Key Points
Deregulation of zebrafish Jagn1 disrupts neutrophil development, affecting steady-state granulopoiesis.
Activation of unfolded protein response and apoptosis alone is insufficient to induce neutropenia in zebrafish.
Visual Abstract
A variety of autosomal recessive mutations in the JAGN1 gene cause severe congenital neutropenia (CN). However, the underlying pathomechanism remains poorly understood, mainly because of the limited availability of primary hematopoietic stem cells from JAGN1-CN patients and the absence of animal models. In this study, we aimed to address these limitations by establishing a zebrafish model of JAGN1-CN. We found 2 paralogs of the human JAGN1 gene, namely jagn1a and jagn1b, which play distinct roles during zebrafish hematopoiesis. Using various approaches such as morpholino-based knockdown, CRISPR/Cas9–based gene editing, and misexpression of a jagn1b harboring a specific human mutation, we successfully developed neutropenia while leaving other hematopoietic lineages unaffected. Further analysis of our model revealed significant upregulation of apoptosis and genes involved in the unfolded protein response (UPR). However, neither UPR nor apoptosis is the primary mechanism that leads to neutropenia in zebrafish. Instead, Jagn1b has a critical role in granulocyte colony-stimulating factor receptor signaling and steady-state granulopoiesis, shedding light on the pathogenesis of neutropenia associated with JAGN1 mutations. The establishment of a zebrafish model for JAGN1-CN represents a significant advancement in understanding the specific pathologic pathways underlying the disease. This model provides a valuable in vivo tool for further investigation and exploration of potential therapeutic strategies.
Introduction
Severe congenital neutropenia (CN) is a preleukemia bone marrow failure syndrome that is characterized by impaired neutrophil development and more than 20 associated mutated genes, including JAGN1.1 Different types of JAGN1 mutations have been reported in patients with CN, including deletions, missense, and, nonsense mutations. The majority of the mutations are located in the cytoplasmic N-terminal part of JAGN1. The severity and symptoms of neutropenia vary among patients. The pathophysiological mechanism in defective granulopoiesis remains poorly understood because of the lack of suitable animal models. The current understanding of JAGN1-associated CN is mostly based on clinical observations and in vitro studies.
JAGN1 encodes an endoplasmic reticulum (ER)-resident protein with 4 transmembrane domains and cytoplasmic N- and C-terminal regions.2 This membrane topology resembles that of 2 known cargo transporters, tetraspanins and endoplasmic reticulum vesicle (Erv) proteins.3 Despite its evolutionary conservation, the precise function of JAGN1 remains to be fully elucidated. In Drosophila melanogaster, the JAGN1 orthologous gene, jagunal, is involved in ER reorganization and vesicular trafficking during oogenesis2 and in the proper asymmetric partitioning of the ER and spindle orientation in embryonic neuroblasts.4 In addition, Jagunal plays a role in signaling and distribution of ER membranes in Drosophila melanogaster stem cells as they adopt their fate.5 In human cells, JAGN1 interacts with tubulin and the coat protein I complex components that are essential for retrograde vesicular trafficking from the Golgi complex to the ER and within the Golgi.6 These interactions occur through the highly conserved N-terminal region of JAGN1. The impact of mutations in JAGN1 on protein stability or its interaction with other binding partners remains unclear. Notably, electron microscopy images have shown ultrastructural ER defects and a lack of granules in neutrophils isolated from patients with JAGN1-CN.6 In vitro studies have demonstrated a connection between JAGN1 deficiency and defective NETs formation,7 reduced myeloperoxidase (MPO) levels,7 and abnormal protein N-glycosylation.6,8 Moreover, transduction experiments with CN-associated JAGN1 mutants in HL-60 cells have suggested the activation of calpain-dependent cell death.9 Furthermore, there are indications of JAGN1 involvement in granulocyte colony-stimulating factor receptor (G-CSFR)-mediated STAT3 phosphorylation in the HeLa cell line and Jagn1–/– mouse neutrophils.6,10 However, it remains uncertain whether these intracellular processes also affect the granulocytic differentiation of bone marrow myeloid progenitor cells, which is impaired in patients with JAGN1-CN. Most studies have been conducted in nonhematopoietic cell lines, artificial in vitro expression systems, terminally differentiated neutrophils, or immortalized lymphocytes of patients with JAGN1-CN.6-9,11 The limited availability of primary bone marrow cells for experimentation hinders our understanding of the disease. Hence, there is an urgent need for suitable animal models to study the role of JAGN1 in granulopoiesis. Complete Jagn1 knockout in mice leads to embryonic lethality, whereas mice with a conditional knockout in hematopoietic cells display normal neutrophil counts.10 Over the past decade, zebrafish has emerged as a promising alternative model system for studying CN in humans.12 Zebrafish deficient in g-csfr,13,14hax1,15srp54,16,17sbds,18g6pc3,19 or taz20 recapitulate phenotypes similar to those observed in CN. In addition, the response to G-CSF is comparable between the zebrafish models and patients with CN.15
In this study, we established a zebrafish model of JAGN1-CN. We demonstrated that either downregulation of jagn1b or misexpression of CN-associated mutated jagn1b induces isolated neutropenia. At the molecular level, we found a robust activation of the unfolded protein response (UPR) upon Jagn1b deregulation, but neither UPR activation nor increased cell death alone hindered neutrophil development in zebrafish. We therefore propose a mechanism through which Jagn1b contributes to the G-CSFR signaling pathway and steady-state granulopoiesis, and its neutropenia phenotype can be rescued by emergency granulopoiesis activation.
Material and methods
Zebrafish maintenance
Zebrafish wild-type (WT) Tübingen TE strain and transgenic reporter lines (tg(mpo:gfp), tg(drl:mCherry)21,22) were maintained according to standard protocols and handled in accordance with the European Union Animal Protection Directive (2010/63/EU). Maintenance and generation of jagn1b mutants, using CRISPR/Cas9 gene editing, was approved by the local government (Tierschutzgesetz§11, Abs.1, Nr.1, husbandry permit 35/9185.46/UniTÜ; Tierschutzgesetz§8, Abs.1, permit M08/20G).
Genetic manipulation
Zebrafish embryos were genetically modified by microinjection of morpholinos (0.5 mM), CRISPR/Cas9 ribonucleoproteins (5 μM), and/or messenger RNAs (mRNAs) (20-50 ng/μL) into the 1 cell-stage.
Whole-mount in situ hybridization
Whole-mount in situ hybridization (WISH) was performed as previously described.23 The antisense probes that were used in this study are listed in supplemental Table 1.
In silico analysis
Genomic synteny was studied by analyzing neighboring genes of human and zebrafish JAGN1 orthologs using ENSEMBL. Multiple protein (supplemental Table 2) alignments and neighbor-joining phylogenetic tree of JAGN1 proteins were performed with 1000 bootstrap replications using Geneious.
Statistical analysis
For statistical analysis, GraphPad Prism (version 8.0.2) was used. The normal distribution was characterized using the Kolmogorov-Smirnov test. When normal distribution was confirmed, an unpaired t test was used for single comparisons between 2 groups and analysis of variance was used for multiple comparisons. When normal distribution was not confirmed, a Mann-Whitney test was used for single comparisons and a Kruskal-Wallis test was used for multiple comparisons.
Additional methods are available online.
Results
Two paralogs of the human JAGN1 gene in zebrafish
Zebrafish has 2 paralogs of the human JAGN1 gene, namely jagn1a and jagn1b. Multiple alignments showed high similarities between the different JAGN1 orthologs (supplemental Figure 1A). Phylogenetic (Figure 1A) and genomic synteny (Figure 1B) analyses indicated that the presence of zebrafish jagn1a and jagn1b was the consequence of species-specific gene duplication.
