Key Points
Pre-HCT CMV reactivation occurred in ∼11% patients and was associated post-HCT CMV reactivation and disease.
Clearance of pre-HCT CMV reactivation was associated with reduced risk of post-HCT CMV reactivation.
Visual Abstract
Cytomegalovirus (CMV) disease occurs occasionally before allogeneic hematopoietic cell transplantation (HCT) and is associated with poor post-HCT outcomes; however, the impact of pre-HCT CMV reactivation is unknown. Pre-HCT CMV reactivation was assessed in HCT candidates from the preemptive antiviral therapy (2007-2017) and letermovir prophylaxis (2018-2021) eras. CMV DNA polymerase chain reaction (PCR) surveillance was routinely performed during the pre-HCT workup period, and antiviral therapy was recommended according to risk of progression to CMV disease. Risk factors for pre-HCT CMV reactivation were characterized, and the associations of pre-HCT CMV reactivation with post-HCT outcomes were examined using logistic regression and Cox proportional hazard models, respectively. A total of 1694 patients were identified, and 11% had pre-HCT CMV reactivation 14 days (median; interquartile range [IQR], 6-23) before HCT. Lymphopenia (≤0.3 × 103/μL) was the strongest risk factor for pre-HCT CMV reactivation at multiple PCR levels. In the preemptive therapy era, patients with pre-HCT CMV reactivation had a significantly increased risk of CMV reactivation by day 100 as well as CMV disease and death by 1 year after HCT. Clearance of pre-HCT CMV reactivation was associated with a lower risk of post-HCT CMV reactivation. Similar associations with post-HCT CMV end points were observed in a cohort of patients receiving letermovir prophylaxis. Pre-HCT CMV reactivation can be routinely detected in high-risk HCT candidates and is a significant risk factor for post-HCT CMV reactivation and disease. Pre-HCT CMV DNA PCR surveillance is recommended in high-risk HCT candidates, and antiviral therapy may be indicated to prevent post-HCT CMV reactivation.
Introduction
Cytomegalovirus (CMV) is a human betaherpesvirus that reactivates in ∼60% to 70% of latently infected, CMV-seropositive hematopoietic cell transplantation (HCT) recipients after HCT.1 CMV reactivation after HCT, as detected by CMV DNA polymerase chain reaction (PCR), is associated with increased HCT-related mortality2 as well as an increased risk of CMV disease after HCT in the absence of preemptive antiviral therapy.3-5 For these reasons, CMV DNAemia is now accepted by regulatory agencies as a surrogate end point for clinical outcomes after HCT.6
Previous studies have examined pre-HCT characteristics as potential risk factors for post-HCT CMV reactivation in CMV-seropositive individuals (supplemental Table 1), but few risk factors were consistently significant across studies. Some larger studies included statistically significant risks associated with newer in vivo T-cell depletion and posttransplantation cyclophosphamide prophylaxis strategies; these therapies can impair T-cell function and possibly increase the risk of CMV reactivation. However, the observed effect sizes in these studies have been relatively small, limiting their clinical value in risk prediction. Other studies were limited by their small sample size or lacked time-to-event or multivariable analytical methods. In addition, the introduction of letermovir prophylaxis has changed how many providers approach risk stratification and treatment of post-HCT CMV reactivation,7 rendering most of these studies inapplicable to the current age of antiviral prophylaxis.
We previously showed that CMV disease diagnosed before HCT, although infrequent (∼0.3%) and often mild, is also associated with an substantially increased risk of CMV disease and mortality after HCT.8 Based on our findings with pretransplant CMV disease8 and anecdotal cases of viremia during the preconditioning workup period resulting in post-HCT complications, in 2007, we instituted routine CMV DNA PCR surveillance and preemptive therapy of all HCT candidates within 4 weeks before the transplant conditioning regimen.
In this study, we sought to systematically evaluate the incidence and risk factors for pre-HCT CMV detection by CMV DNA PCR, examine the impact of pre-HCT CMV PCR DNAemia kinetics as a risk factor for post-HCT CMV events, and explore whether clearance of CMV DNAemia using preemptive antiviral therapy before HCT was associated with better post-HCT outcomes. Furthermore, we independently assessed the influence of pre-HCT CMV on post-HCT CMV in patients who underwent HCT in the current letermovir era.
Methods
Study design and participants
All CMV-seropositive allogeneic HCT recipients who underwent allogeneic HCT at our center from 2007 to 2017 during the preletermovir era were considered for this study. An additional cohort of first allogeneic HCT recipients from 2018 to 2021 who received letermovir prophylaxis after HCT were examined independently.9 Baseline clinical and transplantation data were extracted from the electronic medical record. The study was approved by our institutional review board; all participants provided written informed consent.
Procedures
Beginning in 2007, all HCT recipients underwent routine pre-HCT surveillance regardless of recipient CMV serostatus. Specifically, plasma CMV DNA PCR was performed during the pre-HCT workup period (∼3-4 weeks before HCT at our center). Differences in CMV DNA PCR assay characteristics across the study period are described in the supplemental Methods.
Preemptive antiviral therapy with ganciclovir, valganciclovir, or foscarnet was recommended for pre-HCT CMV reactivation at ≥150 and ≥50 IU/mL in low- and high-risk HCT recipients, respectively. These values were decided based on the testing characteristics of our CMV DNA PCR assays and with the intention to intervene early to prevent progression to disease. Repeat testing was recommended in patients with pre-HCT CMV DNAemia below these thresholds. Patients who received preemptive therapy did not require a documented negative PCR result prior to receiving conditioning, but generally required declining CMV DNAemia to proceed. In addition, delay of transplantation was not routinely recommended, except for pre-HCT CMV disease or in cases of extraordinarily high pre-HCT CMV reactivation (ie, >2500 IU/mL). Institutional post-HCT CMV reactivation surveillance and management guidelines are summarized in the supplemental Methods.
Statistical analysis
Logistic regression was used to evaluate potential risk factors for pre-HCT CMV reactivation at multiple CMV DNAemia levels: any level, ≥150 IU/mL, and ≥500 IU/mL. Demographic and clinical factors evaluated as potential risk factors for pre-HCT CMV reactivation are listed in the supplemental Methods. Underlying disease categories were collapsed in the ≥150 and ≥500 IU/mL models due to a lower number of CMV events at these thresholds and are described further in the supplemental Methods. For all risk factor analyses, patients from the preemptive therapy and letermovir prophylaxis eras were combined, given that letermovir administration occurs after HCT.
Cox proportional hazards regression was used to estimate the association of pre-HCT CMV reactivation with multiple post-HCT CMV DNA PCR end points (ie, any level, ≥150 IU/mL, and ≥500 IU/mL) in the first 100 days after HCT as well as CMV disease, overall, and nonrelapse mortality up to 1 year after HCT. The thresholds of 150 and 500 IU/mL were examined because they represent the levels of preemptive antiviral therapy initiation for low- and high-risk patients with post-HCT CMV reactivation at our center, respectively. In addition, the threshold of 150 IU/mL approximates the limit of quantification of a commonly used assay at other institutions.10 The maximum level of pre-HCT CMV DNAemia was evaluated as a risk factor for post-HCT CMV. In an alternative iteration of these models, pre-HCT CMV was stratified by whether a patient received antiviral treatment for CMV DNAemia. Patients from the preemptive antiviral therapy and letermovir prophylaxis eras were examined independently. Other variables evaluated in the Cox proportional hazards models are listed in the supplemental Methods.
