Key Points
The safety and efficacy benefits of ide-cel were proportionate in young and older patients despite frailty and geriatric characteristics.
Treatment with commercial ide-cel resulted in robust clinical responses and low-grade toxicities in older patients.
Visual Abstract
Frailty and clinical outcomes by age
The safety and efficacy of chimeric antigen receptor T-cell therapy is not well described in older patients, a population that has higher frailty and comorbidities. In this multicenter retrospective study, we evaluated clinical outcomes along with frailty and geriatric characteristics such as comorbidities, polypharmacy, falls, neuropathy, organ dysfunction, and performance status in younger (aged <65 years) vs older (aged ≥65 years) patients who received commercial idecabtagene vicleucel (ide-cel). A total of 156 patients (n = 75, aged ≥65 years) were infused with ide-cel by data cutoff. In older patients (median age: 69 years; range, 65-83; 66.7% frail; 77.3% did not meet KarMMa eligibility criteria), with a median follow-up duration of 14.2 months, best overall response rate (ORR) was 86.7%, which was comparable with pivotal KarMMa study results (ORR: 73%). Median progression-free survival and overall survival in older patients were 9.1 months and 26.5 months, respectively. Grade ≥3 cytokine-release syndrome and immune effector cell–associated neurotoxicity syndrome were observed in 1% and 4% of older patients, respectively. Compared with younger patients, the older patients had significantly higher prevalence of frailty, geriatric characteristics such as polypharmacy (≥5 drugs; 97%), ≥4 comorbidities (69%), and organ dysfunction (35%; P < .05). The safety and efficacy of ide-cel therapy were similar in younger and older patients. Frailty and geriatric characteristics such as polypharmacy, comorbidities, and organ dysfunction in older patients did not confer an inferior overall outcome.
Introduction
Multiple myeloma (MM) remains largely a neoplasm of older persons with the median age of diagnosis being 69 years.1 Among newly diagnosed patients, approximately two-thirds of patients are aged >65 years, and one-third are aged >75 years.2 It is projected that within 15 years nearly 3 of 4 individuals newly diagnosed with MM will be aged 65 to 84 years.3 Research over the last 2 decades has paved the way for several newer therapeutic options for MM leading to substantial improvement in outcomes for patients. However, these significant beneficial advances have been disproportionate, favoring younger patients more than the older ones.4 The outcomes with these novel approaches have been inferior for septuagenarians (aged 75 years) and poorer for octogenarians (aged 80 years).5-7 In fact, the National Cancer Institute Surveillance, Epidemiology, and End Results Program has reported myeloma-related deaths to be ∼19% and ∼80% in patients aged <65 years and aged ≥65 years, respectively.8 The poor outcomes and myeloma-related mortality could be multifactorial including aging-related changes. An increase in frailty, comorbidities, and physical disabilities with age render the management of MM in older patients challenging.9 Use of novel immunotherapies such as chimeric antigen receptor (CAR) T-cell (CART) therapy is especially challenging in this patient population.
There are limited comprehensive data on clinical outcomes in older patients with MM who received CART therapy, particularly as it relates to comorbidities and performance status of these patients in the standard of care setting. The US Food and Drug Administration approval of idecabtagene vicleucel (ide-cel) (Abecma) for relapsed and refractory MM was based on pivotal phase 2 KarMMa clinical trial.10 The study reported ∼70% and ∼85% overall response rate (ORR) in younger (aged <65 years; n = 83) and older (aged ≥65 years; n = 45) patients, respectively. Moreover, the rate of complete remission (CR) was ∼30% for both the age groups. Because of stringent eligibility criteria, the KarMMa patient population was a highly selective group, and therefore, the results are likely not representative of the MM population in real-world clinical practice.
This multicenter retrospective study aimed to assess both safety and efficacy of commercial ide-cel while also examining frailty and various geriatric factors, including comorbidities, polypharmacy, falls, performance status, and organ dysfunction. The study sought to compare these factors between younger and older populations in real-world settings to understand their influence on treatment response and outcomes. Additionally, we assessed physical and physiological limitations that might render older patient’s ineligible for clinical trial participation based on the KarMMa trial inclusion criteria.
Methods
Data collection
Data were retrospectively collected and harmonized from 5 US institutions for this retrospective study. The baseline characteristics including demographics, disease characteristics, prior treatments, time between diagnosis to CART infusion, performance status, and other patient and treatment-related variables were collected along with data for safety, efficacy, survival, frailty, geriatric attributes, and KarMMa eligibility. The data were collected for all patients who underwent leukapheresis followed by ide-cel infusion by 31 August 2022, and after ide-cel infusion follow-up until November 2023. A simplified Facon frailty scale was used, as described previously by Facon et al,11 to evaluate frailty status of the patients at leukapheresis based on age, Charlson Comorbidity Index (CCI), and Eastern Cooperative Oncology Group (ECOG) performance status. The patients whose disease was not evaluable as per International Myeloma Working Group criteria and those who died between ide-cel infusion and before first response assessment were included in response analysis as progressive disease (PD). The patients with partial response or better were considered responders whereas those with stable disease or PD were considered as nonresponders. All geriatric attributes and KarMMa eligibility information were captured at time of leukapheresis. If ide-cel did not meet US Food and Drug Administration specification criteria for release, patients were offered treatment under an expanded access protocol, and their results were included. All supportive care interventions such as blood or platelet transfusion, use of growth factors, and intravenous immunoglobulin infusion were also captured. The definition of organ dysfunction, comorbidities, polypharmacy, renal dysfunction, and list of KarMMa study eligibility criteria to determine patients’ eligibility are included in the supplemental Materials.
