–deficient primary human T cells. (A) Experimental setup for NK-cell recognition assay. (B) Representative flow-cytometric analysis of CD107a+ NK cells (percentage of living CD56+ CD8− lymphocytes) after 5 hours of coincubation with indicated target T cells, K562 cells or PMA/Iono; cells were sorted for successful editing. (C) Quantification of data shown in panel B; n = 3 technical replicates; statistical testing was done using an unpaired 2-tailed t test and results are only shown for comparison of WT and B2M KO T cells; mean ± SD; ∗∗∗P < .001.
Generation of HLA class I reduced primary human T cells
Based on these findings, we aimed to explore whether a reduction, rather than complete elimination, of the diversity of HLA molecules13,14 can be achieved in primary human T cells in a single editing event.
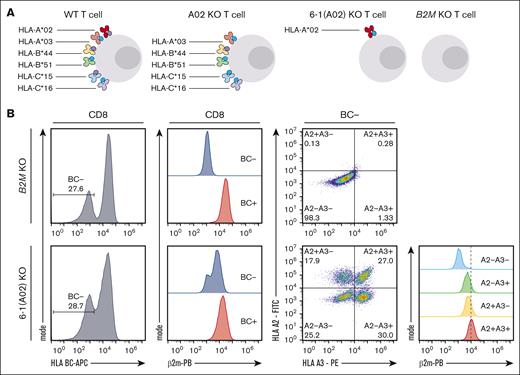
We took T cells of an HLA-A∗02:01+ A∗03:01+ B∗44:03+ B∗51:01+ C∗15:02+ C∗16:01+ donor (Figure 4A) and left these cells unedited, knocked out only 1 individual HLA allele at a time, or individually knocked out all 6 HLA class I alleles, except for 1. The sole preservation of a single HLA allele would allow coverage of 80% of the European White population (African: 78%; Hispanic, South or Central American: 73%; Korean: 84%; Southeast Asian: 80%) if performed for the 10 most frequent individual HLA class I alleles (supplemental Figure 4A).18,19 As additional controls, we knocked out all 6 HLA class I alleles individually in 1 sample or knocked out B2M (supplemental Figure 4B). Flow-cytometric antibody staining can be used to distinguish HLA BC and individual HLA-A∗02 and HLA-A∗03 proteins (supplemental Figure 4B-D). We observed absence of β2m protein only upon B2M KO, but not in any of the other samples in which HLA alleles were edited individually. HLA BC− T cells were generated by B2M KO, but also observed in the 2 samples in which either HLA-A∗02:01 or HLA-A∗03:01-specific gRNAs were left out of the editing cocktail while all gRNAs targeting HLA-B and HLA-C alleles were present. T cells lacking HLA-A∗02:01 or HLA-A∗03:01 were most effectively generated with editing cocktails containing both HLA-A-specific gRNAs, indicating some degree of cross-reactive editing. In fact, T cells lacking HLA-A∗02:01 were more robustly induced by the HLA-A∗03:01–targeting gRNA and vice versa (supplemental Figure 4E-F).
Generation of HLA class I reduced primary human T cells. (A) Experimental scheme showing concept of HLA class I reduction through single HLA allele targeting. (B) Characterization of 6-1 (A2) KO T cells (in which all HLA class I molecules were targeted except for HLA A2) compared with B2M KO T cells; left: HLA BC− population pregated on living CD8+ T cells, second from left: β2m expression of HLA BC− and HLA BC+ populations pregated on living CD8+ T cells; second from right: HLA A2 and HLA A3 expression pregated on HLA BC− CD8+ living T cells; right: β2m expression of HLA BC− subsets as defined in dot plots second from right.
Generation of HLA class I reduced primary human T cells. (A) Experimental scheme showing concept of HLA class I reduction through single HLA allele targeting. (B) Characterization of 6-1 (A2) KO T cells (in which all HLA class I molecules were targeted except for HLA A2) compared with B2M KO T cells; left: HLA BC− population pregated on living CD8+ T cells, second from left: β2m expression of HLA BC− and HLA BC+ populations pregated on living CD8+ T cells; second from right: HLA A2 and HLA A3 expression pregated on HLA BC− CD8+ living T cells; right: β2m expression of HLA BC− subsets as defined in dot plots second from right.