The evolution of JAGN1 gene and the expression patterns of its homologs in zebrafish. (A) A neighbor-joining phylogenetic tree of JAGN1 proteins performed with 1000 bootstrap replications. Branch labels represent consensus support in percentage. (B) Schematic comparison of genomic synteny between human JAGN1 and zebrafish jagn1a and jagn1b loci. (C) Heat map representing gene expression levels in sorted endothelial (EC-1, EC-2) and HSPCs (HSPC-1, HSPC-2) of draculin:mCherry embryos. (D) Representative picture of a GFP+ neutrophil with JAGN1 detection and DAPI staining after immunostaining against JAGN1 and GFP on a 2 dpf transgenic tg(mpo:gfp) reporter embryo. DAPI, 4′,6-diamidino-2-phenylindole; Dm, Drosophila melanogaster; Dr, Danio rerio; Hs, Homo sapiens; Mm, Mus musculus; Ol, Oryzias latipes; Pt, Pan troglodytes; Rn, Rattus norvegicus.
The evolution of JAGN1 gene and the expression patterns of its homologs in zebrafish. (A) A neighbor-joining phylogenetic tree of JAGN1 proteins performed with 1000 bootstrap replications. Branch labels represent consensus support in percentage. (B) Schematic comparison of genomic synteny between human JAGN1 and zebrafish jagn1a and jagn1b loci. (C) Heat map representing gene expression levels in sorted endothelial (EC-1, EC-2) and HSPCs (HSPC-1, HSPC-2) of draculin:mCherry embryos. (D) Representative picture of a GFP+ neutrophil with JAGN1 detection and DAPI staining after immunostaining against JAGN1 and GFP on a 2 dpf transgenic tg(mpo:gfp) reporter embryo. DAPI, 4′,6-diamidino-2-phenylindole; Dm, Drosophila melanogaster; Dr, Danio rerio; Hs, Homo sapiens; Mm, Mus musculus; Ol, Oryzias latipes; Pt, Pan troglodytes; Rn, Rattus norvegicus.
Expression levels of jagn1a and jagn1b were explored in 2 available single-cell RNA sequencing data sets of whole kidney marrow, corresponding to mammalian hematopoietic tissue.24,25 Macrophages expressed low levels of jagn1a, but jagn1b was detected in hematopoietic progenitors, macrophages, erythrocytes, and neutrophils (data not shown). A comparison of the transcriptome data set (GSE7088126) of zebrafish draculin-expressing cells, which mark hematopoietic stem and progenitor cells (HSPCs), with niche cells (endothelial cells) showed that jagn1a was not expressed in the niche cells or the HSPCs, whereas jagn1b RNA was present in both cell types (Figure 1C). Whole-mount immunostaining of transgenic (tg) tg(mpo:gfp) zebrafish embryos using the human α-JAGN1 antibody demonstrated co-localization between green fluorescent protein (GFP) (marking neutrophils) and α-JAGN1 staining (Figure 1D; supplemental Figure 1B).
Jagn1a and Jagn1b have distinct, nonredundant functions in zebrafish hematopoiesis
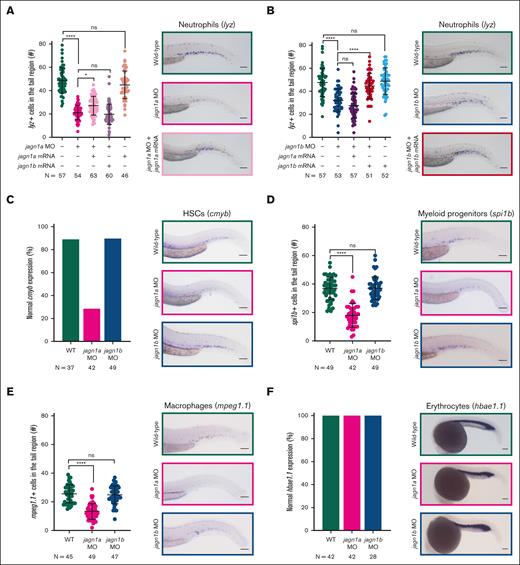
To investigate the importance of Jagn1a and Jagn1b in hematopoiesis, we employed an antisense morpholino (MO)-knockdown strategy. Antisense oligos were designed to bind to the ATG of jagn1a or jagn1b mRNA, thereby preventing their protein translation. We first confirmed the specificity of both morpholinos in zebrafish embryos by injecting them with a GFP mRNA construct containing the ATG of jagn1a or jagn1b (supplemental Figure 2A-B) and used WISH to examine the number of cells that expressed the neutrophil marker lysozymeC (lyz) in the MO-injected embryos (hereafter called morphants). Both jagn1a and jagn1b morphants exhibited a significant reduction in the number of lyz+ neutrophils when compared with the WT embryos (Figure 2A-B). Similar results were obtained when the morpholinos were injected in the transgenic tg(mpo:gfp) reporter line (supplemental Figure 2C). A control MO at the same concentration did not affect neutrophil numbers (supplemental Figure 2D).
Jagn1a and Jagn1b have distinct and nonredundant functions in zebrafish hematopoiesis. (A-B) Effect of jagn1a (A) or jagn1b (B) downregulation on neutrophil counts and the rescue with jagn1a and jagn1b mRNA. The left panels show a quantitative analysis of the cells expressing the neutrophil marker lysozyme C (lyz), stained using WISH. The neutrophils were quantified after interfering with jagn1a (jagn1a MO) or jagn1b function (jagn1b MO), the subsequent rescue with full-length jagn1a or jagn1b mRNA, and jagn1a or jagn1b mRNA overexpression alone. The right panels show representative images of WISH for lysozyme C (lyz) in 2.25 dpf wild-type embryos or jagn1a and jagn1b morphants with or without overexpression of jagn1a or jagn1b mRNA. (C-F) Effect of jagn1a and jagn1b downregulation on other hematopoietic cells. The left panels show quantitative analyses of stained cells with WISH against hematopoietic cell-specific markers. The right panels show representative images of the WISH-stained embryos. The cells were stained against (C) cmyb, staining HSCs, (D) spi1b, staining myeloid progenitors, (E) mpeg1.1, staining macrophages in 2.25 dpf embryos and (F) hbae1.1 staining erythrocytes in 1 dpf embryos. (C,F) Comparison of the percentage (%) of normal gene expression pattern between wild-type, jagn1a and jagn1b morphants. (D-E) Numbers of stained cells in the hematopoietic region. Each dot in panels A-B,D-E represents the number of cells in the hematopoietic tissue of an individual 2.25 dpf embryo. Data are presented as mean ± standard deviation. The data represent the combined results from 2 to 3 independent experiments. N represents the total number of analyzed embryos. (A-E) Images were taken with 10× magnification. Scale bars represent 100 μm. (F) Images were taken with 8× magnification. Scale bars represent 100 μm. ns, not significant; ∗P < .05; ∗∗∗∗P < .0001.
Jagn1a and Jagn1b have distinct and nonredundant functions in zebrafish hematopoiesis. (A-B) Effect of jagn1a (A) or jagn1b (B) downregulation on neutrophil counts and the rescue with jagn1a and jagn1b mRNA. The left panels show a quantitative analysis of the cells expressing the neutrophil marker lysozyme C (lyz), stained using WISH. The neutrophils were quantified after interfering with jagn1a (jagn1a MO) or jagn1b function (jagn1b MO), the subsequent rescue with full-length jagn1a or jagn1b mRNA, and jagn1a or jagn1b mRNA overexpression alone. The right panels show representative images of WISH for lysozyme C (lyz) in 2.25 dpf wild-type embryos or jagn1a and jagn1b morphants with or without overexpression of jagn1a or jagn1b mRNA. (C-F) Effect of jagn1a and jagn1b downregulation on other hematopoietic cells. The left panels show quantitative analyses of stained cells with WISH against hematopoietic cell-specific markers. The right panels show representative images of the WISH-stained embryos. The cells were stained against (C) cmyb, staining HSCs, (D) spi1b, staining myeloid progenitors, (E) mpeg1.1, staining macrophages in 2.25 dpf embryos and (F) hbae1.1 staining erythrocytes in 1 dpf embryos. (C,F) Comparison of the percentage (%) of normal gene expression pattern between wild-type, jagn1a and jagn1b morphants. (D-E) Numbers of stained cells in the hematopoietic region. Each dot in panels A-B,D-E represents the number of cells in the hematopoietic tissue of an individual 2.25 dpf embryo. Data are presented as mean ± standard deviation. The data represent the combined results from 2 to 3 independent experiments. N represents the total number of analyzed embryos. (A-E) Images were taken with 10× magnification. Scale bars represent 100 μm. (F) Images were taken with 8× magnification. Scale bars represent 100 μm. ns, not significant; ∗P < .05; ∗∗∗∗P < .0001.