The American Society for Transplantation and Cellular Therapy criteria were used to grade cytokine-release syndrome (CRS) and immune effector cell–associated neurotoxicity syndrome (ICANS).12 The common terminology criteria for adverse events, version 5.0, was used to grade all hematologic toxicities.13 The International Myeloma Working Group criteria were used by each institution to grade response to therapy; however, confirmatory testing and imaging to confirm CR for patients with extramedullary disease were not mandated because of the retrospective study design.14
For a uniform data collection, the coordinating site provided a data form along with data dictionary for data collection to all participating sites followed by a quality check. Each participating institution obtained institutional review board or ethics committee approval independently, as per individual institutional guidelines.
Statistical analysis
Fisher exact and χ2 tests and Kruskal-Wallis rank sum tests were used to evaluate differences between groups for baseline clinical characteristics, toxicities, use of supportive measures for cytopenias and hypogammaglobulinemia, response, and causes of death. Progression-free survival (PFS) was calculated as time between the date of CART infusion and date of PD or date of death (no PD before death) or date of last contact (no PD). The overall survival (OS) was calculated as time between the date of CART infusion and date of death or date of last contact for those who are alive. The Kaplan-Meier method was used to estimate PFS and OS with the log-rank test to compare survival outcomes between groups with univariate and multivariable Cox proportional hazards models used for estimations of hazard ratios (HRs) and 95% confidence intervals (95% CIs). Descriptive statistics were used to summarize comorbidities and KarMMa study eligibility criteria.
Results
Patient characteristics
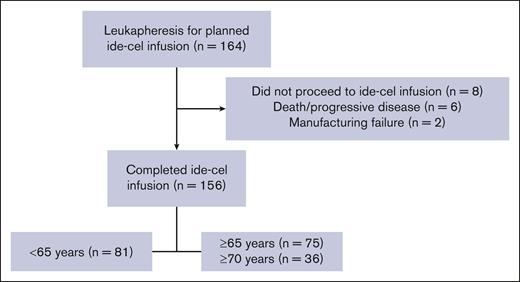
A total of 156 patients (aged <65 years, n = 81; and aged ≥65 years, n = 75) were infused with ide-cel between 12 May 2021 and 31 August 2022, with a median follow-up of 14.2 months. Among 8 patients who did not receive the ide-cel infusion, 2 patients had a manufacturing failure and 6 patients died before infusion (5 because of PD and 1 because of sepsis). Of 75 older patients, 36 were aged ≥70 years (Figure 1).
Baseline characteristics for the younger (aged <65 years) and older (aged ≥65 years) patients are presented in Table 1. The median age at the time of CART infusion for younger and older patients were 58 (range, 42-64) and 69 (range, 65-83) years, respectively (P < .001). A poor ECOG PS of ≥ 2 at time of lymphodepletion chemotherapy (cyclophosphamide and fludarabine) was 13% for both age groups. The older patient group included 61% male patients, 28% non-White patients, 60% with immunoglobulin G–subtype disease, 41% with extramedullary disease, 23% with high marrow burden (≥50% plasma cells in the pre-CART bone marrow biopsy), 25% with Revised International Staging System stage III disease, 39% with any high-risk cytogenetics (del17p, t[4;14], and t[14;16]), and 71% received bridging therapy at discretion of treating physician after leukapheresis. There was no statistically significant difference between younger and older patients for aforementioned baseline characteristics. The median prior lines of therapy (6) and median time from diagnosis to CART infusion (7 years) were similar in both groups. However, younger patients were more likely to have triple class refractory disease (95% vs 84%, P < .05).