Still, editing with individual gRNA cocktails yielded cell products with very specific HLA-allele expression profiles. The number of expressed individual HLA alleles was thereby associated with distinct β2m protein expression levels (Figure 4B). The frequencies of desired cell products containing HLA-A∗02 or -A∗03 only, or containing no HLA class Ia at all, were in the range of 5 % to 10 % (supplemental Figure 4G).
Overall, these data indicate that targeting single HLA alleles with individual gRNAs can generate HLA–reduced T cells.
Escape of HLA–reduced primary human T cells from allogeneic recognition in vitro
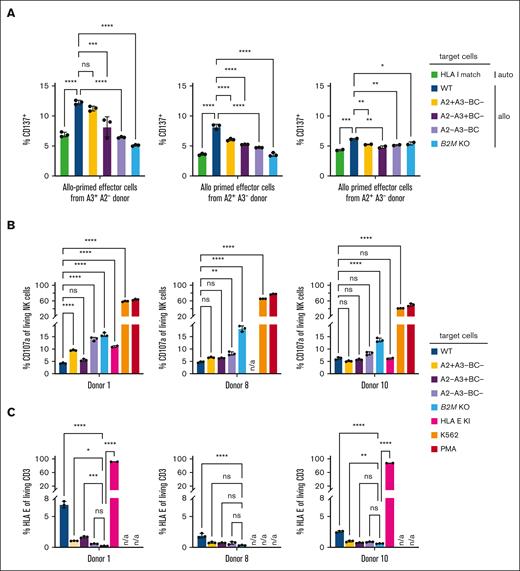
HLA–reduced T cells should not elicit alloreactive T-cell and NK-cell–mediated recognition. To test this, we repeated the mixed lymphocytes reaction assay with sort-purified, HLA–reduced target T cells (expressing HLA-A∗02:01 or HLA-A∗03:01) from donor A, who had a complete HLA mismatch with the 3 donors providing effector T cells, except for HLA-A∗02:01 or HLA-A∗03:01 respectively (Figure 5A). As controls, we also used HLA–mismatched target T cells that were not edited, HLA–matched autologous target cells, as well as sort-purified cells lacking HLA BC and HLA-A∗02:01 or HLA-A∗03:01 or both. The HLA-A2− HLA-A3+ donor shows alloreactivity toward HLA-reduced cells that are HLA-A2+ HLA-A3−. Both HLA-A2+ HLA-A3− donors, on the other hand, do not show such alloreactivity toward HLA-reduced cells that are HLA-A2+ HLA-A3−. The latter donors also do not show alloreactivity toward HLA-reduced cells that are HLA-A2− HLA-A3+, but alloreactivity does not necessarily have to be consistently present against all individual HLA molecules in all donors (Figure 5A). Overall, HLA–reduced T cells seem to be largely protected from allogeneic T-cell recognition.
Escape of HLA–reduced primary human T cells from allogeneic recognition in vitro. (A) Percentage of CD137+ CD8+ effector cells after 48 hours of coculture with indicated target cells. Effector cells were peripheral mononuclear blood cells from indicated donors, cocultured for 7 days together with PBMCs from donor A (allo priming); donors only share an allele for HLA A2 or HLA A3 as indicated; target cells were T cells from donor A without (WT) or with HLA class I reduction, sorted for CD8+ and HLA BC− and A2− A3−, A2+ A3− or A2− A3+; HLA–reduced target cells with HLA BC−A2−A3+ (left) or HLA BC−A2+A3− phenotype, respectively, signify a synthesized HLA-match; statistical testing was done using an ordinary 1-way ANOVA and Tukey multiple comparisons test, and results are only shown for selected comparisons; n = 2-3 technical replicates; ns, not significant; ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. (B) Quantification of CD107a+ NK cells (percentage of living CD56+ CD8− lymphocytes) from 3 different indicated donors after 5 hours of coincubation with indicated target cells; statistical testing was done using an ordinary 2-way ANOVA and Tukey multiple comparisons test and results are only shown for selected comparisons; n = 2-3 technical replicates; ns, not significant; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. (C) Expression of HLA-E for target populations as assessed by flow cytometry; statistical testing was done using an ordinary 2-way ANOVA and Tukey multiple comparisons test and results are only shown for selected comparisons; n = 2-3 technical replicates; ns, not significant; ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. n/a, not performed experimental conditions.