We also performed rescue experiments by coinjecting morpholinos with full-length wild-type jagn1a or jagn1b mRNA containing different sequences around the ATG region, which cannot be targeted by the morpholinos. The neutropenia phenotype induced by jagn1a knockdown was partially rescued by jagn1a mRNA coinjection, but not by coinjection of jagn1b mRNA (Figure 2A; supplemental Figure 2E). Conversely, the neutropenia in jagn1b knockdown embryos were completely restored in embryos coinjected with jagn1b mRNA, but not jagn1a mRNA (Figure 2B; supplemental Figure 2E). The injection of wild-type jagn1a or jagn1b mRNA alone did not affect neutrophil numbers (Figure 2A-B; supplemental Figure 2E). These results indicate that Jagn1a and Jagn1b have nonredundant functions in hematopoiesis.
To explore other hematopoietic cells in jagn1a or jagn1b morphants, we analyzed cmyb+ hematopoietic stem cells (HSCs) and spi1b+ myeloid precursors using WISH (Figure 2C-D). The jagn1a, but not jagn1b morphants exhibited a significant reduction in these cell numbers when compared with their WT counterparts. Moreover, we observed a significant reduction in macrophages (positive for macrophage-expressed gene 1.1 [mpeg1.1]) in jagn1a morphants, but not in jagn1b morphants (Figure 2E). The unchanged expression of the erythrocyte marker hemoglobin, alpha embryonic 1.1 (hbae1.1) (Figure 2F) and the presence of circulating red blood cells (data not shown) in the morphants indicate that jagn1a and jagn1b are not involved in erythropoiesis. These findings strongly suggest that Jagn1a and Jagn1b are regulating distinct mechanisms that might directly or indirectly influence granulopoiesis. Although Jagn1a is involved in early hematopoiesis and myelopoiesis, Jagn1b specifically plays a role in granulopoiesis. Therefore, we decided to focus on Jagn1b in this study.
JAGN1-CN–associated mutations in zebrafish jagn1b cause neutropenia
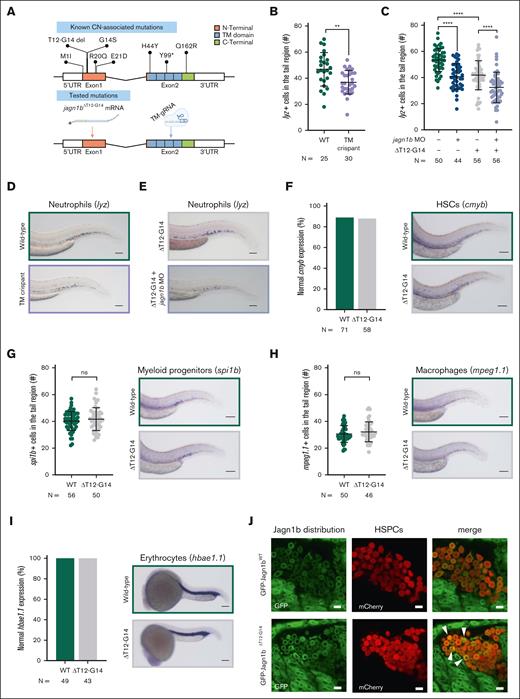
Thus far, our jagn1b MO has successfully disrupted the start codon, preventing the translation of Jagn1b protein. However, only a subset of patients with JAGN1-CN have mutations in the start codon (p.Met1Ile). Patients with JAGN1-CN also have deletions and missense and nonsense mutations (Figure 3A). To mimic these types of JAGN1-CN, we employed 2 different strategies. In the first approach, we designed and tested a guide RNA targeted to the TM domain (Figure 3A). By using CRISPR/Cas9 gene editing (supplemental Figure 3A-B), we detected a significantly reduced number of lyz-expressing neutrophils in 2.25 days postfertilization (dpf) jagn1b crispants when compared with the control group (Figure 3B,D). We also confirmed the reduction of neutrophils in tg(mpo:gfp) reporter embryos (supplemental Figure 3C). Furthermore, similar to the jagn1b morphants, the jagn1b crispants did not show any differences in cmyb+ HSCs, spi1b+ myeloid progenitors, mpeg1.1+ macrophages, and hbae1.1+ erythrocytes when compared with their WT counterparts (supplemental Figure 3D-G).
Expression of zebrafish Jagn1bΔT12-G14 results in neutropenia, without affecting other hematopoietic cells. (A) Top panel shows a schematic representation of identified CN-associated mutations in human JAGN1 gene. The bottom panel shows the tested mutations with the corresponding approach in this study. Note, to mimic the deletion (ΔT12-G14) we overexpressed jagn1bΔT12-G14 mRNA. Further, we targeted the transmembrane domain using CRISPR/Cas9 gene editing to introduce a truncating mutation. (B-C) Quantitative analysis of the cells expressing the neutrophil marker lysozyme C (lyz), stained with WISH in (B) jagn1b TM crispants and (C) after jagn1bΔT12-G14 overexpression alone or in combination with jagn1b morpholino. Each dot represents the number of cells in the hematopoietic tissue of a 2.25 dpf individual embryo. Data are presented as mean ± standard deviation. The data represent combined results from 2 independent experiments. N represents the total number of analyzed embryos. (D-E) Representative images of lyz+ neutrophils in (D) TM crispants and (E) after overexpression of jagn1bΔT12-G14 alone or in combination with jagn1b morpholino. Images were taken with 10× magnification. Scale bars represent 100 μm. (F-I) Effect of overexpression of jagn1bΔT12-G14 on other hematopoietic cells. The left panels show quantitative analyses of stained cells with WISH against hematopoietic cell-specific marker. The right panels show representative images of the WISH-stained embryos. The cells were stained against (F) cmyb, (G) spi1b, (H) mpeg1.1 all 3 in 2.25 dpf embryos and (I) hbae1.1 in 1 dpf embryos. (F,I) Comparison of the percentage (%) of normal gene expression pattern between wild-type embryos compared to that carrying jagn1bΔT12-G14 mutation. (G-H) Numbers of stained cells in the hematopoietic region. Each dot represents the number of cells in the hematopoietic tissue of an individual 2.25 dpf embryo. Data are presented as mean ± standard deviation. The data represent combined results from 2 independent experiments. N represents the total number of analyzed embryos. Pictures were taken with 8× to 10× magnification. Scale bars represent 100 μm. (J) Representative images of intracellular Jagn1 protein localization (GFP signal) after overexpression of gfp-jagn1bWT and gfp-jagn1bΔT12-G14 mRNA in draculin:mCherry-positive HSPCs at 20 hours postfertilization (hpf). ns, not significant; ∗∗P < .01; ∗∗∗∗P < .0001.
Expression of zebrafish Jagn1bΔT12-G14 results in neutropenia, without affecting other hematopoietic cells. (A) Top panel shows a schematic representation of identified CN-associated mutations in human JAGN1 gene. The bottom panel shows the tested mutations with the corresponding approach in this study. Note, to mimic the deletion (ΔT12-G14) we overexpressed jagn1bΔT12-G14 mRNA. Further, we targeted the transmembrane domain using CRISPR/Cas9 gene editing to introduce a truncating mutation. (B-C) Quantitative analysis of the cells expressing the neutrophil marker lysozyme C (lyz), stained with WISH in (B) jagn1b TM crispants and (C) after jagn1bΔT12-G14 overexpression alone or in combination with jagn1b morpholino. Each dot represents the number of cells in the hematopoietic tissue of a 2.25 dpf individual embryo. Data are presented as mean ± standard deviation. The data represent combined results from 2 independent experiments. N represents the total number of analyzed embryos. (D-E) Representative images of lyz+ neutrophils in (D) TM crispants and (E) after overexpression of jagn1bΔT12-G14 alone or in combination with jagn1b morpholino. Images were taken with 10× magnification. Scale bars represent 100 μm. (F-I) Effect of overexpression of jagn1bΔT12-G14 on other hematopoietic cells. The left panels show quantitative analyses of stained cells with WISH against hematopoietic cell-specific marker. The right panels show representative images of the WISH-stained embryos. The cells were stained against (F) cmyb, (G) spi1b, (H) mpeg1.1 all 3 in 2.25 dpf embryos and (I) hbae1.1 in 1 dpf embryos. (F,I) Comparison of the percentage (%) of normal gene expression pattern between wild-type embryos compared to that carrying jagn1bΔT12-G14 mutation. (G-H) Numbers of stained cells in the hematopoietic region. Each dot represents the number of cells in the hematopoietic tissue of an individual 2.25 dpf embryo. Data are presented as mean ± standard deviation. The data represent combined results from 2 independent experiments. N represents the total number of analyzed embryos. Pictures were taken with 8× to 10× magnification. Scale bars represent 100 μm. (J) Representative images of intracellular Jagn1 protein localization (GFP signal) after overexpression of gfp-jagn1bWT and gfp-jagn1bΔT12-G14 mRNA in draculin:mCherry-positive HSPCs at 20 hours postfertilization (hpf). ns, not significant; ∗∗P < .01; ∗∗∗∗P < .0001.
