TO THE EDITOR:
The curative potential of allogeneic hematopoietic stem cell transplantation (allo-HSCT) is limited by the toxic chemotherapy- and irradiation–based conditioning regimens used to prepare the patients for transplantation.1,2 The risk of severe adverse events related to these toxicities may prevent older or infirm patients from accessing the lifesaving benefits of HSCT. Thus, mitigating conditioning-related toxicities will broaden the clinical indications and patient population for which HSCT can be safely used.
Immunotherapeutic conditioning approaches that selectively deplete host hematopoietic stem cells (HSCs) to allow donor engraftment would serve this unmet need. Cellular3,4 and antibody–based conditioning strategies5-7 for making marrow space for HSCT without chemotherapy or irradiation have been explored in preclinical models and early human trials. Several studies have used antibody-drug conjugates (ADC) targeting CD45 or CD117 (c-Kit) to deliver payloads, such as saporin and pyrrolobenzodiazepine dimers, to the host hematopoietic niche, enabling multilineage engraftment in murine allo-HSCT models.6,8-11
However, although ADCs can reduce off-target effects by directing their toxicities toward specific cell types, systemic exposure to ADC payloads nevertheless causes toxicities via ADC internalization by healthy cells and/or premature payload release in circulation.12 Even after on-target ADC uptake, lipophilic small molecule payloads can diffuse into nearby cells and exert bystander toxicities.13,14 Indeed, toxicity concerns halted the phase 3 clinical trials of the pyrrolobenzodiazepine–based CD33-ADC vadastuximab talirine and may impede the application of ADCs to HSCT conditioning. Toxic payload-free antibody–based conditioning would have the most favorable long-term safety profile, making it an especially attractive option if effective.
Preclinical reports targeting CD47 and c-Kit to condition for HSCT15,16 demonstrate the potential of a payload-free antibody–based strategy. Anti-CD47 blocks a “don’t eat me” signal and lowers the threshold for phagocytosis, and anti-c-Kit opsonizes HSCs providing an additional signal for killing by phagocytes. Simultaneous anti-CD47/Kit targeting depleted the HSC niche (supplemental Figure 1) and enabled an efficient syngeneic HSCT.15 However, for allo-HSCT, 4 additional antibodies (CD4, CD8, CD122, and CD40L) are necessary to prevent rejection by T and natural killer cells. This 6-antibody regimen enabled ∼50% donor myeloid lineage chimerism in fully mismatched allo-HSCT.16 Previously, we showed that the balanced Janus kinase 1/2 (JAK1/2) inhibitors ruxolitinib and baricitinib enabled HSCT across major histocompatibility complex barriers when combined with CD45- or c-Kit-ADC via the suppression of host T and natural killer cell responses.10 Therefore, we hypothesized that JAK1/2 inhibition could substitute for the 4 immunosuppressive antibodies used previously in conjunction with anti-CD47/c-Kit, enabling alloengraftment while considerably simplifying this promising conditioning strategy.
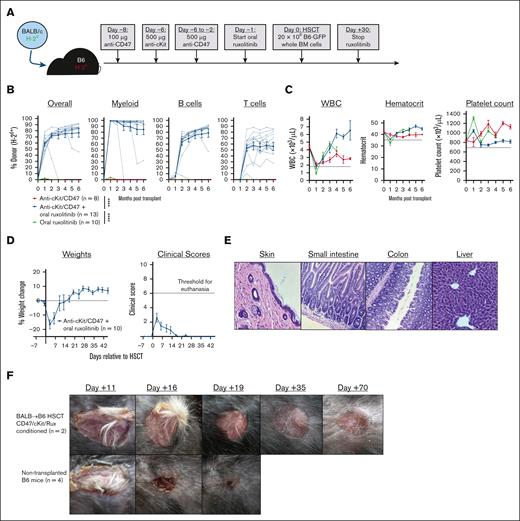
Although neither anti-CD47/c-Kit nor ruxolitinib alone enabled engraftment in fully mismatched (BALB/c→B6) allo-HSCT (Figure 1A-B),15,16 combining these agents allowed stable multilineage engraftment with normal complete blood counts and ∼80% overall donor chimerism, including up to 99% donor chimerism among myeloid cells (Figure 1B-C; supplemental Figure 2). Multilineage engraftment in serial transplantation studies confirmed that marrow from BALB→B6 transplant recipients conditioned with anti-CD47/c-Kit plus ruxolitinib contained functional donor–derived HSCs (supplemental Figure 3). Allo-HSCT experiments combining anti-CD47/c-Kit conditioning with different durations of ruxolitinib treatment showed minimal improvement in donor chimerism from either starting ruxolitinib early (on day –3 vs day –1) or prolonging treatment (to day +60 vs day +30; supplemental Figure 4). Finally, although ruxolitinib was essential for engraftment in allo-HSCT, it was dispensable for syngeneic (B6-GFP→B6) HSCT, which is consistent with our prior finding that JAK1/2 inhibition promotes alloengraftment via immunosuppression (supplemental Figure 5).