The cumulative incidence of post-HCT CMV reactivation in the first 100 days was calculated for each post-HCT CMV DNA PCR end point and CMV disease by 1 year after HCT. Analyses were stratified by the maximum level of pre-HCT CMV DNAemia or by last pre-HCT CMV DNA PCR test result (ie, positive vs negative) in the 7 to 90 days before HCT. Death was treated as a competing risk in all these models. Kaplan-Meier analyses of overall mortality and cumulative incidence of nonrelapse mortality by 1 year after HCT were calculated and stratified by the presence vs absence of any pre-HCT CMV reactivation. In an alternative iteration of these models, patients without pre-HCT CMV reactivation were combined with patients who had low-level (ie, <50 IU/mL) pre-HCT CMV DNAemia.
For all statistical analyses, the window for inclusion was extended from 4 weeks (ie, 28 days) to up to 90 days before HCT to capture pre-HCT CMV reactivation/disease events for which a planned HCT may have been delayed for other reasons.
Results
Patient population and incidence of pre-HCT CMV reactivation and disease
Of the 1536 CMV-seropositive patients who underwent allogeneic HCT at our center from 2007 to 2017, a total of 1367 patients (89%) were included in this study. Of the 343 CMV-seropositive patients who underwent allogeneic HCT at our center from 2018 to 2021 and received letermovir prophylaxis, a total of 342 patients (99.7%) were included in this study. Patients from both cohorts who were excluded due to missing pre-HCT CMV testing are summarized in supplemental Figure 1. Demographics and clinical characteristics for patients from both cohorts are shown in Table 1. There were some differences between patients from both eras (data not shown). For example, because letermovir is only US Food and Drug Administration approved for adult patients, no children aged <18 years are represented in the 2018 to 2021 era. There was a greater proportion of matched-related donors in the 2007 to 2017 era and mismatched/unrelated donors in the 2018 to 2021 era. More patients in the 2018 to 2021 era had underlying myelodysplastic syndrome/myeloproliferative neoplasms, higher Hematopoietic Cell Transplantation-Comorbidity Index (HCT-CI) scores (ie, >1), and received nonmyeloablative/reduced-intensity conditioning regimens as well as modern graft-versus-host disease (GVHD) prophylaxis regimens (eg, posttransplantation cyclophosphamide).
Patient demographics
| Categories . | Letermovir era∗ (n = 327) . | Preemptive therapy era (n = 1367) . | Combined (N = 1694) . |
|---|---|---|---|
| Age at transplant, y | |||
| 0-11 | 0 (0%) | 144 (11%) | 144 (9%) |
| 12-17 | 0 (0%) | 76 (6%) | 76 (4%) |
| 18+ | 327 (100%) | 1147 (84%) | 1474 (87%) |
| Race | |||
| White | 242 (74%) | 1043 (76%) | 1285 (76%) |
| American Indian or Alaska Native | 4 (1%) | 29 (2%) | 33 (2%) |
| Asian | 37 (11%) | 141 (10%) | 178 (11%) |
| Black or African American | 9 (3%) | 40 (3%) | 49 (3%) |
| Multiple | 7 (2%) | 36 (3%) | 43 (3%) |
| Native Hawaiian or other Pacific Islander | 7 (2%) | 25 (2%) | 32 (2%) |
| Unknown | 21 (6%) | 53 (4%) | 74 (4%) |
| Ethnicity | |||
| Hispanic or Latino | 34 (10%) | 130 (10%) | 164 (10%) |
| Not Hispanic or Latino | 274 (84%) | 1194 (87%) | 1468 (87%) |
| Unknown | 19 (6%) | 43 (3%) | 62 (4%) |
| Sex | |||
| Female | 153 (47%) | 645 (47%) | 798 (47%) |
| Male | 174 (53%) | 722 (53%) | 896 (53%) |
| Donor CMV serostatus | |||
| Negative | 176 (54%) | 789 (58%) | 965 (57%) |
| Positive | 151 (46%) | 578 (42%) | 729 (43%) |
| HLA | |||
| Matched related | 61 (19%) | 383 (28%) | 444 (26%) |
| Haploidentical | 31 (9%) | 90 (7%) | 121 (7%) |
| Cord | 38 (12%) | 198 (14%) | 236 (14%) |
| Unrelated/mismatched | 197 (60%) | 696 (51%) | 893 (53%) |
| Acute GVHD | |||
| Grade 0-2 | 261 (80%) | 1186 (87%) | 1447 (85%) |
| Grade 3-4 | 66 (20%) | 181 (13%) | 247 (15%) |
| Underlying disease risk by prognostic scoring† | |||
| High | 25 (8%) | 392 (29%) | 417 (25%) |
| Intermediate | 237 (72%) | 112 (8%) | 349 (21%) |
| Low | 65 (20%) | 863 (63%) | 928 (55%) |
| Lowest ALC in 7-90 d before HCT | |||
| Median (IQR) | 230.0 (20.0-670) | 330.0 (0.0-750) | 315.5 (10.0-720) |
| HCT-CI score | |||
| 0-1 | 126 (39%) | 508 (37%) | 634 (37%) |
| 2 | 41 (13%) | 208 (15%) | 249 (15%) |
| ≥3 | 124 (38%) | 581 (43%) | 705 (42%) |
| Unknown | 36 (11%) | 70 (5%) | 106 (6%) |
| Disease | |||
| AA | 15 (5%) | 51 (4%) | 66 (4%) |
| ALL | 53 (16%) | 191 (14%) | 244 (14%) |
| AML | 129 (39%) | 474 (35%) | 603 (36%) |
| CLL/PLL | 1 (0%) | 58 (4%) | 59 (3%) |
| HL | 3 (1%) | 22 (2%) | 25 (1%) |
| MDS | 70 (21%) | 205 (15%) | 275 (16%) |
| MPN | 46 (14%) | 102 (7%) | 148 (9%) |
| NHL | 2 (1%) | 114 (8%) | 116 (7%) |
| Plasma cell neoplasm (MM/PCL) | 5 (2%) | 67 (5%) | 72 (4%) |
| Primary immune deficiency | 1 (0%) | 38 (3%) | 39 (2%) |
| Nonmalignant disorders | 2 (1%) | 45 (3%) | 47 (3%) |
| GHVD prophylaxis | |||
| MMF + CNI | 64 (20%) | 576 (42%) | 640 (38%) |
| MTX + CNI | 117 (36%) | 495 (36%) | 612 (36%) |
| PTCy | 73 (22%) | 129 (9%) | 202 (12%) |
| Other ± CNI | 0 (0%) | 37 (3%) | 37 (2%) |
| Sirolimus based | 73 (22%) | 130 (10%) | 203 (12%) |
| Conditioning | |||
| MAC | 159 (49%) | 789 (58%) | 948 (56%) |
| NMA | 48 (15%) | 310 (23%) | 358 (21%) |
| RIC | 120 (37%) | 268 (20%) | 388 (23%) |
| Transplant no. | |||
| 1 | 317 (97%) | 1180 (86%) | 1497 (88%) |
| >1 | 10 (3%) | 187 (14%) | 197 (12%) |
| Categories . | Letermovir era∗ (n = 327) . | Preemptive therapy era (n = 1367) . | Combined (N = 1694) . |
|---|---|---|---|
| Age at transplant, y | |||
| 0-11 | 0 (0%) | 144 (11%) | 144 (9%) |
| 12-17 | 0 (0%) | 76 (6%) | 76 (4%) |
| 18+ | 327 (100%) | 1147 (84%) | 1474 (87%) |
| Race | |||
| White | 242 (74%) | 1043 (76%) | 1285 (76%) |
| American Indian or Alaska Native | 4 (1%) | 29 (2%) | 33 (2%) |
| Asian | 37 (11%) | 141 (10%) | 178 (11%) |
| Black or African American | 9 (3%) | 40 (3%) | 49 (3%) |
| Multiple | 7 (2%) | 36 (3%) | 43 (3%) |
| Native Hawaiian or other Pacific Islander | 7 (2%) | 25 (2%) | 32 (2%) |
| Unknown | 21 (6%) | 53 (4%) | 74 (4%) |
| Ethnicity | |||
| Hispanic or Latino | 34 (10%) | 130 (10%) | 164 (10%) |
| Not Hispanic or Latino | 274 (84%) | 1194 (87%) | 1468 (87%) |
| Unknown | 19 (6%) | 43 (3%) | 62 (4%) |
| Sex | |||
| Female | 153 (47%) | 645 (47%) | 798 (47%) |
| Male | 174 (53%) | 722 (53%) | 896 (53%) |
| Donor CMV serostatus | |||
| Negative | 176 (54%) | 789 (58%) | 965 (57%) |
| Positive | 151 (46%) | 578 (42%) | 729 (43%) |
| HLA | |||
| Matched related | 61 (19%) | 383 (28%) | 444 (26%) |
| Haploidentical | 31 (9%) | 90 (7%) | 121 (7%) |