Through breeding the F1 and F2 generations to generate stable zebrafish jagn1b mutants, we were only able to identify adult heterozygous jagn1b mutants (supplemental Figure 3H) with 2 different genotypes, c.347_351del and c.351_357del, which led to truncated Jagn1b proteins with 134 and 133 amino acids, respectively (supplemental Figure 3K). To determine whether homozygous jagn1b mutants were embryonic lethal, we crossed jagn1b heterozygote fish and monitored the development of the offspring from the 1-cell stage until 1 dpf. Interestingly, many embryos exhibited either asymmetrical blastomere division or complete arrest in cell division within the first 2 hours of embryonic development (supplemental Figure 3I). These embryos were unable to proceed to gastrulation, leading to high embryonic lethality (supplemental Figure 3J), which could explain the absence of adult homozygous jagn1b mutants. To confirm that the observed effects were specific to jagn1b mutations, we predicted 13 potential off-target regions for our designed gRNAs using the CCTop tool27 (supplemental Table 10), but none of them was confirmed by Sanger sequencing. These results indicate that Jagn1b is crucial for the correct and symmetrical division of zebrafish blastomeres, and stable jagn1bc.347_351del and jagn1bc.351_357del mutants cannot be generated.
As an alternative strategy to mimic JAGN1-associated CN in zebrafish, we introduced deletion mutation ΔT12-G14 into zebrafish jagn1b full-length mRNA. We aimed to assess the impact of the mutation on its subcellular localization by tagging jagn1bWT or jagn1bΔT12-G14 with GFP at the N-terminal region. Overexpression of GFP-tagged jagn1bWT did not affect the number of neutrophils in WT embryos and rescued the jagn1b morphants (supplemental Figure 4A-B). Interestingly, gfp-jagn1bΔT12-G14 overexpression significantly reduced the number of neutrophils when compared with the wild-type siblings. This effect was even more pronounced after jagn1b knockdown (Figure 3C,E), suggesting a dominant negative effect of mutated Jagn1b. Moreover, we found that the overexpression of jagn1bΔT12-G14 had no effect on cmyb+ HSCs, spi1b+ myeloid progenitors, mpeg1.1+ macrophages, or hbae1.1+ erythrocytes when compared with WT siblings (Figure 3F-I). To determine the subcellular localization of the WT and mutated proteins, we injected the GFP-tagged jagn1b mRNAs into the tg(draculin:mCherry) HSPCs reporter line. Confocal images of injected tg(draculin:mCherry) embryos revealed a symmetric distribution of GFP-Jagn1bWT, most likely in the ER (Figure 3J). In contrast, GFP-Jagn1bΔT12G14 exhibited asymmetric distribution within some HSPCs (arrowheads in Figure 3J; supplemental Videos 1 and 2). This observation suggests that the Jagn1bΔT12G14 could lead to protein aggregation within the ER, resembling the ER-related abnormalities described in patients with JAGN1-CN.6
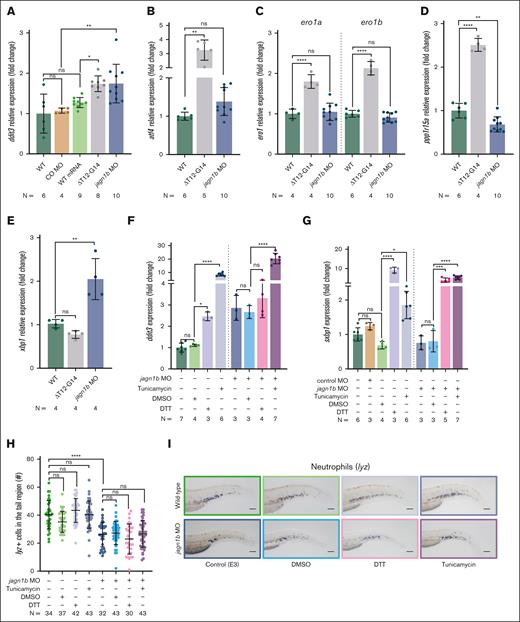
Deregulation of Jagn1b leads to UPR activation, but UPR induction alone is not sufficient to induce neutropenia in zebrafish
Next, we examined whether UPR was activated upon injection of WT and mutated jagn1b mRNA and jagn1b MO. To assess this, we quantified the expression levels of DNA damage-inducible transcript 3 (ddit3), which encodes the UPR marker C/EBP homologous protein (Chop), activated by the 3 different UPR pathways (ATF6, PERK, and IRE1).28 We observed a significant increase in ddit3 expression in embryos injected with either jagn1bΔT12-G14 or jagn1b MO. However, the expression level of ddit3 remained unchanged in embryos injected with jagn1bWT mRNA or control MO (Figure 4A), suggesting that the misexpression of Jagn1bWT or the injection of a nonspecific MO is not sufficient to induce UPR. Because ddit3 expression can be induced by activating transcription factor 4 (ATF4),29 we also quantified the expression levels of the zebrafish ortholog atf4 in embryos injected with jagn1bΔT12-G14 or jagn1b MO. The overexpression of mutated jagn1b, but not the downregulation of jagn1b, led to a significant increase in atf4 expression (Figure 4B). In addition, we analyzed the expression levels of zebrafish orthologs of the Chop target genes, endoplasmic reticulum oxidoreductase 1-alpha (ero1a) and -beta (ero1b) and protein phosphatase 1 regulatory subunit 15A (ppp1r15a encoding Gadd34).30 We found a significant increase in the expression levels of ero1a, ero1b, and ppp1r15a in embryos injected with jagn1bΔT12G14 mRNA but not with jagn1b MO (Figure 4C-D) or control MO (supplemental Figure 5A). Thus, these results suggest that Jagn1bΔT12G14 can induce UPR through the activation of the PERK/ATF4/CHOP pathway. In jagn1b morphants, we saw an increase in the expression of ddit3, which was not induced by Atf4. However, the expression of ddit3 can be induced by several UPR pathways, including the ATF6 pathway. Therefore, we quantified the expression level of unspliced X-box binding protein 1 (xbp1), another downstream ATF6 target. Indeed, the expression levels of unspliced xbp1 were significantly increased in jagn1b morphants when compared with their WT siblings, but not in jagn1bΔT12-G14 overexpressed embryos (Figure 4E). The spliced xbp1 (sxbp1) showed a trend of induction that was not significant (supplemental Figure 5B). However, the upregulation of ddit3 and xbp1 is consistent with the activation of the ATF6 pathway. Although UPR was also induced in jagn1b morphants, the expression of Chop target genes remained unchanged. Interestingly, the jagn1b morphants group showed a slight decrease in ppp1r15a expression (Figure 4D). In addition, mRNA expression of ER to nucleus signaling 1 (ern1) (encoding Ire1), eukaryotic translation initiation factor 2α kinase 3 (eif2ak3) (encoding Perk), and activating transcription factor 6 (atf6) was unaffected upon jagn1b downregulation (supplemental Figure 5B).