Anti-c-Kit/CD47 plus oral ruxolitinib enables fully mismatched murine allogeneic transplantation with high-level multilineage engraftment. (A) Experimental schema for BALB/c→B6 allo-HSCT. (B-C) Longitudinal analysis of donor chimerism by cell lineage (B) and complete blood count (C) in mice that received transplantation. In panel B, the dotted lines represent donor chimerism for individual mice and solid lines are means. In panel C, the horizontal dotted lines indicate the lower end of the reference range for each cell count. (D) Body weight and graft-versus-host disease clinical scoring during the peritransplant period after conditioning and HSCT as per panel (A). (E) Hematoxylin and eosin staining of representative formalin-fixed paraffin-embedded sections (from n = 2 recipient mice) of dorsal skin, small intestine, colon, and liver 45 days after HSCT from mice conditioned and transplanted as per panel A (original magnification ×200). Histology slides were viewed with an Accu-Scope EXC-120 light microscope (10× eyepiece with 18× mm field of view and 20× objective/0.40 numerical aperture, 20°C in air). (F) Successful BALB/c→B6 HSCT recipients or nontransplanted B6 mice were surgically engrafted with skin from BALB/c mice. Photographs of panels E and F were taken using an Apple iPhone 15. Numerical data are presented as mean ± standard error of the mean. Two-way analysis of variance for repeated measures was used in panel B; ∗∗∗∗P < .0001. BM, bone marrow; WBC, white blood cell count.
Anti-c-Kit/CD47 plus oral ruxolitinib enables fully mismatched murine allogeneic transplantation with high-level multilineage engraftment. (A) Experimental schema for BALB/c→B6 allo-HSCT. (B-C) Longitudinal analysis of donor chimerism by cell lineage (B) and complete blood count (C) in mice that received transplantation. In panel B, the dotted lines represent donor chimerism for individual mice and solid lines are means. In panel C, the horizontal dotted lines indicate the lower end of the reference range for each cell count. (D) Body weight and graft-versus-host disease clinical scoring during the peritransplant period after conditioning and HSCT as per panel (A). (E) Hematoxylin and eosin staining of representative formalin-fixed paraffin-embedded sections (from n = 2 recipient mice) of dorsal skin, small intestine, colon, and liver 45 days after HSCT from mice conditioned and transplanted as per panel A (original magnification ×200). Histology slides were viewed with an Accu-Scope EXC-120 light microscope (10× eyepiece with 18× mm field of view and 20× objective/0.40 numerical aperture, 20°C in air). (F) Successful BALB/c→B6 HSCT recipients or nontransplanted B6 mice were surgically engrafted with skin from BALB/c mice. Photographs of panels E and F were taken using an Apple iPhone 15. Numerical data are presented as mean ± standard error of the mean. Two-way analysis of variance for repeated measures was used in panel B; ∗∗∗∗P < .0001. BM, bone marrow; WBC, white blood cell count.
Notably, anti-CD47/c-Kit-conditioned mice showed constitutional signs of distress (fur ruffling, reduced activity) beginning 1 to 2 days pre-HSCT and resolving by 1 week after HSCT. However, the mice showed no clinical signs (diarrhea, skin lesions) or histopathological evidence of graft-versus-host disease during the peritransplant period (Figure 1D-E). The observed morbidity likely resulted from the transient but profound anemia that has been reported with anti-CD47/c-Kit conditioning.15,16 CD47 blockade induces phagocytic red blood cell (RBC) clearance,17 but because both anti-CD47 and anti-c-Kit were necessary for the development of severe anemia (supplemental Figure 1D), we speculate that the anemia results from RBC destruction combined with the inability of the depleted HSC niche to compensate via increased erythropoiesis. Although anti-CD47/c-Kit conditioned mice had similarly low hematocrits on day 0 with or without ruxolitinib starting on day –1 (supplemental Figure 1C), we considered that longer pre-HSCT exposure to ruxolitinib may exacerbate anemia due to inhibition of JAK2-mediated erythropoietin signaling. Consistently, mice conditioned with anti-CD47/c-Kit plus ruxolitinib starting at day –3 had mildly worsened anemia by day 0 compared with mice starting ruxolitinib on day –1, but this did not significantly increase mortality (supplemental Figure 6).