| Cord | 38 (12%) | 198 (14%) | 236 (14%) |
| Unrelated/mismatched | 197 (60%) | 696 (51%) | 893 (53%) |
| Acute GVHD | |||
| Grade 0-2 | 261 (80%) | 1186 (87%) | 1447 (85%) |
| Grade 3-4 | 66 (20%) | 181 (13%) | 247 (15%) |
| Underlying disease risk by prognostic scoring† | |||
| High | 25 (8%) | 392 (29%) | 417 (25%) |
| Intermediate | 237 (72%) | 112 (8%) | 349 (21%) |
| Low | 65 (20%) | 863 (63%) | 928 (55%) |
| Lowest ALC in 7-90 d before HCT | |||
| Median (IQR) | 230.0 (20.0-670) | 330.0 (0.0-750) | 315.5 (10.0-720) |
| HCT-CI score | |||
| 0-1 | 126 (39%) | 508 (37%) | 634 (37%) |
| 2 | 41 (13%) | 208 (15%) | 249 (15%) |
| ≥3 | 124 (38%) | 581 (43%) | 705 (42%) |
| Unknown | 36 (11%) | 70 (5%) | 106 (6%) |
| Disease | |||
| AA | 15 (5%) | 51 (4%) | 66 (4%) |
| ALL | 53 (16%) | 191 (14%) | 244 (14%) |
| AML | 129 (39%) | 474 (35%) | 603 (36%) |
| CLL/PLL | 1 (0%) | 58 (4%) | 59 (3%) |
| HL | 3 (1%) | 22 (2%) | 25 (1%) |
| MDS | 70 (21%) | 205 (15%) | 275 (16%) |
| MPN | 46 (14%) | 102 (7%) | 148 (9%) |
| NHL | 2 (1%) | 114 (8%) | 116 (7%) |
| Plasma cell neoplasm (MM/PCL) | 5 (2%) | 67 (5%) | 72 (4%) |
| Primary immune deficiency | 1 (0%) | 38 (3%) | 39 (2%) |
| Nonmalignant disorders | 2 (1%) | 45 (3%) | 47 (3%) |
| GHVD prophylaxis | |||
| MMF + CNI | 64 (20%) | 576 (42%) | 640 (38%) |
| MTX + CNI | 117 (36%) | 495 (36%) | 612 (36%) |
| PTCy | 73 (22%) | 129 (9%) | 202 (12%) |
| Other ± CNI | 0 (0%) | 37 (3%) | 37 (2%) |
| Sirolimus based | 73 (22%) | 130 (10%) | 203 (12%) |
| Conditioning | |||
| MAC | 159 (49%) | 789 (58%) | 948 (56%) |
| NMA | 48 (15%) | 310 (23%) | 358 (21%) |
| RIC | 120 (37%) | 268 (20%) | 388 (23%) |
| Transplant no. | |||
| 1 | 317 (97%) | 1180 (86%) | 1497 (88%) |
| >1 | 10 (3%) | 187 (14%) | 197 (12%) |
AA, aplastic anemia; ALC, absolute lymphocyte count; ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; CLL, chronic myelogenous leukemia; CNI, calcineurin inhibitor; HL, Hodgkin lymphoma; IQR, interquartile range; MAC, myeloablative conditioning; MDS, myelodysplastic syndrome; MM, multiple myeloma; MMF, mycophenolate mofetil; MPN, myeloproliferative neoplasm; MTX, methotrexate; NHL, non-Hodgkin lymphoma; NMA, nonmyeloablative conditioning; PLL, prolymphocytic leukemia; PTCy, posttransplantation cyclophosphamide; RIC, reduced-intensity conditioning.
A total of 342 patients from the letermovir era were evaluated for risk factors for pre-HCT CMV, but only the subset included in the predictive analyses for post-HCT CMV reactivation (n = 327) are shown in Table 1.
Underlying disease risk was based upon prognostic scoring systems used institutionally for each respective disease.
A total of 155 patients (11%) from the 2007 to 2017 era had a positive CMV DNA PCR test before HCT, and 97 patients (7%) started preemptive antiviral therapy for CMV (Table 2). Similarly, 37 patients (11%) from the 2018 to 2021 era had a positive CMV DNA PCR test before HCT, and 23 (7%) were initiated on preemptive antiviral therapy. The median day onset for positive CMV DNA PCR test before HCT in either era was 14 days (interquartile range, 6-23 days) before HCT. The proportion of patients with pre-HCT CMV DNAemia at multiple levels, with or without treatment, are also shown in Table 2. Nine patients from the 2007 to 2017 era met criteria for the diagnosis of pre-HCT CMV disease (8 pulmonary and 1 gastrointestinal). Although no patients from the 2018 to 2021 era met definitive criteria for pre-HCT CMV disease, 1 patient had probable CMV pneumonia based on a CMV DNA PCR of 1098 IU/mL from bronchoscopic alveolar lavage. Of note, 35 patients in the letermovir era did not receive letermovir prophylaxis and were not considered for this study. Only 1 of 35 (3%) had pre-HCT CMV detection requiring preemptive antiviral therapy through HCT. Reasons for not receiving letermovir prophylaxis for the remaining patients were previously published.9
Proportion of patients who received PET or LET with pre-HCT CMV detected by CMV DNA PCR and median day of detection in the 90 days before HCT at each evaluated CMV DNA PCR threshold
| . | Any CMV detection within 7 to 90 d before HCT . | CMV detection ≥150 IU/mL within 7 to 90 d before HCT . | CMV detection ≥500 IU/mL within 7 to 90 d before HCT . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PET∗ (n = 1367) . | LET∗ (n = 342) . | PET∗ (n = 1367) . | LET∗ (n = 342) . | PET∗ (n = 1367) . | LET∗ (n = 342) . | |||||||
| n . | % . | n . | % . | n . | % . | n . | % . | n . | % . | n . | % . | |
| No | 1212 | 89% | 305 | 89% | 1314 | 96% | 326 | 95% | 1343 | 98% | 331 | 97% |
| Yes without anti-CMV treatment | 58 | 4% | 14 | 4% | ||||||||
| Yes with anti-CMV treatment | 97 | 7% | 23 | 7% | 53 | 4% | 16 | 5% | 24 | 2% | 11 | 3% |
| Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | |||||||
| Day of CMV onset closest to transplant (transplant day 0) ≤90 d before transplant | –14.0 (–23 to –6) | –12.5 (–25 to –8) | –20.0 (–33 to –10) | –15.0 (–21 to –8) | –32.0 (–43 to –20) | –21.0 (–41 to –18) | ||||||
| Day of CMV onset with maximum viral load (transplant day 0) ≤90 d before transplant | N/A | N/A | –26.0 (–52 to –16) | –21.0 (–34 to –15) | –39.0 (–70 to –20) | –22.0 (–41 to –18) | ||||||
| . | Any CMV detection within 7 to 90 d before HCT . | CMV detection ≥150 IU/mL within 7 to 90 d before HCT . | CMV detection ≥500 IU/mL within 7 to 90 d before HCT . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PET∗ (n = 1367) . | LET∗ (n = 342) . | PET∗ (n = 1367) . | LET∗ (n = 342) . | PET∗ (n = 1367) . | LET∗ (n = 342) . | |||||||
| n . | % . | n . | % . | n . | % . | n . | % . | n . | % . | n . | % . | |
| No | 1212 | 89% | 305 | 89% | 1314 | 96% | 326 | 95% | 1343 | 98% | 331 | 97% |
| Yes without anti-CMV treatment | 58 | 4% | 14 | 4% | ||||||||
| Yes with anti-CMV treatment | 97 | 7% | 23 | 7% | 53 | 4% | 16 | 5% | 24 | 2% | 11 | 3% |
| Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | |||||||
| Day of CMV onset closest to transplant (transplant day 0) ≤90 d before transplant | –14.0 (–23 to –6) | –12.5 (–25 to –8) | –20.0 (–33 to –10) | –15.0 (–21 to –8) | –32.0 (–43 to –20) | –21.0 (–41 to –18) | ||||||
| Day of CMV onset with maximum viral load (transplant day 0) ≤90 d before transplant | N/A | N/A | –26.0 (–52 to –16) | –21.0 (–34 to –15) | –39.0 (–70 to –20) | –22.0 (–41 to –18) | ||||||
Note that the most (75%) of any CMV detection occurred in the first 30 days before HCT, which approximates the recommended window for pre-HCT CMV surveillance during the pre-HCT workup period at our center (ie, 3-4 weeks before HCT).