Deregulation of Jagn1b results in UPR activation, but UPR induction alone is not sufficient to induce neutropenia in zebrafish. (A-E) Relative expression levels (fold change) of UPR-associated genes measured with quantitative RT-PCR after interference with jagn1b by overexpressing jagn1bΔT12-G14 mRNA or inhibiting jagn1b with morpholino. Relative expression levels of (A) ddit3, coding the UPR marker Chop, (B) atf4, (C) ero1a and ero1b, (D) ppp1r15a, and (E) xbp1 were measured, corresponding groups are indicated. Expression levels were normalized to the housekeeping gene b-actin and fold change was calculated with respect to the expression levels of wild-type embryos. Each dot represents the value of the relative expression of 1 sample. Data are presented as mean ± standard deviation. The data represent combined results from 2 to 3 independent experiments. N represents the total number of analyzed complimentary DNA samples. (F-G) Relative expression (fold change) quantified by quantitative RT-PCR, normalized to the housekeeping gene b-actin and fold changes were calculated respect the expression levels of wild-type embryos of (F) UPR-marker ddit3 (G) and spliced xbp1 after UPR induction with tunicamycin (1 μg/mL), DTT (0.7 mM) or DMSO control (0.7%) alone or in combination with jagn1b morpholino. Data are presented as mean ± standard deviation. Each dot represents the value of relative expression of one sample. The data represent combined results from 2 to 3 independent experiments. N represents the total number of analyzed complimentary DNA samples. (H-I) Effect of chemically induced UPR on neutrophil counts. (H) Quantification of lyz+ neutrophils stained with WISH after UPR induction with tunicamycin (1 μg/mL), DTT (0.7 mM) or DMSO control (0.7%) alone or in combination with jagn1b morpholino. Each dot represents the number of cells in the hematopoietic tissue of a 2 dpf individual embryo after 24 hours of treatment. Data are presented as mean ± standard deviation. The data represent the combined results from 2 to 3 independent experiments. N represents the total number of analyzed embryos. (I) Representative images were taken with 10× magnification. Scale bars represents 100 μm. ns, not significant; ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001.
Deregulation of Jagn1b results in UPR activation, but UPR induction alone is not sufficient to induce neutropenia in zebrafish. (A-E) Relative expression levels (fold change) of UPR-associated genes measured with quantitative RT-PCR after interference with jagn1b by overexpressing jagn1bΔT12-G14 mRNA or inhibiting jagn1b with morpholino. Relative expression levels of (A) ddit3, coding the UPR marker Chop, (B) atf4, (C) ero1a and ero1b, (D) ppp1r15a, and (E) xbp1 were measured, corresponding groups are indicated. Expression levels were normalized to the housekeeping gene b-actin and fold change was calculated with respect to the expression levels of wild-type embryos. Each dot represents the value of the relative expression of 1 sample. Data are presented as mean ± standard deviation. The data represent combined results from 2 to 3 independent experiments. N represents the total number of analyzed complimentary DNA samples. (F-G) Relative expression (fold change) quantified by quantitative RT-PCR, normalized to the housekeeping gene b-actin and fold changes were calculated respect the expression levels of wild-type embryos of (F) UPR-marker ddit3 (G) and spliced xbp1 after UPR induction with tunicamycin (1 μg/mL), DTT (0.7 mM) or DMSO control (0.7%) alone or in combination with jagn1b morpholino. Data are presented as mean ± standard deviation. Each dot represents the value of relative expression of one sample. The data represent combined results from 2 to 3 independent experiments. N represents the total number of analyzed complimentary DNA samples. (H-I) Effect of chemically induced UPR on neutrophil counts. (H) Quantification of lyz+ neutrophils stained with WISH after UPR induction with tunicamycin (1 μg/mL), DTT (0.7 mM) or DMSO control (0.7%) alone or in combination with jagn1b morpholino. Each dot represents the number of cells in the hematopoietic tissue of a 2 dpf individual embryo after 24 hours of treatment. Data are presented as mean ± standard deviation. The data represent the combined results from 2 to 3 independent experiments. N represents the total number of analyzed embryos. (I) Representative images were taken with 10× magnification. Scale bars represents 100 μm. ns, not significant; ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001.
Previous studies have proposed that ER stress and UPR activation are involved in diminished granulocytic differentiation downstream of CN-associated mutations such as ELANE31,32 or G6PC3.33 Elevated ER-stress was also described in JAGN1-mutant granulocytes.6 Therefore, we aimed to investigate whether UPR activation could impair granulopoiesis in our zebrafish model. To induce UPR in vivo, we treated embryos with dithiothreitol (DTT) or tunicamycin, 2 compounds known to disrupt protein folding by reducing disulfide bonds and inhibiting N-linked glycosylation, respectively.34 These reagents have been used previously to induce UPR in cell-based,35 zebrafish,17 and mice neutropenia models31 and the zebrafish UPR reporter.36,37 To confirm the activation of UPR in zebrafish embryos, we treated WT embryos with DTT or tunicamycin from 24 to 48 hours postfertilization (hpf) and quantified the expression levels of ddit3 (Figure 4F) and sxbp1 (Figure 4G) using reverse transcriptase polymerase chain reaction (RT-PCR) at 48 hpf. As a negative control, we used dimethylsulfoxide (DMSO) treatment. Our results showed that both, DTT and tunicamycin treatments were sufficient to induce UPR as evidenced by an increased expression of ddit3 and sxbp1 in treated WT embryos (Figure 4F-G). However, there was no significant difference in the number of neutrophils between DTT-treated, tunicamycin-treated, DMSO-treated, and untreated embryos (Figure 4H-I). Next, we treated jagn1b morphants. Despite the strong UPR activation (Figure 4F-G), we did not observe any additional decrease in neutrophil numbers in jagn1b morphants after treatment with tunicamycin or DTT (Figure 4H-I).
Together, we detected UPR activation after the interference with Jagn1b function. However, the implicated UPR pathways varied depending on whether jagn1b was downregulated or upon overexpression of jagn1bΔT12-G14. Nevertheless, the chemically induced UPR response alone did not affect the number of neutrophils, arguing that UPR induction alone is insufficient to cause neutropenia in zebrafish.
Apoptosis is increased upon jagn1b knockdown, but it is insufficient to induce neutropenia
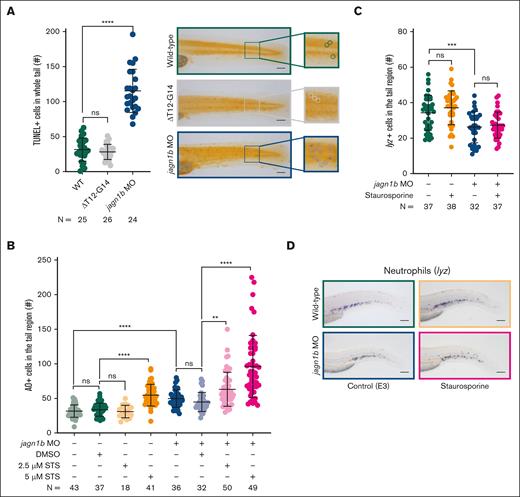
There are several connections between chronic ER stress, CHOP activation, its targets, and apoptosis.28,30,38 Therefore, we investigated apoptosis in embryos injected with jagn1bΔT12-G14 mRNA and jagn1b MO using the terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling (TUNEL) assay. Surprisingly, we found that the number of apoptotic cells was increased only in jagn1b morphants and not with jagn1bΔT12-G14 (Figure 5A). TUNEL-positive cells were distributed throughout the body, including the tail region. However, we did not observe a significant increase in apoptotic cells within the hematopoietic tissue (data not shown). This finding was further confirmed by acridine orange (AO) staining (Figure 5B).