Baseline characteristics of patients infused with ide-cel
| . | Total . | Age of <65 y . | Age of ≥65 y . | P value . |
|---|---|---|---|---|
| Number of patients, n (%) | 156 | 81 (51.9) | 75 (48.1) | |
| Age at time of CART infusion, y (median) | 63.5 | 58 | 69 | <.001 |
| Range | 42-83 | 42-64 | 65-83 | |
| Sex | .71 | |||
| Male, n (%) | 98 (62.8) | 52 (64.2) | 46 (61.3) | |
| Female, n (%) | 58 (37.2) | 29 (35.8) | 29 (38.7) | |
| ECOG PS at time of lymphodepletion | .92 | |||
| 0-1, n (%) | 131 (86.8) | 67 (87.0) | 64 (86.5) | |
| ≥2, n (%) | 20 (13.3) | 10 (13.0) | 10 (13.5) | |
| Unknown | 5 | 4 | 1 | |
| Race/ethnicity | .78 | |||
| White, n (%) | 109 (69.9) | 55 (67.9) | 54 (72.0) | |
| Black, n (%) | 26 (16.7) | 13 (15.1) | 13 (17.3) | |
| Hispanic, n (%) | 9 (5.8) | 5 (6.2) | 4 (5.3) | |
| Other, n (%) | 12 (7.6) | 8 (9.9) | 4 (5.3) | |
| Myeloma subtype | .31 | |||
| IgG, n (%) | 88 (57.1) | 44 (54.3) | 44 (60.3) | |
| IgA, n (%) | 34 (22.1) | 22 (27.2) | 12 (16.4) | |
| IgM, n (%) | 1 (0.65) | 0 (0.0) | 1. (1.4) | |
| FLC, n (%) | 31 (20.1) | 15 (18.5) | 16 (21.9) | |
| Extramedullary disease, n (%) | 73 (46.8) | 42 (51.9) | 31 (41.3) | .19 |
| High marrow burden∗ (≥50%), n (%) | 42 (28.2) | 25 (33.3) | 17 (23.0) | .16 |
| R-ISS disease stage | .99 | |||
| I, n (%) | 28 (21.1) | 15 (21.4) | 13 (20.6) | |
| II, n (%) | 72 (54.1) | 38 (54.3) | 34 (54.0) | |
| III, n (%) | 33 (24.8) | 17 (24.3) | 16 (25.4) | |
| Unknown, n (%) | 23 | 11 | 12 | |
| Cytogenetic abnormality | ||||
| Any high-risk cytogenetics†, n (%) | 50 (36.5) | 24 (33.8) | 26 (39.4) | .50 |
| Unknown | 19 | 10 | 9 | |
| del(17p), n (%) | 34 (24.8) | 16 (22.5) | 18 (27.3) | .52 |
| Unknown | 18 | 10 | 9 | |
| t(4;14), n (%) | 18 (13.7) | 9 (13.2) | 9 (14.3) | .86 |
| Unknown | 25 | 13 | 12 | |
| t(14;16), n (%) | 6 (4.6) | 3 (4.5) | 3 (4.8) | .94 |
| Unknown | 26 | 14 | 12 | |
| Bridging therapy, n (%) | 116 (74.4) | 63 (77.8) | 53 (70.7) | .31 |
| Refractory status | ||||
| Immunomodulatory agent, n (%) | 144 (92.3) | 75 (92.6) | 69 (92.0) | .89 |
| Proteasome inhibitor, n (%) | 140 (89.7) | 76 (93.8) | 64 (85.3) | .081‡ |
| Anti-CD38 antibody, n (%) | 146 (93.6) | 78 (96.3) | 68 (90.7) | .15 |
| Triple-refractory§, n (%) | 140 (89.7) | 77 (95.1) | 63 (84.0) | .023 |
| Penta-refractory||, n (%) | 62 (39.7) | 38 (46.9) | 24 (32.0) | .057‡ |
| Number of prior therapies | ||||
| Median (range) | 6 (4-18) | 6 (4-16) | 6 (4-18) | .85 |
| Average | 7.1 | 6.9 | 7.2 | |
| Time between diagnosis to CART infusion, y (average, n = 131) | 7.2 | 7 | 7.4 | .49 |
| . | Total . | Age of <65 y . | Age of ≥65 y . | P value . |
|---|---|---|---|---|
| Number of patients, n (%) | 156 | 81 (51.9) | 75 (48.1) | |
| Age at time of CART infusion, y (median) | 63.5 | 58 | 69 | <.001 |
| Range | 42-83 | 42-64 | 65-83 | |
| Sex | .71 | |||
| Male, n (%) | 98 (62.8) | 52 (64.2) | 46 (61.3) | |
| Female, n (%) | 58 (37.2) | 29 (35.8) | 29 (38.7) | |
| ECOG PS at time of lymphodepletion | .92 | |||
| 0-1, n (%) | 131 (86.8) | 67 (87.0) | 64 (86.5) | |
| ≥2, n (%) | 20 (13.3) | 10 (13.0) | 10 (13.5) | |
| Unknown | 5 | 4 | 1 | |
| Race/ethnicity | .78 | |||
| White, n (%) | 109 (69.9) | 55 (67.9) | 54 (72.0) | |
| Black, n (%) | 26 (16.7) | 13 (15.1) | 13 (17.3) | |
| Hispanic, n (%) | 9 (5.8) | 5 (6.2) | 4 (5.3) | |
| Other, n (%) | 12 (7.6) | 8 (9.9) | 4 (5.3) | |
| Myeloma subtype | .31 | |||
| IgG, n (%) | 88 (57.1) | 44 (54.3) | 44 (60.3) | |
| IgA, n (%) | 34 (22.1) | 22 (27.2) | 12 (16.4) | |
| IgM, n (%) | 1 (0.65) | 0 (0.0) | 1. (1.4) | |
| FLC, n (%) | 31 (20.1) | 15 (18.5) | 16 (21.9) | |
| Extramedullary disease, n (%) | 73 (46.8) | 42 (51.9) | 31 (41.3) | .19 |
| High marrow burden∗ (≥50%), n (%) | 42 (28.2) | 25 (33.3) | 17 (23.0) | .16 |
| R-ISS disease stage | .99 | |||
| I, n (%) | 28 (21.1) | 15 (21.4) | 13 (20.6) | |
| II, n (%) | 72 (54.1) | 38 (54.3) | 34 (54.0) | |