IQR, interquartile range; LET, letermovir prophylaxis; PET, preemptive antiviral therapy; N/A, data unavilable.
Note that PET patients had a total of 2880 pre-HCT samples submitted for CMV DNA PCR testing; whereas LET patients had a total of 1234 pre-HCT samples submitted for CMV DNA PCR testing.
Risk factors for pre-HCT CMV reactivation
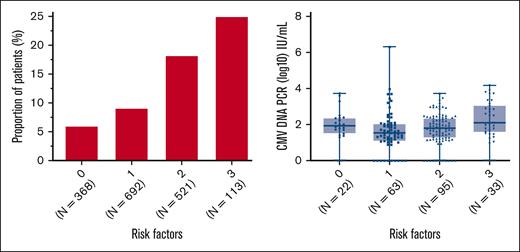
We evaluated multiple demographic and clinical factors as potential risk factors for pre-HCT CMV reactivation at multiple CMV DNA PCR levels (supplemental Table 2). Some of the strongest identified risk factors for pre-HCT CMV included HCT-CI scores ≥3, underlying lymphoid malignancy, and lymphopenia at 0.1-0.3 × 103/μL within 90 days before HCT (with a greater risk observed at <0.05-0.1× 103/μL). The proportion positive and levels of pre-HCT CMV DNAemia stratified by these risk factors are summarized for the combined cohort in supplemental Figure 2. The concurrent presence of all risk factors resulted in an approximate fivefold proportional increase in pre-HCT CMV reactivation compared with patients without any risk factors (Figure 1).
Proportion of patients with pre-HCT CMV reactivation and pre-HCT CMV DNA PCR levels by number of risk factors. Proportion of patients with detectable CMV DNAemia by PCR from 1 to 90 days before HCT (left) and corresponding levels of pre-HCT CMV DNAemia in these patients (right) stratified by the number of pre-HCT risk factors present, including HCT-CI score ≥3, absolute lymphocyte count ≤0.3× 103/μL, and underlying lymphoid malignancy.
Proportion of patients with pre-HCT CMV reactivation and pre-HCT CMV DNA PCR levels by number of risk factors. Proportion of patients with detectable CMV DNAemia by PCR from 1 to 90 days before HCT (left) and corresponding levels of pre-HCT CMV DNAemia in these patients (right) stratified by the number of pre-HCT risk factors present, including HCT-CI score ≥3, absolute lymphocyte count ≤0.3× 103/μL, and underlying lymphoid malignancy.
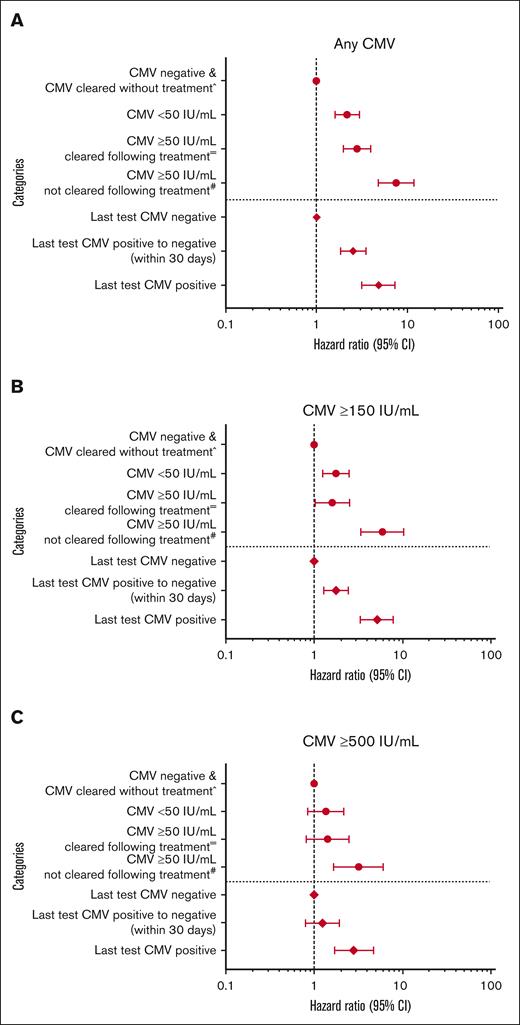
Multivariable logistic regression showed an increased risk of CMV reactivation before HCT for any level of lymphopenia (ie, ≤0.3× 103/μL), HCT-CI scores ≥3, and an underlying diagnosis of either lymphoma, primary immune deficiency, or other nonmalignant disorders (excluding aplastic anemia; Figure 2). Results for multivariable logistic regression models of pre-HCT CMV DNAemia at ≥150 and ≥500 IU/mL were similar overall to the “any” pre-HCT CMV reactivation analyses; however, underlying disease was not significant for ≥500 IU/mL. A proportional increase of pre-HCT CMV was observed in patients who self-reported as Asian race (supplemental Figure 3). In an alternative iteration of the logistic regression models (supplemental Figure 4), recipient race was included as an adjustment variable, and Asian race was associated with an increased risk of pre-HCT CMV reactivation at any level of CMV DNAemia alongside the previously mentioned variables.