The long-term coexistence of donor- and recipient–derived T cells in successfully transplanted mice without graft rejection or graft-versus-host disease suggested achievement of stable cross-tolerance. Indeed, BALB→B6 HSCT recipients conditioned with anti-CD47/c-Kit plus ruxolitinib tolerated the engraftment of BALB/c skin (Figure 1F). As a secondary tolerance test, we infused carboxyfluorescein diacetate succinimidyl ester-labeled T cells purified from BALB/c→B6 transplant recipients (containing B6- and BALB/c-derived T cells) into new cohorts of B6, BALB/c, and SJL mice (supplemental Figure 7). Transferred T cells of BALB/c (H-2Kd+) or B6 (H-2Kb+) origin did not proliferate upon adoptive transfer to BALB/c or B6 mice but both proliferated upon infusion into the third-party SJL strain. As expected, T cells from B6 mice that failed to engraft BALB/c HSCs after anti-CD47/c-Kit conditioning alone remained alloreactive to BALB/c (supplemental Figure 8). Thus, JAK1/2 inhibitor treatment during the peritransplant period suppresses the acute host T-cell response to alloantigen and promotes durable donor-specific tolerance. We are investigating several mechanistic aspects of tolerance induction by JAK1/2 inhibitors, including the role of specific JAKs and the effect of JAK inhibitors on donor- and host–derived conventional and regulatory T-cell responses.
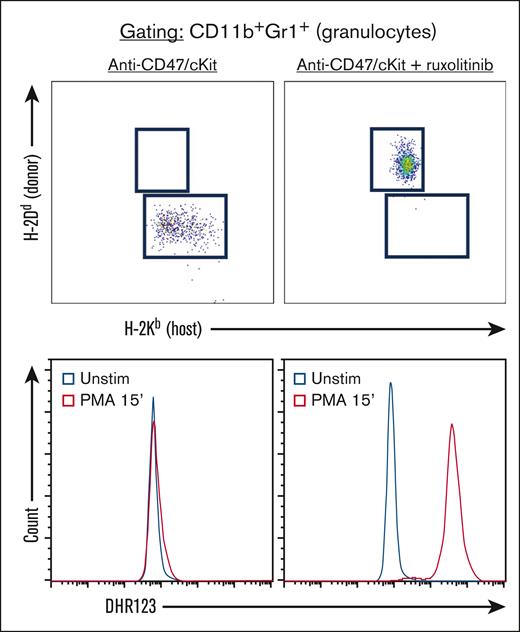
Finally, the high donor chimerism among myeloid cells, particularly granulocytes, suggested that anti-CD47/c-Kit plus ruxolitinib would be effective conditioning for allo-HSCT aimed at correcting inherited deficits in this lineage. We, therefore, applied this approach to the gp91phox–/Y mouse model of chronic granulomatous disease,18 in which affected hemizygous males lack the catalytic subunit of NADPH oxidase necessary for phagocyte oxidative burst. Mice conditioned with anti-CD47/c-Kit plus ruxolitinib robustly converted to full donor granulopoiesis upon engraftment of BALB/c HSCs, with the restoration of an intact oxidative burst (Figure 2). Although stable mixed chimerism among myeloid cells may be sufficient to resolve the infectious and inflammatory sequelae of chronic granulomatous disease,19,20 maximizing donor chimerism remains beneficial for minimizing graft failure risk and potentially for autologous gene therapies, in which a low efficiency of gene correction would limit the frequency of functionally normal cells even if full donor chimerism was achieved. Future studies will assess whether successfully transplanted gp91phox–/Y mice mount appropriate responses to sterile inflammation and live pathogen challenge.21
Anti-CD47/c-Kit plus ruxolitinib enables fully mismatched allo-HSCT, which corrects the defective phagocyte oxidative burst in the gp91phox–/Y mouse model of chronic granulomatous disease. Peripheral blood donor chimerism (top panels) and Dihydrorhodamine 123 staining after stimulation with phorbol 12-myristate-13-acetate (PMA) or treatment with dimethyl sulfoxide vehicle control (Unstim; bottom panels) in gp91phox–/Y mice transplanted as per the protocol in Figure 1A, using the indicated conditioning treatments (t = 3 months after HSCT, n = 4 mice per group). All plots are gated for CD11b+Gr1hi cells.