Apoptosis is increased upon jagn1b knockdown, but it is not sufficient to induce neutropenia. (A) Assessment of TUNEL+ apoptotic cells upon jagn1b interference. The left panel shows the quantification of the TUNEL+ cells in the whole trunk region of 2.25 dpf embryos after overexpression of jagn1bΔT12-G14 or jagn1b morpholino. Data shows mean and standard deviation. Each dot represents the number of TUNEL+ cells in the tail region of an individual embryo. The data represent the combined results from 2 independent experiments. N represents the total number of analyzed embryos. The right panel shows representative images of the tail region of wild-type, jagn1bΔT12-G14 embryos, and jagn1b morphants. Images were taken with 10× magnification. Scale bars represent 100 μm. The square at the right of each image represents a magnification of the marked tail region where each apoptotic cell is encircled. (B) Apoptosis induction in staurosporine-treated embryos using AO staining. Quantification of AO-positive apoptotic cells in the tail region of 2 dpf wild-type embryos and jagn1b morphants treated with DMSO, or staurosporine (STS). Each dot represents the number of AO+ cells in the tail region of an individual 2 dpf embryo. Data are presented as mean ± standard deviation. The data represent the combined results from 3 independent experiments. N represents the total number of analyzed embryos. (C-D) Effect of staurosporine-induced apoptosis on the neutrophil count. (C) Quantification of lyz+ neutrophils stained with WISH after apoptosis induction with staurosporine (5 μM) alone or combined with jagn1b morpholino. Data are presented as mean ± standard deviation. Each dot represents the number of stained cells in the hematopoietic region of an individual 2 dpf embryo. The data represent the combined results from 2 independent experiments. N represents the total number of analyzed embryos. (D) Representative images were taken with 10× magnification. Scale bars represent 100 μm. ns, not significant; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001.
Apoptosis is increased upon jagn1b knockdown, but it is not sufficient to induce neutropenia. (A) Assessment of TUNEL+ apoptotic cells upon jagn1b interference. The left panel shows the quantification of the TUNEL+ cells in the whole trunk region of 2.25 dpf embryos after overexpression of jagn1bΔT12-G14 or jagn1b morpholino. Data shows mean and standard deviation. Each dot represents the number of TUNEL+ cells in the tail region of an individual embryo. The data represent the combined results from 2 independent experiments. N represents the total number of analyzed embryos. The right panel shows representative images of the tail region of wild-type, jagn1bΔT12-G14 embryos, and jagn1b morphants. Images were taken with 10× magnification. Scale bars represent 100 μm. The square at the right of each image represents a magnification of the marked tail region where each apoptotic cell is encircled. (B) Apoptosis induction in staurosporine-treated embryos using AO staining. Quantification of AO-positive apoptotic cells in the tail region of 2 dpf wild-type embryos and jagn1b morphants treated with DMSO, or staurosporine (STS). Each dot represents the number of AO+ cells in the tail region of an individual 2 dpf embryo. Data are presented as mean ± standard deviation. The data represent the combined results from 3 independent experiments. N represents the total number of analyzed embryos. (C-D) Effect of staurosporine-induced apoptosis on the neutrophil count. (C) Quantification of lyz+ neutrophils stained with WISH after apoptosis induction with staurosporine (5 μM) alone or combined with jagn1b morpholino. Data are presented as mean ± standard deviation. Each dot represents the number of stained cells in the hematopoietic region of an individual 2 dpf embryo. The data represent the combined results from 2 independent experiments. N represents the total number of analyzed embryos. (D) Representative images were taken with 10× magnification. Scale bars represent 100 μm. ns, not significant; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001.
Next, we investigated whether induced apoptosis could cause neutropenia by treating WT embryos and jagn1b morphants with the protein kinase inhibitor staurosporine (STS) for 24 hours. Apoptosis was evaluated using AO staining (Figure 5B) and the TUNEL assay (supplemental Figure 5C-D). We found that the jagn1b morphants were more susceptible to the STS treatment than the WT embryos. The jagn1b morphants already showed an increased number of AO+ apoptotic cells in the tail region at a low STS concentration (2.5 μM) (Figure 5B) when compared with the DMSO-treated jagn1b morphants, whereas the WT embryos only showed an increased number of AO+ apoptotic cells at the high concentration (5 μM) when compared with DMSO-treated WT siblings (Figure 5B). To investigate whether apoptosis led to neutropenia, we quantified the lyz+ neutrophils in WT and morphants treated with 5 μM STS. Interestingly, the induction of apoptosis did not affect the number of neutrophils in either WT embryos or jagn1b morphants (Figure 5C-D).
Deregulation of Jagn1b affects steady-state and emergency granulopoiesis.
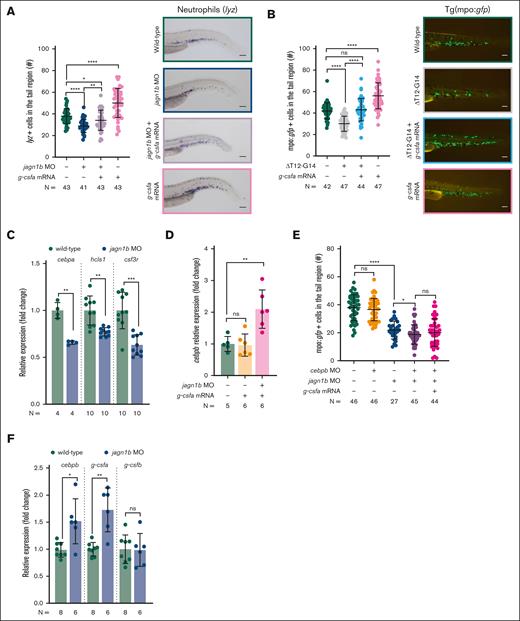
We also tested whether JAGN1 is involved in G-CSFR-triggered steady-state and emergency granulopoiesis because several indications support this hypothesis.6,10 It was previously shown that the G-CSFR pathway and the relationship between its activation and CCAAT/enhancer-binding protein alpha (CEBPA) induction is evolutionarily conserved between human and zebrafish.15,39 To investigate whether overexpression of g-csfa rescues neutropenia in jagn1b morphants, we used 2 different approaches. First, we injected g-csfa mRNA into zebrafish embryos, allowing for protein translation at an early embryonic stage, and quantified lyz+ neutrophils (Figure 6A). Second, we induced g-csfa expression in 44 hpf embryos using a heat-inducible construct15,40 and quantified mpo+ neutrophils (supplemental Figure 6A). Both approaches showed a significantly increased number of neutrophils in jagn1b morphants when compared with WT embryos at 2.25 dpf and successfully rescued the neutropenia induced by jagn1b knockdown (Figure 6A; supplemental Figure 6A). Similarly, overexpression of g-csfa in transgenic tg(mpo:gfp) reporter embryos, together with jagn1bΔT12-G14 mRNA, rescued neutropenia induced by Jagn1bΔT12-G14 (Figure 6B).
Jagn1b deregulation affects steady-state and emergency granulopoiesis. (A-B) G-csfa rescue after jagn1b interference. (A) G-csfa rescue in jagn1b morphants. The left panel shows the quantification of lyz+ neutrophils stained with WISH after g-csfa overexpression in 2.25 dpf wild-type embryos and jagn1b morphants. Right panel shows representative images taken with 10× magnification. Scale bars represent 100 μm. (B) G-csfa rescue in jagn1bΔT12-G14 embryos. The left panel shows the quantification of mpo:gfp+neutrophils after g-csfa overexpression in 2.25 dpf wild-type and jagn1bΔT12-G14 embryos. Right panel shows representative images taken with 8× magnification. Scale bars represent 100 μm. (C) Relative expression (fold change) of cebpa, hcls1 and csf3r in wild-type embryos and jagn1b morphants, quantified by quantitative RT-PCR, normalized to the housekeeping gene b-actin. Fold change was calculated in respect to the wild-type expression. (D) Relative expression (fold change) of cebpb after g-csfa overexpression in wild-type embryos and jagn1b morphants, quantified by quantitative RT-PCR, normalized to the housekeeping gene b-actin. Fold change was calculated in respect to the WT expression. (E) Quantification of mpo:gfp+ neutrophils after interfering with the function of jagn1b (jagn1b MO), cebpb (cebpb MO) and overexpression of g-csfa (g-csfa mRNA) alone or in combination. (F) Relative expression (fold change) of cebpb, g-csfa and g-csfb in wild-type embryos and jagn1b morphants, quantified by quantitative RT-PCR and normalized to b-actin. The fold change difference was calculated respective to the wild-type expression. (A-B,E) Each dot represents the number of cells in the hematopoietic tissue of a 2.25 dpf individual embryo. Data are presented as mean ± standard deviation. The data represent combined results from 2-3 independent experiments. N represents the total number of analyzed embryos. (C,D,F) Each dot represents the relative expression of 1 analyzed sample. Data are presented as mean ± standard deviation. The data represent combined results from 2 to 3 independent experiments. N represents the total number of analyzed biological samples. ns, not significant; ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001.