Escape of HLA–reduced primary human T cells from allogeneic recognition in vitro. (A) Percentage of CD137+ CD8+ effector cells after 48 hours of coculture with indicated target cells. Effector cells were peripheral mononuclear blood cells from indicated donors, cocultured for 7 days together with PBMCs from donor A (allo priming); donors only share an allele for HLA A2 or HLA A3 as indicated; target cells were T cells from donor A without (WT) or with HLA class I reduction, sorted for CD8+ and HLA BC− and A2− A3−, A2+ A3− or A2− A3+; HLA–reduced target cells with HLA BC−A2−A3+ (left) or HLA BC−A2+A3− phenotype, respectively, signify a synthesized HLA-match; statistical testing was done using an ordinary 1-way ANOVA and Tukey multiple comparisons test, and results are only shown for selected comparisons; n = 2-3 technical replicates; ns, not significant; ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. (B) Quantification of CD107a+ NK cells (percentage of living CD56+ CD8− lymphocytes) from 3 different indicated donors after 5 hours of coincubation with indicated target cells; statistical testing was done using an ordinary 2-way ANOVA and Tukey multiple comparisons test and results are only shown for selected comparisons; n = 2-3 technical replicates; ns, not significant; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. (C) Expression of HLA-E for target populations as assessed by flow cytometry; statistical testing was done using an ordinary 2-way ANOVA and Tukey multiple comparisons test and results are only shown for selected comparisons; n = 2-3 technical replicates; ns, not significant; ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. n/a, not performed experimental conditions.
Importantly, HLA–reduced T cells also did not activate NK cells (Figure 5B). Next to T cells expressing only HLA-A∗02:01 or HLA-A∗03:01, cells lacking HLA-A∗02:01, HLA-A∗03:01 and HLA BC did not stimulate NK cells, protecting from missing-self recognition to a similar degree as HLA-E knock-in T cells did. We hypothesize that protection of HLA-reduced cells from NK-cell recognition could be due to preservation of noncanonical HLA expression. Indeed, cells lacking HLA-A∗02:01, HLA-A∗03:01 and HLA BC could be stained for HLA-E (Figure 5C), which is in line with preserved β2m protein expression upon 6xKO of canonical HLA class I alleles (supplemental Figure 4B-C). In summary, HLA–reduced T cells escape both alloreactive T-cell and NK-cell–mediated recognition in vitro.
Improved functionality of HLA–reduced primary human T cells in the presence of HLA–mismatched T and NK cells in vivo
Finally, we aimed to investigate the functionality of HLA–reduced primary human T cells in vivo. Studying allogeneic T-cell and NK-cell–mediated recognition of human T cells in vivo is challenging. Usually, for in vivo testing of allogeneic rejection of human T cells, the individual cell products (T cells from 1 donor, HLA mismatched T cells from another donor, as well as NK cells) are actively injected into mice, often preprimed before ex vivo, and then only monitored over short periods of time due to poor maintenance of transferred T cells. For this reason, we humanized mice after transplantation with CD34 (hCD34)+ hematopoietic stem and progenitor cells that build up a human endogenous immune-cell repertoire encompassing B, T and NK cells (supplemental Figure 5). In addition to this, recipient mice had an NSG-SGM3 background, which allows more rapid and more complete reconstitution of human immune-cell lineages compared with conventional NSG mice.20
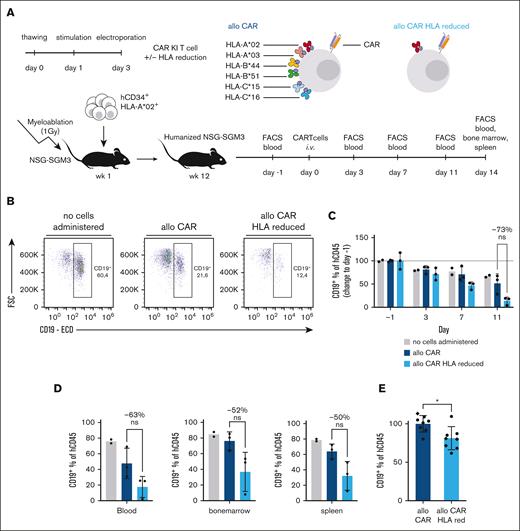
To probe the functionality of HLA–reduced primary human T cells, we reduced HLA diversity so that HLA-A∗02:01 would be the sole matching HLA allele compared with the donor that was used for recipient mouse humanization (Figure 6A). Simultaneously, we knocked-in an anti-CD19 CAR into the endogenous TRAC locus. In contrast to our previous in vivo analysis, we here chose an anti-CD19 CAR as an antigen-specific receptor since the CAR would recognize the reconstituted human B cells in a continuous manner, and thereby renders the transgenic T cells a good target of allogeneic recognition through reconstituted T cells and NK cells for a period of at least 1 to 2 weeks.