| III, n (%) | 33 (24.8) | 17 (24.3) | 16 (25.4) | |
| Unknown, n (%) | 23 | 11 | 12 | |
| Cytogenetic abnormality | ||||
| Any high-risk cytogenetics†, n (%) | 50 (36.5) | 24 (33.8) | 26 (39.4) | .50 |
| Unknown | 19 | 10 | 9 | |
| del(17p), n (%) | 34 (24.8) | 16 (22.5) | 18 (27.3) | .52 |
| Unknown | 18 | 10 | 9 | |
| t(4;14), n (%) | 18 (13.7) | 9 (13.2) | 9 (14.3) | .86 |
| Unknown | 25 | 13 | 12 | |
| t(14;16), n (%) | 6 (4.6) | 3 (4.5) | 3 (4.8) | .94 |
| Unknown | 26 | 14 | 12 | |
| Bridging therapy, n (%) | 116 (74.4) | 63 (77.8) | 53 (70.7) | .31 |
| Refractory status | ||||
| Immunomodulatory agent, n (%) | 144 (92.3) | 75 (92.6) | 69 (92.0) | .89 |
| Proteasome inhibitor, n (%) | 140 (89.7) | 76 (93.8) | 64 (85.3) | .081‡ |
| Anti-CD38 antibody, n (%) | 146 (93.6) | 78 (96.3) | 68 (90.7) | .15 |
| Triple-refractory§, n (%) | 140 (89.7) | 77 (95.1) | 63 (84.0) | .023 |
| Penta-refractory||, n (%) | 62 (39.7) | 38 (46.9) | 24 (32.0) | .057‡ |
| Number of prior therapies | ||||
| Median (range) | 6 (4-18) | 6 (4-16) | 6 (4-18) | .85 |
| Average | 7.1 | 6.9 | 7.2 | |
| Time between diagnosis to CART infusion, y (average, n = 131) | 7.2 | 7 | 7.4 | .49 |
Bold indicates statistically significant difference between age groups, P < .05.
FLC, free light chain; IgA, immunoglobulin A; IgG, immunoglobulin G; IgM, immunoglobulin M; R-ISS, revised International Staging System.
High tumor burden defined by ≥50% clonal plasma cells in prelymphodepletion chemotherapy bone marrow biopsy.
As defined by the presence of del(17p), t(4;14) or t(14;16).
Borderline significance between age groups, P < .10.
Defined as refractory to ≥1 immunomodulatory drug, ≥1 proteasome inhibitor, and ≥1 anti-CD38 monoclonal antibody.
Penta-refractory defined as refractory to ≥2 immunomodulatory drugs, ≥2 proteasome inhibitors, and ≥1 anti-CD38 monoclonal antibody.
Safety
The overall incidence of any grade CRS for the younger and older patients were 81.5% and 88.0% (P = .53), respectively (Table 2). Grade ≥3 CRS was observed in only 2 younger patients (2.4%) and 1 older patient (1.2%). The median time to maximum grade CRS was 1 day for both groups. The overall incidence of any grade ICANS was not significantly different by age (P = .14), although it was slightly higher for the older patients (25.3%) than in younger patients (13.6%). Grade ≥3 ICANS was observed in 4 younger (4.9%) and 3 older patients (4.0%). The median time to maximum grade ICANS was shorter at 1.5 days for older patients compared with 3 days in younger patients and the difference reached borderline significance (P < .1). As with CRS and ICANS, the prevalence of cytopenias (grade ≥3 neutropenia, anemia, and thrombocytopenia) at day 30, day 60, and day 90 did not differ significantly between these 2 age groups. Similarly, rates of growth factor use for neutropenia, blood transfusion for anemia, and platelet transfusion for thrombocytopenia within the first 90 days after CART therapy also did not differ significantly between these 2 age categories. During month 3 (day 61-90) after CART therapy, the number of patients requiring growth factor support, blood transfusion, or platelet transfusion were comparable between younger and older patients (15.6%, 11.7%, and 7.8% vs 15.3%, and 12.5%, 9.7% respectively). The rate of intravenous immunoglobulin use for hypogammaglobulinemia was 27.2% in younger patients and 37.3% in older patients. The median duration of hospitalization was 9 days for both the age groups. Moreover, the number of patients requiring intensive care unit stay were 7.4% (n = 6) and 4.0% (n = 3) for younger and older patients, respectively. A total of 11 types of viral infections were reported in our study population (supplemental Table 1). Total number of patients with viral infections were numerically higher in the older group (24.0%, n = 18) than in the younger group (17.2%, n = 14).