Jagn1b deregulation affects steady-state and emergency granulopoiesis. (A-B) G-csfa rescue after jagn1b interference. (A) G-csfa rescue in jagn1b morphants. The left panel shows the quantification of lyz+ neutrophils stained with WISH after g-csfa overexpression in 2.25 dpf wild-type embryos and jagn1b morphants. Right panel shows representative images taken with 10× magnification. Scale bars represent 100 μm. (B) G-csfa rescue in jagn1bΔT12-G14 embryos. The left panel shows the quantification of mpo:gfp+neutrophils after g-csfa overexpression in 2.25 dpf wild-type and jagn1bΔT12-G14 embryos. Right panel shows representative images taken with 8× magnification. Scale bars represent 100 μm. (C) Relative expression (fold change) of cebpa, hcls1 and csf3r in wild-type embryos and jagn1b morphants, quantified by quantitative RT-PCR, normalized to the housekeeping gene b-actin. Fold change was calculated in respect to the wild-type expression. (D) Relative expression (fold change) of cebpb after g-csfa overexpression in wild-type embryos and jagn1b morphants, quantified by quantitative RT-PCR, normalized to the housekeeping gene b-actin. Fold change was calculated in respect to the WT expression. (E) Quantification of mpo:gfp+ neutrophils after interfering with the function of jagn1b (jagn1b MO), cebpb (cebpb MO) and overexpression of g-csfa (g-csfa mRNA) alone or in combination. (F) Relative expression (fold change) of cebpb, g-csfa and g-csfb in wild-type embryos and jagn1b morphants, quantified by quantitative RT-PCR and normalized to b-actin. The fold change difference was calculated respective to the wild-type expression. (A-B,E) Each dot represents the number of cells in the hematopoietic tissue of a 2.25 dpf individual embryo. Data are presented as mean ± standard deviation. The data represent combined results from 2-3 independent experiments. N represents the total number of analyzed embryos. (C,D,F) Each dot represents the relative expression of 1 analyzed sample. Data are presented as mean ± standard deviation. The data represent combined results from 2 to 3 independent experiments. N represents the total number of analyzed biological samples. ns, not significant; ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001.
To assess the impact of Jagn1b alterations on steady-state granulopoiesis, we examined the zebrafish ortholog of CEBPA.41,42 Indeed, we found a significant reduction in cebpa-expressing cells (supplemental Figure 6B) and cebpa expression level (Figure 6C) in jagn1b morphants when compared to control siblings. Furthermore, we found that the expression levels of CEBPA targets, hematopoietic cell-specific lyn substrate 1 (hcls1) and csf3r (encoding zebrafish G-CSFR), were significantly reduced in jagn1b morphants when compared with WT embryos (Figure 6C). These results strongly suggest impaired G-CSF signaling upon jagn1b downregulation.
Given that steady-state granulopoiesis was impaired, the rescue observed upon g-csfa induction in jagn1b morphants is likely explained by the activation of emergency granulopoiesis. To examine this hypothesis, we measured the expression level of CCAAT/enhancer-binding protein beta (cebpb), a key regulator of emergency granulopoiesis43,44 and found a substantial increase in its expression in jagn1b morphants upon g-csfa induction (Figure 6D). To further confirm the essential role of cebpb in emergency granulopoiesis activation, we interfered with cebpb function in the g-csfa-rescued embryos using a MO knockdown strategy. As a control, we injected cebpb MO into WT embryos. As expected, cebpb knockdown alone did not affect the number of neutrophils in WT embryos, confirming that cebpb does not play a vital role in steady-state granulopoiesis.43 However, in sharp contrast, cebpb knockdown further decreased the number of neutrophils in jagn1b morphants, and g-csfa overexpression was no longer sufficient to rescue the neutropenia phenotype when cebpb was downregulated (Figure 6E). These effects were rescued by overexpressing cebpb mRNA (supplemental Figure 6C-D). Together, our results demonstrate that g-csfa induction rescues neutrophil development in jagn1b morphants by activating the emergency granulopoiesis.
We also noticed a significant increase in the expression level of cebpb (Figure 6F) and the number of cebpb-expressing cells (supplemental Figure 6E) in jagn1b morphants when compared with WT counterparts. The same trend was observed for the expression of endogenous g-csfa, but not g-csfb (Figure 6F), the second ortholog of human G-CSF.39 This suggests that jagn1b knockdown alone is capable of elevating the expression of g-csfa and cebpb, although the level of induction is insufficient to completely rescue neutropenia. Similarly, embryos injected with jagn1bΔT12-G14 mRNA showed a decrease in cebpa- (supplemental Figure 6B) and an increased number of cebpb-expressing cells (supplemental Figure 6E). These observations suggest that, consistent with the results obtained in jagn1b morphants, the steady-state granulopoiesis is impaired and the emergency granulopoiesis is activated downstream of mutated Jagn1b.
Discussion
Thus far, the main focus of research on the mechanism of JAGN1-associated CN has been restricted to clinical observation and in vitro studies.6-10,45,46 In this study, we report the successful establishment of a zebrafish model of JAGN1-associated CN, demonstrating neutropenia induction by interfering with JAGN1 function using jagn1b-specific MO, CRISPR/Cas9, or misexpression of a JAGN1-CN specific mutation. Our analyses revealed 4 new findings. First, JAGN1 is required for cell division. It was initially discovered to be essential for Drosophila oocyte development by controlling ER reorganization, vesicular trafficking, and asymmetric ER partitioning during cell mitosis.2,4 Similarly, we observed a strong disruption in the cell division of blastomeres during early zebrafish development in the Jagn1b CRIPSR/Cas9-mutant model. Therefore, a JAGN1 deficiency in the whole organism during early embryonic development is lethal, as shown in zebrafish (this study) and mice.10 It is worth noting that knockdown of jagn1b using MO does not usually lead to complete loss of protein, and crispants (transient knockout) contain maternal WT transcripts until gastrulation, which enable normal embryonic development. The mutations in JAGN1 may alter the protein’s structure, leading to impaired ER-membrane binding or altered interactions between JAGN1 and its interaction partners, which seems important for cell division.
Second, our results suggest that ER stress and UPR pathways are not the primary and sole cause of neutropenia downstream of mutated Jagn1b because UPR induction alone did not cause neutropenia in WT embryos, and it did also not lead to a further decrease in neutrophil numbers in jagn1b morphants. It was described previously that specific mutations in SEC61A1 can cause CN through dysregulation of the UPR.35 Furthermore, the expression of mutant ELANE in human primary granulocytic precursors or myeloid cell lines led to UPR activation and apoptosis.47,48 In addition, myeloid cell lines that express mutant ELANE48 and granulocytic precursors of G193X Elane mice showed increased susceptibility to tunicamycin treatment.31 These results show that there is a direct link between ER stress and neutropenia. In vitro, JAGN1-deficient granulocytes also exhibit increased levels of binding immunoglobulin protein, suggesting the activation of ER stress.6 In addition, under conditions of ER stress, JAGN1 was induced in insulinoma cells and the sperm proteome of men with obesity, contributing to alleviating protein folding load in the ER and restoring ER homeostasis.49,50 These findings also suggest a connection between ER stress and JAGN1. Nevertheless, UPR activation is not the primary cause of JAGN1-associated neutropenia. In this study, we observed elevated expression levels of the UPR-associated genes atf4 and ddit3 and the Chop targets ero1a, ero1b, and ppp1r15a following overexpression of jagn1bΔT12-G14. The upregulation of these genes is consistent with the activation of the PERK/ATF4/CHOP pathway. In jagn1b morphants, we saw upregulation of ddit3 and unspliced xbp1, downstream targets of the ATF6 pathway.51,52 However, there was no significant increase in atf4 expression. These results are consistent with the activation of the ATF6 pathway. Furthermore, we saw a trend of induction of spliced xbp1 in jagn1b morphants, which could be a hint for the activated IRE1 pathway. Surprisingly, the Chop target genes ero1a and ero1b were unaffected upon jagn1b knockdown. However, we need to consider that the chosen time point for UPR measurement may not have captured or reflected the expression of these target genes. Our results indicate that different JAGN1 mutations activate different UPR pathways. These data are consistent with our previous observations of ELANE mutant-specific activation of different UPR pathways.32 The different mutations may also alter the structure of JAGN1 differently, leading to various degrees of accumulation of misfolded proteins inducing UPR. JAGN1 has also been described as essential for proper protein trafficking.3 Its absence or mutation can affect the interaction with transport-related proteins, for example components of the coat protein I complex,6 and affect the transport of different proteins, leading to stagnation and subsequent activation of UPR. Further experiments are required to elucidate JAGN1 mutation-specific activated UPR pathways. In addition, functional inhibition of the UPR pathways is required to demonstrate conclusively that the activation is not responsible for neutropenia. Based on our results, however, we would expect that the inhibition of PERK/ATF4/CHOP or ATF6 pathway would not restore granulopoiesis. It is, therefore, very likely that UPR activation is one of the multiple affected processes downstream of mutated JAGN1.