Improved functionality of HLA–reduced primary human T cells in the presence of HLA mismatched T and NK cells in vivo. (A) Experimental setup for in vivo transfer of HLA–reduced aCD19-CAR T cells; effector cells from HLA-A∗02+ donor A (same donor as in Figures 4 and 5) were administered IV 1 day after assessment of humanization of hu-CD34 NSG-SGM3 mice; humanization was performed with cord blood derived hCD34+ cells from donor C, which were different from cells of donor A for every HLA class I allele except for HLA-A∗02; every 3 days, blood samples were drawn and analyzed via flow cytometry; CAR T cells used as effector cells all received an aCD19-CAR knock-in into the endogenous TRAC gene locus and were simultaneously edited for all HLA class I alleles except for HLA∗02, and were administered directly after electroporation without prior sorting. (B) CD19+ B cells (percentage of living hCD45+ lymphocytes) on day 11 after administration of no cells or aCD19 CAR T cells with unedited or reduced HLA alleles into humanized mice. (C) CD19+ B cells shown as change in percentage relative to 1 day prior to administration of no cells or aCD19 CAR T cells with unedited or reduced HLA alleles into humanized mice; statistical testing by ordinary 2-way ANOVA and Tukey multiple comparisons test; n = 2-3 mice. (C) CD19+ B cells (percentage of hCD45+ living lymphocytes) recovered in indicated organs on day 14 after aCD19 CAR T-cell administration with unedited or reduced HLA alleles into humanized mice; statistical testing was done using an unpaired t test; n = 2-3 mice. (E) Numbers of CD19+ B cells recovered in blood of humanized mice on day 7 after aCD19 CAR T-cell administration with unedited or reduced HLA alleles, pooled data from 2 independent experiments (dot or diamond symbol barcode), normalized to mean of allogeneic CAR T cells in both experiments; statistical testing was done using an unpaired t test; n = 8 mice; ∗P < .05. Bar height indicates mean, error bars indicate SD for panels C-E. ns, not significant.
Improved functionality of HLA–reduced primary human T cells in the presence of HLA mismatched T and NK cells in vivo. (A) Experimental setup for in vivo transfer of HLA–reduced aCD19-CAR T cells; effector cells from HLA-A∗02+ donor A (same donor as in Figures 4 and 5) were administered IV 1 day after assessment of humanization of hu-CD34 NSG-SGM3 mice; humanization was performed with cord blood derived hCD34+ cells from donor C, which were different from cells of donor A for every HLA class I allele except for HLA-A∗02; every 3 days, blood samples were drawn and analyzed via flow cytometry; CAR T cells used as effector cells all received an aCD19-CAR knock-in into the endogenous TRAC gene locus and were simultaneously edited for all HLA class I alleles except for HLA∗02, and were administered directly after electroporation without prior sorting. (B) CD19+ B cells (percentage of living hCD45+ lymphocytes) on day 11 after administration of no cells or aCD19 CAR T cells with unedited or reduced HLA alleles into humanized mice. (C) CD19+ B cells shown as change in percentage relative to 1 day prior to administration of no cells or aCD19 CAR T cells with unedited or reduced HLA alleles into humanized mice; statistical testing by ordinary 2-way ANOVA and Tukey multiple comparisons test; n = 2-3 mice. (C) CD19+ B cells (percentage of hCD45+ living lymphocytes) recovered in indicated organs on day 14 after aCD19 CAR T-cell administration with unedited or reduced HLA alleles into humanized mice; statistical testing was done using an unpaired t test; n = 2-3 mice. (E) Numbers of CD19+ B cells recovered in blood of humanized mice on day 7 after aCD19 CAR T-cell administration with unedited or reduced HLA alleles, pooled data from 2 independent experiments (dot or diamond symbol barcode), normalized to mean of allogeneic CAR T cells in both experiments; statistical testing was done using an unpaired t test; n = 8 mice; ∗P < .05. Bar height indicates mean, error bars indicate SD for panels C-E. ns, not significant.