Risk factors for pre-HCT CMV reactivation at multiple levels. Multivariable logistic regression of any (A), ≥150 IU/mL (B), or ≥500 IU/mL (C) viral load CMV in the 90 days before HCT transplant in patients from both eras. +Denotes the lowest pretransplant lymphocyte counts within 7 to 90 days before transplant. ∗Denotes inclusion of unknown or unreported HCT-CI scores. Note that underlying disease categories were collapsed in the pre-HCT reactivation ≥150 and ≥500 IU/mL models due to a lower number of events in each. Specifically, categories include the following: (1) lymphoid malignancies (acute lymphocytic leukemia [ALL], chronic lymphocytic leukemia [CLL], Hodgkin lymphoma [HL], non-Hodgkin lymphoma [NHL], and plasma cell neoplasms); (2) myeloid malignancies (acute myelogenous leukemia [AML], myelodysplastic syndrome [MDS], and other myeloproliferative neoplasms [MPNs]); and (3) Nonmalignant diseases (a variety of diseases such as aplastic anemia [AA] or primary immune deficiencies). 95% CI, 95% confidence interval; PLL, prolymphocytic leukemia.
Risk factors for pre-HCT CMV reactivation at multiple levels. Multivariable logistic regression of any (A), ≥150 IU/mL (B), or ≥500 IU/mL (C) viral load CMV in the 90 days before HCT transplant in patients from both eras. +Denotes the lowest pretransplant lymphocyte counts within 7 to 90 days before transplant. ∗Denotes inclusion of unknown or unreported HCT-CI scores. Note that underlying disease categories were collapsed in the pre-HCT reactivation ≥150 and ≥500 IU/mL models due to a lower number of events in each. Specifically, categories include the following: (1) lymphoid malignancies (acute lymphocytic leukemia [ALL], chronic lymphocytic leukemia [CLL], Hodgkin lymphoma [HL], non-Hodgkin lymphoma [NHL], and plasma cell neoplasms); (2) myeloid malignancies (acute myelogenous leukemia [AML], myelodysplastic syndrome [MDS], and other myeloproliferative neoplasms [MPNs]); and (3) Nonmalignant diseases (a variety of diseases such as aplastic anemia [AA] or primary immune deficiencies). 95% CI, 95% confidence interval; PLL, prolymphocytic leukemia.
Pre-HCT CMV reactivation as a predictor for post-HCT CMV reactivation and disease
The cumulative incidences of CMV DNAemia in the first 100 days after HCT were independently estimated at multiple levels for patients in both eras (Figure 3). To identify differences in the incidence of post-HCT CMV reactivation and disease in patients with and without pre-HCT CMV reactivation, cumulative incidence curves were stratified by the presence of any positive CMV DNA PCR test before HCT. For all examined post-HCT CMV end points, patients with any level of pre-HCT CMV reactivation had a greater incidence of post-HCT CMV in the first 100 days after HCT than patients without pre-HCT CMV (Figure 3A-C). In addition, we examined whether any differences in cumulative incidence were present in adults (≥18 years) vs children (<18 years), and a larger effect size was noted in children with pre-HCT CMV reactivation (supplemental Figure 5).
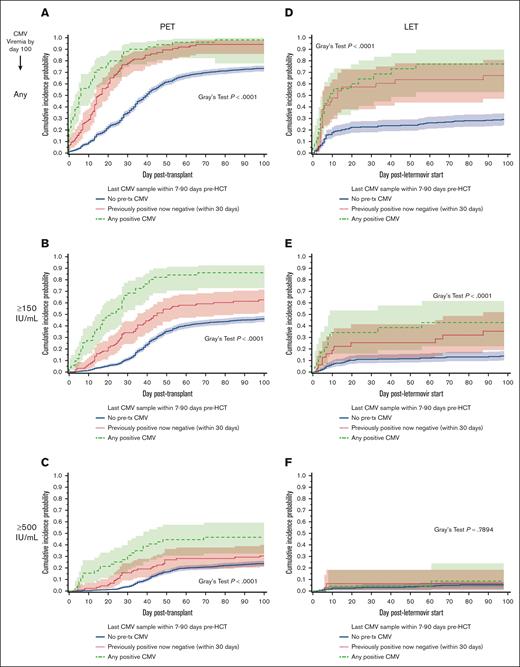
Cumulative incidences of CMV reactivation in the first 100 days post-HCT stratified by the presence of pre-HCT CMV DNAemia. Cumulative incidence curve showing the time to any CMV infection (A,D), to ≥150 IU/mL CMV infection (B,E), or to ≥500 IU/mL CMV infection (C,F), among preemptive therapy (PET)–era patients (n = 1367; A-C) or among participants receiving letermovir (LET) prophylaxis (n = 332; D-F) within the first 100 days after transplant, separated by whether any pretransplant CMV was detected by PCR (yes in red [top line]; no in blue [bottom line]). Lighter colored shading indicates the 95% confidence interval. In each graph, the difference between those with any pretransplant CMV PCR detection was calculated using Gray’s test; associated P values are listed on the right of the graphs.
Cumulative incidences of CMV reactivation in the first 100 days post-HCT stratified by the presence of pre-HCT CMV DNAemia. Cumulative incidence curve showing the time to any CMV infection (A,D), to ≥150 IU/mL CMV infection (B,E), or to ≥500 IU/mL CMV infection (C,F), among preemptive therapy (PET)–era patients (n = 1367; A-C) or among participants receiving letermovir (LET) prophylaxis (n = 332; D-F) within the first 100 days after transplant, separated by whether any pretransplant CMV was detected by PCR (yes in red [top line]; no in blue [bottom line]). Lighter colored shading indicates the 95% confidence interval. In each graph, the difference between those with any pretransplant CMV PCR detection was calculated using Gray’s test; associated P values are listed on the right of the graphs.
A total of 133 patients (10%) from the 2007 to 2017 era met the criteria for the diagnosis of CMV disease by 1 year after HCT; 74 (5%) in the first 100 days after HCT and 59 (4%) after post-HCT day 100. Of these patients, 24 (18%) had pre-HCT CMV reactivation detected by PCR. Patients with any level of pre-HCT CMV reactivation had a greater incidence of CMV disease up to 1 year after HCT than patients without pre-HCT CMV (supplemental Figure 6). The associations of pre-HCT CMV reactivation with CMV disease were supported by multivariable Cox regression models (supplemental Figure 7).
To examine whether clearance of CMV DNAemia before HCT may be associated with a decreased incidence of CMV in patients with pre-HCT CMV, cumulative incidence curves stratified by the last pre-HCT CMV DNA PCR test result were constructed (Figure 4). Specifically, patients were categorized as “positive,” “negative,” or “previously positive, now negative within 30 days,” based on their last CMV DNA PCR result before HCT. For all examined post-HCT CMV end points, patients with positive CMV DNAemia who proceeded to HCT had a greater incidence of post-HCT CMV than patients without pre-HCT CMV (Figure 4A-C). In addition, patients “previously positive, now negative within 30 days” also had a greater incidence of post-HCT CMV reactivation, although the incidence was lower than patients with positive CMV DNAemia who proceed to HCT.
Cumulative incidences of CMV reactivation in the first 100 days post-HCT stratified by the last pre-HCT CMV DNA PCR test result. Cumulative incidence curve showing the time to any CMV infection (A,D), time to CMV viral load ≥150 IU/mL (B,E), or time to CMV viral load ≥500 IU/mL (C,F), among PET era patients (n = 1367; A-C) or LET era patients (n = 332; D-F) in the first 100 days after HCT, dependent on the presence (green dashed line), absence (blue solid line), or clearance (red dotted line) of pre-HCT CMV DNAemia. Lighter colored shading indicates the 95% confidence interval. In each graph, the difference between those with any vs no pretransplant CMV PCR detection was calculated using Gray’s test; P values are listed on the right of the graphs.