Third, our experiments revealed that cell death alone cannot induce neutropenia in zebrafish. The mechanism proposed for neutropenia in CN-associated genes, such as HAX1,53G6PC3,54WAS,55SBDS,56GFI1,57 and ELANE,48 converge in a final common process involving apoptosis of granulocytic precursors. Similarly, there is evidence that suggests that JAGN1 plays a role in apoptosis. Experiments conducted with JAGN1-CN–associated mutations in HL-60 cells demonstrated calpain-dependent cell death activation.9 In addition, JAGN1 promotes the expression of TRAIL-R1 in primary effusion lymphoma cell lines.11 When apoptosis was induced with STS in JAGN1-deficient neutrophils, a higher frequency of susceptibility was observed when compared with neutrophils from healthy individuals.6 In line with these findings, we observed enhanced apoptosis in jagn1b morphants and a higher susceptibility to apoptosis induction with STS. However, it did not affect neutrophil development when we induced apoptosis pharmacologically by treating WT or jagn1b morphants from 24 to 48 hpf with STS. It is worth noting that a previous study has shown that continuous administration of STS from 6 hpf to 72 hpf in zebrafish embryos lead to neutropenia.58 The induction of apoptosis at very early stages of embryonic development affects granulopoiesis and impairs organogenesis (eg, heart, liver, and vascularization).58 The main reason for selecting the 24 hpf time window for our treatment was to minimize the side-effects on early embryonic development and primitive granulopoiesis. The fact that apoptosis was induced in jagn1b morphants but not in embryos injected with jagn1bΔT12-G14 mRNA can be explained by their degree of UPR response and ER stress. For example, the downregulation of ppp1r15a, as seen in jagn1b morphants, but not in jagn1bΔT12-G14 mRNA-injected embryos, has been associated with increased apoptosis.59 Whether Jagn1bΔT12-G14 leads to apoptosis at a later time point of granulopoiesis needs to be elucidated. Our observations suggest that it is likely that the activation of UPR and subsequent cell death following jagn1b impairment is not the primary mechanism that leads to neutropenia in zebrafish. Nevertheless, further studies are required to evaluate the implication of apoptosis in neutrophil development conclusively.
Fourth, there is valid evidence of deregulated G-CSFR signaling in patients with JAGN1-CN. Boztug et al demonstrated a significant reduction in G-CSFR expression on neutrophils among patients with JAGN1-CN, indicating potential G-CSFR glycosylation defects.6 Wirnsberger et al found reduced phosphorylation of the signal transducer and activator 3 (STAT3) in G-CSF–treated Jagn1-deficient neutrophils, despite normal G-CSFR levels. Consistent with the findings of Boztug et al, we observed a substantial decrease in csf3r mRNA expression following jagn1b downregulation. This coincided with the downregulation of cebpa mRNA levels and an increase in cebpb expression. The elevated expression of the latter factor in untreated jagn1b morphants is likely a compensatory mechanism employed by hematopoietic cells to counterbalance the reduced cebpa expression and to attempt to initiate granulopoiesis in the absence of jagn1b, albeit with limited efficiency. Interestingly, the induced expression of cebpb correlates with activated csf3a but not csf3r expression. These findings confirm the differential signaling outcomes between emergency granulopoiesis triggered by Cebpb- and Cebpa-regulated steady-state granulopoiesis. These data align with our previous observations that showed a diminished steady-state and enhanced emergency granulopoiesis in patients with CN.44
Notably, the expression levels of the studied candidate genes in this study were quantified from whole embryo mRNA and not specifically in hematopoietic cells. Granulopoiesis in patients with CN is known to arrest at the promyelocytic stage, and sorting of promyelocytic cells would be essential to analyze the effects of Jagn1b alteration on granulopoiesis. However, the corresponding developmental stages of neutrophils in zebrafish are not as distinct as in humans. Therefore, we hypothesized that the changes in expression levels seen in the whole embryo would, at least, partially recapitulate the situation in promyelocytic cells. Additional WISH examination, solely focusing our analysis on the hematopoietic tissue, also confirmed some of the RT-PCR results.
Despite the evolutionary conservation of granulopoiesis among vertebrates,60-62 there might be species-specific adaption during the evolution. In mice, conditional knockout of Jagn1 or transgenic expression of JAGN1-CN–specific mutations had no effect on neutrophil development.10 Similarly, mice deficient in Hax163 or G6pc364 or Elane (p.G193X) transgenic mice31 displayed either no or mild neutropenia. In contrast, zebrafish are a suitable model organisms to study neutropenia. One potential limitation of the zebrafish model is the challenge of identifying orthologs of human genes within its genome. There are 2 zebrafish homologs of human JAGN1, namely jagn1a and jagn1b, with high sequence similarity but nonredundant functions in hematopoiesis. Although jagn1a plays a role in immature HSCs, jagn1b expression and its specific involvement in granulopoiesis strongly support its function as the ortholog of human JAGN1.
Acknowledgments
The authors thank Advaita Dick and Anna-Sophia Hellmuth (University Hospital Tuebingen) for technical support, Patrick Mueller (University of Konstanz) for providing the draculin:mCherry reporter line, Daniel Capek (University of Konstanz) for technical help, and the Institute of Medical Virology and Microbiology for continuous support in confocal microscopy. The authors also acknowledge Euro-BioImaging for providing access to Zeiss LSM880 and services at the Austrian BioImaging/CMI node. This research was supported using resources of the VetCore Facility (VetImaging) of the University of Veterinary Medicine Vienna.
This work was supported by the German Research Foundation, Madeleine Schickedanz Kinderkrebsstiftung, and EuNet-INNOCHRON COST action.
Some of the graphs, including the visual abstract, were created using BioRender.com.
Authorship
Contribution: L.D. and B.B. made initial observations; L.D. designed the work, performed the experiments, and analyzed the data; J.S. and K.W. provided useful insights and interpreted the data; B.B. supervised the work and interpreted the data; and B.B., L.D., and J.S. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for B.B. is Austrian BioImaging/CMI, Vienna, Austria.
Correspondence: Julia Skokowa, Department of Oncology, Hematology, Clinical Immunology and Rheumatology, University Hospital Tuebingen, Otfried-Mueller Str 10, Tuebingen 72076, Germany; email: Julia.skokowa@med.uni-tuebingen.de; and Baubak Bajoghli, Austrian Bioimaging/CMI, Vienna Biocenter Campus, Dr Bohr-Gasse-3, 1030-Vienna, Austria; email: Baubak.bajoghli@vbcf.ac.at.
References
Author notes
J.S. and B.B. contributed equally to this study.
Data are available on reasonable request from the corresponding authors, Julia Skokowa (Julia.skokowa@med.uni-tuebingen.de) and Baubak Bajoghli (Baubak.bajoghli@vbcf.ac.at).
The full-text version of this article contains a data supplement.