To maximize engraftment of allogeneic HLA-reduced donor T cells, we directly transferred them after editing without further in vitro culture or purity sorting. As a control, we transferred either allogeneic CAR KI T cells without HLA reduction or no cells. We observed a mild decrease in CD19+ B cells when allogeneic CAR T cells were infused (Figure 6B-C). This elimination of B cells was, however, enhanced when allogeneic CAR T cells were HLA-reduced. HLA–reduced allogeneic CAR T cells consistently led to more effective B-cell elimination also in the bone marrow and spleen at the end point analysis (Figure 6D) as well as in a second independent experiment (Figure 6E).
In summary, HLA–reduced allogeneic CAR T cells showed improved functionality in the presence of HLA–mismatched T and NK cells in a humanized in vivo model of chronic antigen exposure.
Discussion
Just as B2M KO cells, HLA–reduced T cells circumvent allogeneic T-cell recognition in case of HLA mismatches. However, in contrast to B2M KO cells, HLA-reduced T cells do not elicit NK-cell-mediated recognition through “missing self.” While transgenic expression of HLA-E8 or HLA-G13 has been proposed to counteract NK-cell reactivity, HLA reduction entails the unique advantage of preserving cellular physiology.
We here show proof-of-concept for HLA reduction to a single, matching HLA molecule, representing the most extreme case for which a match in canonical HLA class I molecules can still be reached through engineering. To demonstrate how much this improves the likelihood of finding suitable cell donors, we calculated that HLA reduction to the 10 most prevalent HLA class I molecules would already cover about 80% of the European Caucasian population (78%-84% of other populations) (supplemental Figure 4A).
It is also a possibility to leave more HLA molecules intact. Although more donors are then needed to cover substantial parts of a population, the probability of finding a suitable donor is still much enhanced. For example, calculations with donors that have naturally occurring HLA class I homozygocities for HLA-A, HLA-B and HLA-C (ie, donors that are homozygous on HLA-A, HLA-B as well as HLA-C) from German blood donor registries showed that 20 donors with the most frequent homozygous HLA class I haplotypes could already cover about two-thirds of the German population (supplemental Figure 6).
Importantly, HLA reduction is generally applicable to all HLA alleles and should also be implemented for common HLA alleles in diverse ethnic groups. The lack of monoclonal antibodies for additional individual HLA molecules may pose a problem to making HLA reduction a broadly applicable strategy. However, it is also conceivable to perform HLA reduction and then adoptive cell transfers without further selection on defined HLA-reduced populations. In this case, cells that show residual HLA mismatches should be rejected in vivo. The possibility to omit sorting steps also facilitates the practical implementation of such cellular therapies.
Eliminating all canonical HLA alleles is also a possible strategy for generating broadly applicable cells, thereby preserving only noncanonical HLA expression. We here show that T cells that are negative for HLA-A2 HLA-A3 and HLA-BC prevent both NK cell as well as allogeneic T-cell activation just as HLA-reduced T cells do. Fittingly, T cells that are negative for HLA-A2 HLA-A3 and HLA-BC sustain HLA-E expression. However, as reported before by others, HLA-ABC− T cells do have lower HLA-E expression levels than unedited cells.13,14 This can be explained by HLA-E’s function to present signal peptides from canonical and noncanonical HLA class I molecules.21 Consistent with this, we observe a correlation between HLA-E and canonical HLA class I expression levels also in unedited cells and see that HLA-A2+ HLA-A3− HLA-BC− T cells have higher HLA-E expression levels than HLA-ABC− T cells (Figure 5C). Robust inhibition of NK-cell recognition therefore appears more likely when at least 1 canonical HLA molecule is preserved. In line with this, others have seen that HLA-ABC− iPSC-derived blood cells did not elicit NK-cell reactivity when HLA-C7 was still expressed.14 In any case, preserving at least 1 canonical HLA class I molecule represents an important safeguard in case engineered T cells are infected or undergo malignant transformation.