Cumulative incidences of CMV reactivation in the first 100 days post-HCT stratified by the last pre-HCT CMV DNA PCR test result. Cumulative incidence curve showing the time to any CMV infection (A,D), time to CMV viral load ≥150 IU/mL (B,E), or time to CMV viral load ≥500 IU/mL (C,F), among PET era patients (n = 1367; A-C) or LET era patients (n = 332; D-F) in the first 100 days after HCT, dependent on the presence (green dashed line), absence (blue solid line), or clearance (red dotted line) of pre-HCT CMV DNAemia. Lighter colored shading indicates the 95% confidence interval. In each graph, the difference between those with any vs no pretransplant CMV PCR detection was calculated using Gray’s test; P values are listed on the right of the graphs.
The association of pre-HCT CMV reactivation with post-HCT CMV reactivation and disease were supported by univariable (supplemental Table 3) and multivariable (Figure 5) Cox regression models. Matched-related donor and high-grade (ie, grade 3-4) acute GVHD were also associated with a decreased and increased risk of post-HCT CMV reactivation/disease in multivariable models, respectively (data not shown).
Associations of pre-HCT CMV reactivation (with or without clearance after preemptive antiviral therapy) and of last pre-HCT CMV DNA PCR test result with the risk of post-HCT CMV reactivation at multiple levels. Multivariable Cox regression of the risk of post-HCT CMV reactivation associated with the absence or presence of pre-HCT CMV with or without clearance after preemptive antiviral therapy (above demarcation line in each plot, as circles) or by the last pre-HCT CMV PCR test (below demarcation line in each plot, as diamonds). Evaluated post-HCT CMV end points include any level (A), ≥150 IU/mL (B), or ≥500 IU/mL (C). ˆReference (ie, no CMV detection within 7-90 days before HCT) includes a small number of patients with pre-HCT CMV reactivation ≥50 and <150 IU/mL who became negative before HCT without preemptive antiviral therapy (n = 17). =Indicates inclusion of patients with pre-HCT CMV reactivation ≥50 IU/mL who received preemptive antiviral therapy and became negative before HCT. #Indicates inclusion of patients with pre-HCT CMV reactivation ≥50 IU/mL who received preemptive antiviral therapy and did not become negative before HCT. Models were also adjusted for recipient age, race, underlying disease, HCT-CI score, transplantation year, donor CMV serostatus, HLA matching, GVHD prophylaxis, and acute GVHD grade (time-dependent variable). 95% CI, 95% confidence interval.
Associations of pre-HCT CMV reactivation (with or without clearance after preemptive antiviral therapy) and of last pre-HCT CMV DNA PCR test result with the risk of post-HCT CMV reactivation at multiple levels. Multivariable Cox regression of the risk of post-HCT CMV reactivation associated with the absence or presence of pre-HCT CMV with or without clearance after preemptive antiviral therapy (above demarcation line in each plot, as circles) or by the last pre-HCT CMV PCR test (below demarcation line in each plot, as diamonds). Evaluated post-HCT CMV end points include any level (A), ≥150 IU/mL (B), or ≥500 IU/mL (C). ˆReference (ie, no CMV detection within 7-90 days before HCT) includes a small number of patients with pre-HCT CMV reactivation ≥50 and <150 IU/mL who became negative before HCT without preemptive antiviral therapy (n = 17). =Indicates inclusion of patients with pre-HCT CMV reactivation ≥50 IU/mL who received preemptive antiviral therapy and became negative before HCT. #Indicates inclusion of patients with pre-HCT CMV reactivation ≥50 IU/mL who received preemptive antiviral therapy and did not become negative before HCT. Models were also adjusted for recipient age, race, underlying disease, HCT-CI score, transplantation year, donor CMV serostatus, HLA matching, GVHD prophylaxis, and acute GVHD grade (time-dependent variable). 95% CI, 95% confidence interval.
Association of preemptive antiviral therapy for pre-HCT CMV with the risk of post-HCT CMV reactivation
To investigate whether initiation of preemptive antiviral therapy for pre-HCT CMV DNA PCR influences the risk of post-HCT CMV, alternative multivariable Cox regression models were constructed (Figure 5). Specifically, patient pre-HCT CMV reactivation status was categorized as “negative,” <50 IU/mL, ≥50 IU/mL, and cleared with preemptive antiviral therapy; or ≥50 IU/mL and did not clear with preemptive antiviral therapy. Each category was then analyzed as a risk factor for post-HCT CMV reactivation at multiple levels by 100 days after HCT. Patients with pre-HCT CMV and who cleared their DNAemia with preemptive antiviral therapy before HCT were at significantly increased risk of post-HCT CMV DNAemia at any level and at ≥150 IU/mL. Patients with pre-HCT CMV reactivation and who cleared their DNAemia with preemptive antiviral therapy trended toward an increased risk of post-HCT CMV DNAemia at ≥500 IU/mL compared with patients negative for pre-HCT for CMV reactivation. However, patients who proceeded to HCT with pre-HCT CMV without clearing their CMV DNAemia despite preemptive antiviral therapy were at even greater risk of CMV DNAemia at any level, ≥150 IU/mL, and ≥500 IU/mL, highlighting the profound modulation in the risk of post-HCT CMV after clearance of pre-HCT CMV DNAemia via the use of preemptive antiviral therapy. In addition, patients who received sirolimus–based GVHD prophylaxis had a 30% decreased risk of post-HCT CMV reactivation at any level and ≥150 IU/mL after adjustment for pre-HCT CMV.
Association of pre-HCT CMV reactivation with overall and nonrelapse mortalities
The impact of pre-HCT CMV DNAemia on overall and nonrelapse mortalities by 1 year is shown in supplemental Figures 8 and 9. Any CMV reactivation was associated with an increased risk of overall and nonrelapse mortalities. The statistical significance was more pronounced when patients without pre-HCT CMV reactivation were combined with patients who had low-level (ie, <50 IU/mL) pre-HCT CMV DNAemia.
Relevance of pre-HCT CMV in the era of letermovir prophylaxis
Cumulative incidence analyses of post-HCT CMV DNAemia stratified by the presence of pre-HCT CMV were repeated in patients from the 2018 to 2021 era (Figure 3D-F). Similar to the 2007 to 2017 era, patients from the 2018 to 2021 era with any pre-HCT CMV reactivation detected had a greater incidence of post-HCT CMV in the first 100 days after HCT than patients without pre-HCT CMV for all evaluated end points (Figure 3D-F).
A total of 18 patients (5%) from the 2018 to 2021 era met criteria for the diagnosis of CMV disease by 1 year after HCT; 3 (1%) in the first 100 days after HCT and 15 (4%) after post-HCT day 100. Of these patients, only 1 patient (6%) had pre-HCT CMV reactivation detected by PCR. Statistical modeling of post-HCT CMV disease could not be replicated in patients from the 2018 to 2021 era due to the insufficient number of CMV disease events for analysis.
The cumulative incidence of post-HCT CMV DNAemia stratified by the last pre-HCT CMV DNA PCR test result was also examined in letermovir-era HCT recipients independently (Figure 4D-F). Analogously, patients with positive CMV testing who proceeded to HCT had a greater incidence of post-HCT CMV than patients without pre-HCT CMV for the any level and ≥150 IU/mL viral end points. However, the difference was no longer statistically significant at ≥500 IU/mL, although the number of events at this viral load was low. Univariable Cox regression analyses confirmed qualitatively similar findings as the preemptive antiviral therapy era; however, multivariable analysis was limited due to the low number of post-HCT CMV reactivation events during letermovir prophylaxis (data not shown). In addition, overall and nonrelapse mortality analyses could not be performed due to the small sample size of that cohort.