Adverse events
| Event and grade . | Total . | Age of <65 y . | Age of ≥65 y . | P value . |
|---|---|---|---|---|
| CRS, n (%) | 156 | 81 (51.9) | 75 (48.1) | |
| Any, n (%) | 132 (84.6) | 66 (81.5) | 66 (88.0) | .53 |
| Grade ≥3, n (%) | 3 (1.9) | 2 (2.4) | 1 (1.2) | |
| Median time to maximum severity, d (range) | 1 (0-21) | 1 (0-14) | 1 (0-21) | .28 |
| ICANS | ||||
| Any, n (%) | 30 (23.8) | 11 (13.6) | 19 (25.3) | .14 |
| Grade ≥3, n (%) | 7 (4.5) | 4 (4.9) | 3 (4.0) | |
| Median time to maximum severity, d (range) | 2 (0-24) | 3 (0-24) | 1.5 (0-4) | .054∗ |
| Neutropenia (grade ≥3) | ||||
| 30 d, n = 154, n (%) | 48 (31.4) | 29 (36.3) | 19 (26.0) | .17 |
| 60 d, n = 117, n (%) | 28 (23.9) | 18 (30.0) | 10 (17.5) | .11 |
| 90 d, n = 130, n (%) | 13 (10.0) | 6 (8.8) | 7 (11.3) | .64 |
| Anemia (grade ≥3) | ||||
| 30 d, n = 154, n (%) | 29 (18.8) | 13 (16.3) | 16 (21.6) | .39 |
| 60 d, n = 118, n (%) | 18 (15.3) | 11 (18.0) | 7 (12.3) | .39 |
| 90 d, n = 131, n (%) | 13 (9.9) | 6 (8.7) | 7 (11.3) | .62 |
| Thrombocytopenia (grade ≥3) | ||||
| 30 d, n = 154, n (%) | 44 (28.6) | 23 (28.8) | 21 (28.4) | .96 |
| 60 d, n = 118, n (%) | 42 (35.6) | 21 (34.4) | 21 (36.8) | .78 |
| 90 d, n = 131, n (%) | 31 (23.7) | 16 (23.2) | 15 (24.2) | .89 |
| Supportive care for cytopenias | ||||
| G-CSF | ||||
| 1-30 d, n = 156, n (%) | 115 (73.7) | 59 (72.8) | 56 (74.7) | .80 |
| 31-60 d, n = 153, n (%) | 57 (37.3) | 30 (38.5) | 27 (36.0) | .75 |
| 61-90 d, n = 149, n (%) | 23 (15.4) | 12 (15.6) | 22 (15.3) | .96 |
| Blood transfusion | ||||
| 1-30 d, n = 156, n (%) | 72 (46.2) | 39 (48.2) | 33 (44.0) | .60 |
| 31-60 d, n = 153, n (%) | 32 (20.9) | 17 (21.8) | 15 (20.0) | .79 |
| 61-90 d, n = 149, n (%) | 18 (12.1) | 9 (11.7) | 9 (12.5) | .88 |
| Platelet transfusion | ||||
| 1-30 d, n = 156, n (%) | 58 (37.2) | 30 (37.0) | 28 (37.3) | .97 |
| 31-60 d, n = 153, n (%) | 33 (21.6) | 19 (24.4) | 14 (18.7) | .39 |
| 61-90 d, n = 149, n (%) | 13 (8.7) | 6 (7.8) | 7 (9.7) | .68 |
| IVIG infusion, n (%) | 50 (32.1) | 22 (27.2) | 28 (37.3) | .17 |
| Hospitalization | ||||
| Median hospital stay, d (range) | 9 (5-69) | 9 (5-68) | 9 (6-69) | .84 |
| Intensive care unit stay, n (%) | 9 (5.8) | 6 (7.4) | 3 (4.0) | .36 |
| Event and grade . | Total . | Age of <65 y . | Age of ≥65 y . | P value . |
|---|---|---|---|---|
| CRS, n (%) | 156 | 81 (51.9) | 75 (48.1) | |
| Any, n (%) | 132 (84.6) | 66 (81.5) | 66 (88.0) | .53 |
| Grade ≥3, n (%) | 3 (1.9) | 2 (2.4) | 1 (1.2) | |
| Median time to maximum severity, d (range) | 1 (0-21) | 1 (0-14) | 1 (0-21) | .28 |
| ICANS | ||||
| Any, n (%) | 30 (23.8) | 11 (13.6) | 19 (25.3) | .14 |
| Grade ≥3, n (%) | 7 (4.5) | 4 (4.9) | 3 (4.0) | |
| Median time to maximum severity, d (range) | 2 (0-24) | 3 (0-24) | 1.5 (0-4) | .054∗ |
| Neutropenia (grade ≥3) | ||||