The T cells that we HLA-reduced to a single remaining HLA allele were edited in 7 different genetic loci (5 HLA class I alleles and 2 TCR targets; including knock-in into 1 TCR locus). Although this underlines the feasibility of highly multiplexed engineering through CRISPR/Cas9, it may also raise concerns of an increased risk of chromosomal loss and translocations.22,23 Such concerns are somewhat mitigated by a favorable safety profile of T cells with multiplexed CRISPR/Cas9 editing in clinical trials24 and long-term preclinical in vivo analyses.25 Furthermore, it has recently been shown that Cas12a nuclease-assisted knock-in can be combined with Cas9-derived base editing for generation of double strand breaks to generate T cells with multiplexed editing without elevated translocation frequencies.26
Previous studies that investigated the functionality of universal or broadly applicable human donor T cells in vivo actively supplemented immunodeficient mice with target cells, previously primed T cells (to mimic the endogenous recipient repertoire) and monitored survival of donor T cells no longer than 8 days.8,13,14,27 Furthermore, human NK cells were usually not present. In our study, we aimed to perform in vivo studies in a setting with endogenous human immune-cell repertoires (encompassing both T and NK cells) that developed as naturally as possible. In addition, we wanted to monitor human donor T-cell maintenance for as long as possible. Finally, we aimed to study donor T-cell function against endogenous targets that also did not have to be supplied exogenously. Therefore, on the one hand, we humanized mice with CD34+ hematopoietic stem and progenitor cells. This led to the development of B-, T- and NK-cell populations, providing a system with endogenous target and effector cells to study the maintenance of later applied donor T cells. On the other hand, we used NSG-SGM3 mice as a background. In these mice, the presence of IL-3, GM-CSF SCF enables a more complete humanization and thereby enhanced maintenance of human immune cells.20 Using this system, we observed enhanced functionality (ie, elimination of endogenous B cells) with HLA-reduced compared with HLA nonreduced allogeneic donor T cells in the presence of allogeneic endogenous T and NK cells. In the future, it will be relevant to study whether this enhanced functionality is due to enhanced T-cell maintenance, and whether such improved maintenance is linked to escape from T- or NK-cell–mediated rejection. As of now, our data indicate superior functionality of HLA–reduced allogeneic T cells in a preclinical in vivo system which mimics the actual in vivo situation in patients as closely as anyhow possible.
In summary, we here provide proof-of-concept that HLA reduction is a feasible strategy to circumvent both allogeneic T-cell as well as NK-cell–mediated rejection, while simultaneously preserving T-cell physiology and canonical HLA class I expression. HLA reduction thereby extends the toolbox of cellular engineering for therapy of tumor diseases, infections, and autoimmunity.
Acknowledgments
The authors thank members of the Busch and Schober laboratory for experimental help and critical discussion.
This work was mainly supported by the German Federal Ministry of Education and Research (BMBF, project 01KI2013) and by Else Kröner-Fresenius-Stiftung (Promotionskolleg Translationale Medizin, Technical University of Munich). Further support was received by D.H.B. from the German Center for Infection Research and the Deutsche Forschungsgemeinschaft (SFB-TRR 338/1 2021 -452881907 (project A01); SFB 1054/B09 and SFB 1371/TP04), and by K.S. from the Else Kröner-Fresenius-Stiftung (project 2020_EKEA.127).
The funders had no influence on the study design and data interpretation.
Authorship
Contribution: D.H.B. and K.S. conceptualized the study; P.M.W., D.H.B., and K.S. developed methodology; P.M.W. and K.S. conducted formal analysis of the data; P.M.W., L.W., and P.H. performed experiments; J.M.S., S.D., T.T., L.C.-S., and D.H.B. contributed resources; P.M.W., D.H.B. and K.S. wrote the manuscript; all authors read and approved the manuscript; D.H.B. and K.S. acquired the funding; D.H.B. and K.S. supervised the study and provided project administration.