Discussion
In this study, we identified pre-HCT CMV DNAemia as a strong pre-HCT risk factor for post-HCT reactivation, with its importance maintained in the letermovir era. Pre-HCT CMV DNAemia was detected in 11% of CMV-seropositive HCT candidates before proceeding to HCT. Clearance of pre-HCT CMV DNAemia before HCT with or without preemptive antiviral treatment was associated with a decreased incidence of CMV reactivation and end-organ disease after HCT, whereas persistent CMV DNAemia at the time of transplant drastically increased the risk. The association of pre-HCT reactivation with an increased risk of CMV after HCT was replicated in an independent cohort of recipients of letermovir prophylaxis. Patients with profound pre-HCT lymphopenia and higher HCT-CI scores were at the highest risk for pre-HCT CMV reactivation at multiple CMV DNA PCR thresholds.
Our study is, to our knowledge, the first to estimate the incidence of pre-HCT CMV reactivation in CMV-seropositive allogeneic HCT candidates using CMV DNA PCR. The incidence of pre-HCT CMV reactivation was 11%, which was unchanged throughout the preemptive therapy era (2007-2017) and the more recent era of letermovir prophylaxis (2018-2021). Lymphopenia within 90 days before HCT was the most significant risk factor for pre-HCT CMV reactivation at all evaluated CMV DNA PCR levels (ie, any level, ≥150 IU/mL, and ≥500 IU/mL), with the greatest risk associated with absolute lymphocyte counts <0.05× 103/μL and the concurrent presence of multiple risk factors. The well-described importance of total lymphocyte counts11 and functional CMV-specific T-cell responses12 in controlling CMV reactivation lend further credence to these results.13 Higher HCT-CI scores were associated with an increased risk of pre-HCT CMV reactivation at all evaluated thresholds. The HCT-CI score is a measure of comorbidities and the overall health status of HCT candidates; scores ≥3 have been associated with worse clinical outcomes after HCT, including increased mortality and CMV disease by 1 year.14 Patients with lymphoid malignancies were also at increased risk of pre-HCT CMV reactivation at multiple levels. These patients may have received corticosteroids as part of their chemotherapy regimens.15 Furthermore, the risk in patients with lymphoma may be attributed to the increased use of alkylating chemotherapy agents and/or B-cell depleting therapies (eg, rituximab) in these patients before undergoing HCT.16-18 In addition, a large proportion of patients with acute lymphoblastic leukemia received treatment with tyrosine kinase inhibitors, which have been associated with an increased risk of CMV infection.19 The presence of multiple risk factors for pre-HCT concurrently increased the risk of pre-HCT reactivation, which could be used to design targeted testing or interventional strategies.
HCT recipients of self-identified Asian descent were found to be at an increased risk of pre-HCT CMV at multiple levels. The finding was statistically significant in univariable and adjusted multivariable regression models. Other studies hypothesized that non-Caucasian, CMV-seropositive HCT recipients may possess fewer CMV immunodominant alleles, leading to diminished T-cell responses and increased rates of CMV.20 Therefore, it is possible that race was reflective of the overrepresentation of this specific immune genotype in our study. We recently observed a similar increased risk of CMV reactivation after HCT during letermovir prophylaxis in non-Caucasian, CMV-seropositive HCT recipients at our center, which could be caused by a similar mechanism.21
Studies that evaluated clinical pre-HCT variables as potential risk factors for clinically significant and subclinical CMV infection after HCT consistently identified transplants from haploidentical, HLA mismatched, or unrelated donors as important risk factors; however, the effect size was relatively small.22-24 The newly identified risk factor of CMV detection before HCT adds another robust independent risk factor for posttransplant CMV events, even in letermovir recipients. The risk of post-HCT CMV DNAemia was particularly high when CMV DNAemia persisted at the time of transplantation, an observation that would be expected in a large proportion of patients if pre-HCT DNAemia is unknown due to lack of surveillance. Perhaps the most striking aspect of our results was the ability to replicate our findings in the era of letermovir prophylaxis, including the risk reduction associated with viral clearance before HCT. We also found an effect of pre-HCT CMV reactivation on the risk of overall and nonrelapse mortalities in the preemptive therapy era, a finding that deserves further exploration.
Our study provides the rationale for optimized prevention strategies both before and after HCT. Historically, some CMV prevention strategies included pre-HCT administration of ganciclovir as part of universal prophylaxis strategies, yet widespread adoption of these strategies has not occurred.25-28 This study included preemptive treatment of pre-HCT CMV DNAemia, and although it may appear to be beneficial, this study was not randomized. Our management recommendations included delay of transplantation only in cases of CMV disease or unusually high levels of CMV DNAemia, although this strategy could be further explored in the future. The substantial posttransplant CMV reactivation risk associated with pre-HCT CMV in both eras suggests that post-HCT strategies could be improved. For instance, there may be a role for earlier letermovir initiation after HCT in patients with pre-HCT CMV reactivation, similar to what is done for highest risk patients, such as cord blood transplant recipients.21,29 Alternatively, GVHD prevention strategies associated with lower CMV reactivation risk, including the use of mammalian target of rapamycin (mTOR) inhibitors (eg, sirolimus), could be considered in patients with pre-HCT CMV detection.24,30,31 Randomized trials are needed to evaluate such strategies.
Our study has limitations. First, CMV PCR testing was only ordered at a minimum of once before HCT according to our institutional guidelines. Therefore, there is a possibility that the incidence of pre-HCT CMV reactivation could be underestimated due to heterogenous testing. In addition, we could not examine age-related differences in the incidence of post-HCT CMV in the letermovir prophylaxis era, given that the drug is currently only approved in HCT recipients aged ≥18 years.32 We were unable to account for specific mechanisms and reasons of pretransplant lymphopenia (eg, steroids, B-cell depleting therapies, and tyrosine kinase inhibitors) or reasons for the observed association of self-reported race with pre-HCT CMV reactivation. Lastly, our analysis of pre-HCT preemptive therapy on post-HCT CMV outcomes was not derived from a randomized controlled trial, and there were some statistically significant differences in demographic and transplantation characteristics between patients from both eras.
In conclusion, in this large cohort of CMV-seropositive HCT recipients who received routine CMV DNA PCR testing before allogeneic HCT in both the preemptive antiviral therapy and letermovir eras, we established, for the first time, that the incidence of pre-HCT CMV detection is ∼11%. We identified multiple risk factors for pre-HCT CMV reactivation, including lymphopenia, underlying lymphoid malignancy, and higher HCT-CI scores, and we determined that concurrent occurrence of these risk factors increases the risk to up to ∼25%. We showed that pre-HCT CMV detection at multiple viral load levels is a strong risk factor for post-HCT CMV PCR DNAemia, including the risk of post-HCT CMV disease and mortality up to 1 year after HCT, with effects sizes infrequently seen with other pre-HCT factors. Furthermore, the risk of post-HCT CMV reactivation appeared modifiable with clearance of CMV DNAemia before proceeding to HCT, highlighting the importance of CMV DNA PCR surveillance before HCT. Importantly, we replicated our findings related to CMV end points in the era of letermovir prophylaxis. Taken together, our data support the testing for CMV DNAemia during the pretransplantation workup, especially in those with lymphopenia, a high HCT-CI score, lymphoid malignancy, or a combination of these.