| 30 d, n = 154, n (%) | 48 (31.4) | 29 (36.3) | 19 (26.0) | .17 |
| 60 d, n = 117, n (%) | 28 (23.9) | 18 (30.0) | 10 (17.5) | .11 |
| 90 d, n = 130, n (%) | 13 (10.0) | 6 (8.8) | 7 (11.3) | .64 |
| Anemia (grade ≥3) | ||||
| 30 d, n = 154, n (%) | 29 (18.8) | 13 (16.3) | 16 (21.6) | .39 |
| 60 d, n = 118, n (%) | 18 (15.3) | 11 (18.0) | 7 (12.3) | .39 |
| 90 d, n = 131, n (%) | 13 (9.9) | 6 (8.7) | 7 (11.3) | .62 |
| Thrombocytopenia (grade ≥3) | ||||
| 30 d, n = 154, n (%) | 44 (28.6) | 23 (28.8) | 21 (28.4) | .96 |
| 60 d, n = 118, n (%) | 42 (35.6) | 21 (34.4) | 21 (36.8) | .78 |
| 90 d, n = 131, n (%) | 31 (23.7) | 16 (23.2) | 15 (24.2) | .89 |
| Supportive care for cytopenias | ||||
| G-CSF | ||||
| 1-30 d, n = 156, n (%) | 115 (73.7) | 59 (72.8) | 56 (74.7) | .80 |
| 31-60 d, n = 153, n (%) | 57 (37.3) | 30 (38.5) | 27 (36.0) | .75 |
| 61-90 d, n = 149, n (%) | 23 (15.4) | 12 (15.6) | 22 (15.3) | .96 |
| Blood transfusion | ||||
| 1-30 d, n = 156, n (%) | 72 (46.2) | 39 (48.2) | 33 (44.0) | .60 |
| 31-60 d, n = 153, n (%) | 32 (20.9) | 17 (21.8) | 15 (20.0) | .79 |
| 61-90 d, n = 149, n (%) | 18 (12.1) | 9 (11.7) | 9 (12.5) | .88 |
| Platelet transfusion | ||||
| 1-30 d, n = 156, n (%) | 58 (37.2) | 30 (37.0) | 28 (37.3) | .97 |
| 31-60 d, n = 153, n (%) | 33 (21.6) | 19 (24.4) | 14 (18.7) | .39 |
| 61-90 d, n = 149, n (%) | 13 (8.7) | 6 (7.8) | 7 (9.7) | .68 |
| IVIG infusion, n (%) | 50 (32.1) | 22 (27.2) | 28 (37.3) | .17 |
| Hospitalization | ||||
| Median hospital stay, d (range) | 9 (5-69) | 9 (5-68) | 9 (6-69) | .84 |
| Intensive care unit stay, n (%) | 9 (5.8) | 6 (7.4) | 3 (4.0) | .36 |
G-CSF, growth-colony stimulating factor; IVIG, Intravenous immunoglobulin.
Borderline significance between age groups, P < .10.
Frailty
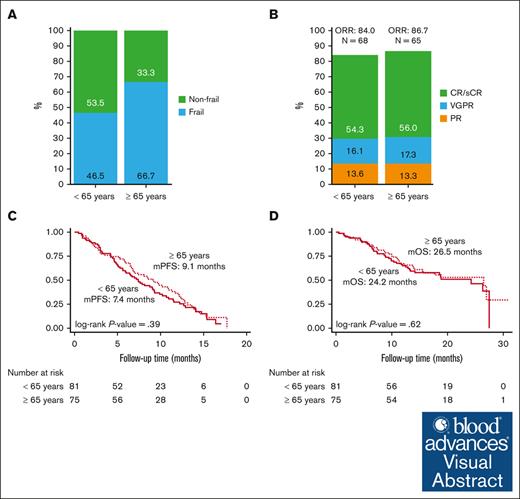
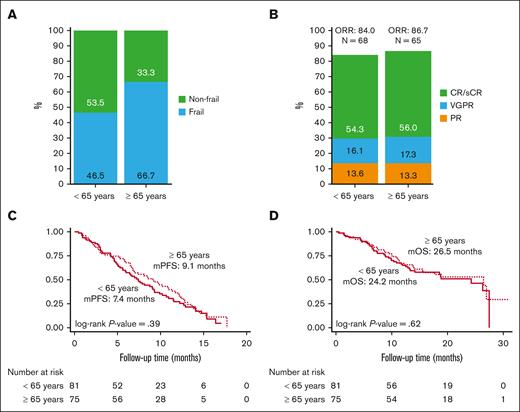
A total of 137 of 156 patients were evaluable for frailty. As shown in Figure 2A, 46.5% younger patients (n = 33) and 66.7% older patients (n = 44) were identified as being frail (P = .017). The remaining 19 patients were not included in analysis because of missing data for either ECOG or CCI.
Frailty and clinical outcomes by age. (A) Frailty, (B) ORR, (C) PFS, and (D) OS in patients receiving ide-cel by age category. mPFS, median PFS; mOS, median OS.
Frailty and clinical outcomes by age. (A) Frailty, (B) ORR, (C) PFS, and (D) OS in patients receiving ide-cel by age category. mPFS, median PFS; mOS, median OS.
Treatment response
A total of 156 patients infused with ide-cel were evaluable for treatment response. The rates of best ORR (84.0 % in those aged <65 years vs 86.7% in those aged ≥65 years) and CR or better (54.3% in those aged <65 years vs 56.0% in those aged ≥65 years) were similar. There was no significant difference in best ORR between the age groups (P = .92; Figure 2B).