Anti-CD47/c-Kit plus ruxolitinib enables fully mismatched allo-HSCT, which corrects the defective phagocyte oxidative burst in the gp91phox–/Y mouse model of chronic granulomatous disease. Peripheral blood donor chimerism (top panels) and Dihydrorhodamine 123 staining after stimulation with phorbol 12-myristate-13-acetate (PMA) or treatment with dimethyl sulfoxide vehicle control (Unstim; bottom panels) in gp91phox–/Y mice transplanted as per the protocol in Figure 1A, using the indicated conditioning treatments (t = 3 months after HSCT, n = 4 mice per group). All plots are gated for CD11b+Gr1hi cells.
In conclusion, anti-CD47/c-Kit plus ruxolitinib provided a simple, effective conditioning regimen enabling fully mismatched allo-HSCT without radiation, chemotherapy, or toxic ADC payloads. However, anemia remains a major cause of morbidity and mortality after anti-CD47/c-Kit targeting, warranting further optimization to improve the safety of this strategy. Because the Fc region of anti-CD47 is required for erythrocyte toxicity,22,23 we are developing fragment crystallizable (Fc)-silenced anti-CD47 targeting reagents that we hypothesize will enable HSC depletion in combination with anti-c-Kit but without inducing anemia. Selective JAK inhibitors devoid of activity against JAK2, if effective as allo-HSCT conditioning agents, could also be beneficial by avoiding undesired antagonism of erythropoietin signaling. Such modifications would bolster the utility of this conditioning strategy for inherited RBC diseases, with great potential to reduce the global burden of diseases, such as sickle cell anemia.
Acknowledgments: The authors thank the Flow Cytometry Core Facility of the Alvin J. Siteman Cancer Center at the Washington University School of Medicine and Barnes-Jewish Hospital in St. Louis, MO, for providing flow cytometer access (supported in part by the NCI Cancer Center support grant P30CA091842). The authors also thank Mary Dinauer for helpful discussions regarding the experiments with the gp91phox–/Y mouse model.
This study was funded by the National Institutes of Health (NIH)/National Cancer Institute (NCI) grant R35CA210084 (J.F.D., including a Research Supplement to Promote Diversity to S.P.P.), NIH/NCI Leukemia SPORE grant (P50CA171963; J.F.D.), NIH/NCI Leukemia SPORE Career Enhancement and Developmental Research Awards (P50CA171063; S.P.P.), ASTCT New Investigator Award (S.P.P.), American Society of Hematology (ASH) Scholar Award (S.P.P.), and awards from Gabrielle's Angel Foundation for Cancer Research (S.P.P.).
Contribution: S.P.P. and J.F.D. designed the research; S.P.P., A.R.Y., and J.K.R. performed the experiments; S.P.P. and J.F.D. wrote the manuscript; and all authors read and approved the manuscript before submission.
Conflict-of-interest disclosure: J.F.D discloses the following conflicts of interest: equity stock/ownership in Magenta Therapeutics and WUGEN; consulting fees from Incyte; board or advisory committee membership from RiverVest Venture Partners, hC Bioscience, Inc; research funding from NeoImmune Tech, Macrogenics, Incyte, BioLineRx, and WUGEN; and holds patents with WUGEN. The remaining authors declare no competing financial interests.
Correspondence: John F. DiPersio, Division of Oncology, Department of Medicine, Washington University School of Medicine, 660 S. Euclid Ave, Campus Box 8007, St. Louis, MO 63110; email: jdipersi@wustl.edu.
References
Author notes
Materials and protocols will be available upon reasonable request from the authors, Stephen P. Persaud (persaud@wustl.edu) and John F. DiPersio (jdipersi@wustl.edu).
The full-text version of this article contains a data supplement.