Acknowledgments
The authors thank Madison Kennedy and Jessica Lawler for their technical writing assistance in preparing this manuscript. Funding for their assistance was provided by the National Institutes of Health (NIH; grant T32AI118690-08S1). The authors also thank Rachel Blazevic, Marta Levkova, Jordan Sheldon, and Anna Santa Elena for their help with conducting manual chart reviews as well as Ryan Basom and Chris Davis for their help with additional data collection and management. The authors are also indebted to the patients and providers at Fred Hutchinson Cancer Center for their continued commitment to research participation.
The study was supported by the Fred Hutchinson Cancer Center Quality Assurance Program. Additional resources for the study were provided by the NIH (grant CA15704). D.Z., M.L.G., and M.J.B. also received support from the NIH (grants K23AI163343, K23AI097234, and K24HL093294, respectively). G.R.H. is supported by NIH R01AI175535. A.S.-K. received support from the Polish National Agency for Academic Exchange, Medical University of Gdansk, and the Joel D. Meyers Endowment Scholarship (Fred Hutchinson Cancer Center). E.K. received support from the Swiss National Science Foundation (grant number P500PM_202961) and the SICPA Foundation. M.A.B. is an Amy Strelzer Manasevit Research Program scholar (administered by the Be The Match Foundation).
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Authorship
Contribution: D.Z. and M.J.B. were responsible for the design of the study and interpretation of the data. D.Z., W.M.L., and H.X. analyzed the data and created the figures; data were directly accessed and verified by D.Z., A.S.-K., E.K., M.L.G., and H.X.; A.S.-K. helped with categorizing disease risk; M.A.B. and M.U.O. helped with categorizing conditioning, graft-versus-host disease prophylaxis, and disease risk; E.K. confirmed the receipt of antiviral prophylaxis; M.L.G. helped with generating study end points; and all authors contributed to the writing and revision of the manuscript and approved the final version.
Conflict-of-interest disclosure: L.H. reports research funding from Bristol Myers Squibb, Merck, Millennium-Takeda, and Sanofi; royalties from UpToDate; and has consulted for BioLineRX outside the submitted work. A.W. reports grants and personal fees from AlloVir, Ansun Biopharma, Kyorin Pharmaceutical, Pfizer, and Vir/GlaxoSmithKline outside the submitted work. A.L.G. reports central laboratory testing contracts from Abbott, Cepheid, Gilead, Hologic, Novavax, and Pfizer outside the submitted work. G.R.H. has consulted for GENERON Corporation, NapaJen Pharma, iTeos Therapeutics, Neoleukin Therapeutics, Commonwealth Serum Laboratories, and Cynata Therapeutics; and has received research funding from Compass Therapeutics, Syndax Pharmaceuticals, Applied Molecular Transport, SerPlus Technology, Heat Biologics, Laevoroc Oncology, iTeos Therapetics, and Genentech unrelated to the subject matter of this manuscript. J.A.H. reports research support from AlloVir, Merck, Oxford Immunotec, and Takeda; and personal fees from AlloVir, Moderna, Karius, and Gilead. M.J.B. reports personal fees from Merck, AlloVir, Symbio, Helocyte, Takeda, Moderna, and Evrys Bio outside the submitted work; and research support from Merck and Moderna. The remaining authors declare no competing financial interests.
Correspondence: Michael J. Boeckh, Vaccine and Infectious Disease Division, Fred Hutchinson Cancer Center, 1100 Fairview Ave North, E4-100, Seattle, WA 98109; email: mboeckh@fredhutch.org.
References
Author notes
Deidentified participant level data will be made available upon request after necessary funding has been secured and a mutually agreed upon data use agreement has been completed. Data will be available for sharing from the time of publication for 3 years. Requests should be directed to the corresponding author, Michael J. Boeckh (mboeckh@fredhutch.org).
The full-text version of this article contains a data supplement.

![Risk factors for pre-HCT CMV reactivation at multiple levels. Multivariable logistic regression of any (A), ≥150 IU/mL (B), or ≥500 IU/mL (C) viral load CMV in the 90 days before HCT transplant in patients from both eras. +Denotes the lowest pretransplant lymphocyte counts within 7 to 90 days before transplant. ∗Denotes inclusion of unknown or unreported HCT-CI scores. Note that underlying disease categories were collapsed in the pre-HCT reactivation ≥150 and ≥500 IU/mL models due to a lower number of events in each. Specifically, categories include the following: (1) lymphoid malignancies (acute lymphocytic leukemia [ALL], chronic lymphocytic leukemia [CLL], Hodgkin lymphoma [HL], non-Hodgkin lymphoma [NHL], and plasma cell neoplasms); (2) myeloid malignancies (acute myelogenous leukemia [AML], myelodysplastic syndrome [MDS], and other myeloproliferative neoplasms [MPNs]); and (3) Nonmalignant diseases (a variety of diseases such as aplastic anemia [AA] or primary immune deficiencies). 95% CI, 95% confidence interval; PLL, prolymphocytic leukemia.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/8/17/10.1182_bloodadvances.2023012234/2/m_blooda_adv-2023-012234-gr2.jpeg?Expires=1767728883&Signature=LXnvE1uHSlQmTo5gGB8-ZP0xZlOjGdrjPKCjmAF-K2tchVWSzio4bdSdtsS6BWubNT8xALnBddvvxoUE26mNAldHiJzTRC2xYzkmTkhgS5zOIrbKSNC6jfBaJfKDmPHVWpweEp6VzzBtvXVmL1uciIvqC0LCMfj0zkLIEi-j-c-7BOMqUFrwFmUcuN61k~lGsjIGa4SPRzRrWmkUUU5HpYc47L1iBRgfkjC8JY-gr9EslF-rcJiLk1UmFlWn7rSfq5nLYjqZb9BJYZ~J4zyHq32kPDAfHlIWjEQUePmrbpwlHBGDyDOLFaiaYr8tANcklqNjctB9cSft777fEfzq0w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Cumulative incidences of CMV reactivation in the first 100 days post-HCT stratified by the presence of pre-HCT CMV DNAemia. Cumulative incidence curve showing the time to any CMV infection (A,D), to ≥150 IU/mL CMV infection (B,E), or to ≥500 IU/mL CMV infection (C,F), among preemptive therapy (PET)–era patients (n = 1367; A-C) or among participants receiving letermovir (LET) prophylaxis (n = 332; D-F) within the first 100 days after transplant, separated by whether any pretransplant CMV was detected by PCR (yes in red [top line]; no in blue [bottom line]). Lighter colored shading indicates the 95% confidence interval. In each graph, the difference between those with any pretransplant CMV PCR detection was calculated using Gray’s test; associated P values are listed on the right of the graphs.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/8/17/10.1182_bloodadvances.2023012234/2/m_blooda_adv-2023-012234-gr3.jpeg?Expires=1767728883&Signature=EEhT~~cRnOYMqlHKRoI~bgzkC-GwueRN8oJSvCTJMC5T6gdqK8dBvdnjWS5jRaIZWbYM7CvnOp6nFXlmRRbznuyOzPahDU-QT0Tidwbk8I1PtQrt51i9SVyAGAhnSFbp9xWtdt9GvpIUtVu2QJ15prVTNBrMl5dYoH2GgfAs6ecdr1mnHVzTZO0X84h71GbiUaHHv8nvgAxsffG8HqaCsAHhHbPfmNqiQwlkk4G97PgWgLahOXSH~7CqOl5ht~JmeCYpt~wYYsohe0b2xRTHO8tqGfqtYxozcGkO72BvFS6Deau2AkCMs0MxGpZY0I3IU~rr6QF-qqCn7wMD9zWJDg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)