Conflict-of-interest disclosure: D.H.B. is cofounder of STAGE Cell Therapeutics GmbH (now Juno Therapeutics/Celgene) and T Cell Factory B.V. (now Kite/Gilead). D.H.B. has a consulting contract with and receives sponsored research support from Juno Therapeutics/Celgene. P.M.W., D.H.B., and K.S. are currently filing patents related to this work. The remaining authors declare no competing financial interests.
Correspondence: Kilian Schober, Microbiological Institute – Institute of Clinical Microbiology, Immunology and Hygiene, University Hospital of Erlangen, Wasserturmstr. 3-5, Erlangen 91054, Germany; email: kilian.schober@uk-erlangen.de; and Dirk H. Busch, Institute for Medical Microbiology, Immunology and Hygiene, Technische Universität München, Munich, Germany; email: dirk.busch@tum.de.
References
Author notes
P.M.W. and L.W. contributed equally to the study.
D.H.B. and K.S. jointly supervised the study.
All data generated or analyzed during this study are included in this article, its supplementary information files, and/or are available upon reasonable request from the corresponding authors, Dirk H. Busch (dirk.busch@tum.de) and Kilian Schober (kilian.schober@uk-erlangen.de)
The full-text version of this article contains a data supplement.

![Intrinsic in vitro functionality of HLA class I and II–deficient primary human T cells. (A) Representative flow-cytometric intracellular cytokine staining of interferon gamma (IFN-γ) and tumor necrosis factor α (TNFα) in response to no antigen, increasing amounts of antigen or phorbol 12-myristate 13-acetate (PMA) and ionomycin; human PBMCs as effector cells underwent orthotopic TCR replacement with CMV NLV–specific TCR 6-2 (TCR KI) and were simultaneously edited with B2M gRNA (B2M KO) or CIITA gRNA (CIITA KO); K562 cells loaded with NLV-peptide pp65495-503 as antigen were used for stimulation. (B) Quantification of data from panel A (n = 2-3 technical replicates, mean ± standard deviation [SD]), x-axis showing logarithmic molar peptide concentration; neg, negative control. (C) Killing of target cells (HepG2 cells pulsed with NLV-peptide pp65495-503) by HLA class II KO T cells as measured through changes in cell index over time (left panel) in an xCELLigence assay and quantified as the area under the curve (right panel) calculated over the entire time period shown; addition of effector cells indicated by dashed line; positive control of target-cell lysis achieved through addition of detergent Triton-X; negative control (neg. ctrl.) shows uninhibited target-cell growth through absence of effector cells; mock-edited effector cells were used as control to show nonspecific effect on target-cell growth through superseding; CD8+ T cells from panels A and B were used as effector cells and sorted for successful editing (CD8+ hTCR− mTRBC+ β2m−/CIITA−); statistical testing by ordinary 1-way analysis of variance (ANOVA) and Tukey multiple comparisons test), n = 3 technical replicates, mean ± SD; ns, not significant; ∗∗∗∗P < .0001. (D) As in panel C, with HLA class I KO T cells as investigated effector cells.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/8/13/10.1182_bloodadvances.2023011496/2/m_blooda_adv-2023-011496-gr2.jpeg?Expires=1765919888&Signature=GNaz4wMWM4GRC8gEyFNn~t5aW4~Rt~YNA24J-9pDfSGbkTYdfo2hDMdhdgVnq1U~ZRIO6mAN8~j-AhdfQLs57WgNM9QdpPV8oivXJS1HIVPqxjnBefwhAdjjsqwAx0fJcPk7YpqXXONk8fyzhhq-aTijT2AomirfSoi-6d4UP-Zg7PB-ZVKasIKP4yGHF4v1PzSXmoeYmgZN-OpJNXb~B5BjM-89YoT0h0qlE4-08it7KQ5ayT0nJ5vHS6nwvLeiMu2tRiW5m0bzpFS84Jzx~PSm-kNg1t2a76NeBox1hae7pChcF6pybF7YKek~Ih0TooCrviLiDs0RAJcnKf23HQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)