PFS and survival outcomes
There was no significant difference in PFS by age category (P = .39), although the median PFS of 7.4 months for younger patients was numerically shorter compared with 9.1 months for older patients (Figure 2C). High-risk cytogenetics (HR, 1.8; 95% CI, 1.0-3.2; P = .044) and triple refractory status (HR, 0.4; 95% CI, 0.2-0.8; P = .010) were significant in univariate analysis whereas prior B-cell maturation antigen (BCMA)-directed therapy (HR, 5.1; 95% CI, 1.1-22.7; P = .034) and triple refractory status (HR, 0.2; 95% CI, 0.08-0.46; P < .001) were significantly associated with risk of PD for older patients in multivariable analysis (supplemental Table 2). Similarly, OS was not different by age group (P = .62; Figure 2D), the 12-month rates were 76.5% and 68% for the younger and older groups, respectively. ECOG PS score of ≥2 at lymphodepletion chemotherapy (HR, 2.7; 95% CI, 1.1-6.3; P = .026) on univariate analysis whereas triple refractory status (HR, 0.3; 95% CI, 0.1-0.8; P = .023) on multivariable analysis were significantly associated with inferior OS in older patients (supplemental Table 3).
Causes of death
At time of data cutoff, 73 (46.7%) patients died, which included 39 (53.4%) younger and 34 (46.5%) older patients (Table 3). Most of the deaths (n = 55; 75.3%) were myeloma-related due to PD, which included 31 (79.5%) younger and 24 (70.6%) older patients. The number of nonrelapse mortality events was 18 (24.7%), which included 8 (20.5%) younger and 10 (29.4%) older patients. The causes of nonrelapse mortality varied among both the groups. Cardiac etiology contributed to deaths of 3 older patients (8.8%) (1 each due to cardiac failure, cardiac arrest, and cardiac amyloidosis). Ten patients (5 in each group) died because of infections, which included 4 related to COVID-19 (2 in each group), 3 related to unknown infections in the older group, and 3 related to bacterial infection in younger group. Two of the younger patients died because of toxicities including 1 related to CRS grade 5 and 1 related to unspecified neurotoxicity. One older patient died because of Lewy-body dementia. Two patients (1 in each group) died because of unspecified cause.
Causes of death
| . | Total . | Age of <65 y . | Age of ≥65 . | P value . |
|---|---|---|---|---|
| Total deaths | 73 | 39 (53.4) | 34 (46.5) | .37 |
| Myeloma-related deaths | 55 (75.34) | 31 (79.49) | 24 (70.59) | .31 |
| Nonrelapse mortality | 18 (24.66) | 8 (20.51) | 10 (29.41) | |
| Nonrelapse mortality categories | .22 | |||
| Cardiac | 3 (4.11) | 0 (0.0) | 3 (8.82) | |
| Infection (COVID-19, bacteremia, etc) | 10 (13.70) | 5 (12.82) | 5 (14.71) | |
| Toxicity (CRS/ICANS) | 1 (1.37) | 1 (2.56) | 0 (0.0) | |
| Neurotoxicity, unspecified | 1 (1.37) | 1 (2.56) | 0 (0.0) | |
| Lewy-body dementia | 1 (1.37) | 0 (0.0) | 1 (2.94) | |
| Unspecified | 2 (2.74) | 1 (2.56) | 1 (2.94) |
| . | Total . | Age of <65 y . | Age of ≥65 . | P value . |
|---|---|---|---|---|
| Total deaths | 73 | 39 (53.4) | 34 (46.5) | .37 |
| Myeloma-related deaths | 55 (75.34) | 31 (79.49) | 24 (70.59) | .31 |
| Nonrelapse mortality | 18 (24.66) | 8 (20.51) | 10 (29.41) | |
| Nonrelapse mortality categories | .22 | |||
| Cardiac | 3 (4.11) | 0 (0.0) | 3 (8.82) | |
| Infection (COVID-19, bacteremia, etc) | 10 (13.70) | 5 (12.82) | 5 (14.71) | |
| Toxicity (CRS/ICANS) | 1 (1.37) | 1 (2.56) | 0 (0.0) | |
| Neurotoxicity, unspecified | 1 (1.37) | 1 (2.56) | 0 (0.0) | |
| Lewy-body dementia | 1 (1.37) | 0 (0.0) | 1 (2.94) | |
| Unspecified | 2 (2.74) | 1 (2.56) | 1 (2.94) |
Geriatric characteristics
A set of selected geriatric characteristics, such as polypharmacy (≥5 medications), excessive polypharmacy (≥10 medications), comorbidities (≥4), polyneuropathy, falls, and organ dysfunction, were analyzed to understand their prevalence in both age groups (Table 4). Polypharmacy, comorbidities, and organ dysfunction were significantly higher in the older cohort (97.3%, 69.3%, and 34.7%, respectively) than in the younger cohort (87.7%, 53.1%, and 17.3%, respectively; P < .05 for all). The prevalence of excessive polypharmacy (70.7%), polyneuropathy (74.7%), and falls (26.7%) were numerically higher in older patients than in younger patients, but this difference was not significant.
Geriatric characteristics
| . | Total . | Age of <65 y . | Age of ≥65 y . | P value . |
|---|---|---|---|---|
| Number of patients, n (%) | 156 | 81 (51.9) | 75 (48.1) | |
| Polypharmacy (≥5) | .033 | |||
| Yes, n (%) | 144 (92.3) | 71 (87.7) | 73 (97.3) | |
| No, n (%) | 12 (7.7) | 10 (12.3) | 2 (2.7) | |
| Excessive polypharmacy (≥10) | .10 | |||
| Yes, n (%) | 100 (64.1) | 47 (58.0) | 53 (70.7) | |
| No, n (%) | 56 (35.9) | 34 (42.0) | 22 (29.3) | |
| Comorbidities (≥4) | .038 | |||
| Yes, n (%) | 95 (60.9) | 43 (53.1) | 52 (69.3) | |
| No, n (%) | 61 (39.1) | 38 (46.9) | 23 (30.7) | |
| Neuropathy | .21 | |||
| Yes, n (%) | 109 (69.9) | 53 (65.4) | 56 (74.7) | |
| No, n (%) | 47 (30.1) | 28 (34.6) | 19 (25.3) | |
| Falls | .16 | |||
| Yes, n (%) | 34 (21.8) | 14 (17.3) | 20 (26.7) | |
| No, n (%) | 122 (78.2) | 67 (82.7) | 55 (73.3) | |
| Organ dysfunction∗ | .013 | |||
| Yes, n (%) | 40 (25.6) | 14 (17.3) | 26 (34.7) | |
| No, n (%) | 116 (74.4) | 67 (82.7) | 49 (65.3) |
| . | Total . | Age of <65 y . | Age of ≥65 y . | P value . |
|---|---|---|---|---|
| Number of patients, n (%) | 156 | 81 (51.9) | 75 (48.1) | |
| Polypharmacy (≥5) | .033 | |||
| Yes, n (%) | 144 (92.3) | 71 (87.7) | 73 (97.3) | |
| No, n (%) | 12 (7.7) | 10 (12.3) | 2 (2.7) | |
| Excessive polypharmacy (≥10) | .10 | |||
| Yes, n (%) | 100 (64.1) | 47 (58.0) | 53 (70.7) | |
| No, n (%) | 56 (35.9) | 34 (42.0) | 22 (29.3) | |
| Comorbidities (≥4) | .038 | |||
| Yes, n (%) | 95 (60.9) | 43 (53.1) | 52 (69.3) | |
| No, n (%) | 61 (39.1) | 38 (46.9) | 23 (30.7) | |
| Neuropathy | .21 | |||
| Yes, n (%) | 109 (69.9) | 53 (65.4) | 56 (74.7) | |
| No, n (%) | 47 (30.1) | 28 (34.6) | 19 (25.3) | |
| Falls | .16 | |||
| Yes, n (%) | 34 (21.8) | 14 (17.3) | 20 (26.7) | |
| No, n (%) | 122 (78.2) | 67 (82.7) | 55 (73.3) | |
| Organ dysfunction∗ | .013 | |||
| Yes, n (%) | 40 (25.6) | 14 (17.3) | 26 (34.7) | |
| No, n (%) | 116 (74.4) | 67 (82.7) | 49 (65.3) |
Bold indicates statistically significant difference between age groups, P < .05.
Refer to supplemental Materials for organ dysfunction definition.
Comorbidities and KarMMa trial eligibility
Besides evaluating comorbidity burden (≥4) as a geriatric characteristic, the prevalence of specific and individual comorbidities (≥1) was also analyzed to understand patients’ physical and physiological status. As shown in Table 5, the prevalence of comorbidities was 94.0% in younger and 97.5% in older patients. A total of 87 vs 102 comorbidities were recorded for younger and older patient groups, respectively. The most frequent comorbidities that were present in at least >10% of the patients in each group were captured. Neuropathy, hypertension, pain, diabetes, hyperlipidemia, immunodeficiency, renal dysfunction, and gastroesophageal reflux disease were common across the 2 age categories; however, the prevalence of these comorbidities, except gastroesophageal reflux disease, was higher in older patients. Anxiety, depression, and arthritis were more frequent in younger patients, whereas anemia, obstructive sleep apnea, and hypothyroidism were observed commonly in older patients. As shown in supplemental Table 4, a total of 18 (11.5%) patients in our study population had renal impairment (RI; creatinine clearance of <50 mL/min), including 7 (4.5%) patients with severe RI (creatinine clearance of <30 mL/min). The majority of these patients with RI and severe RI were older (n = 17, 22.7% and n = 6, 8.0%, respectively). Among all cardiac ailments, congestive heart failure was reported in 1 (1.2%) younger and 5 (6.6%) older patients. Moreover, cardiac arrythmias were reported in 7 (8.6%) younger and 12 (16%) older patients.
Comorbidities and eligibility for KarMMa trial
| . | Total . | Age of <65 y . | Age of ≥65 y . |
|---|---|---|---|
| Number of patients, n (%) | 156 | 81 (51.9) | 75 (48.0) |
| Comorbidity burden | |||
| No comorbidities | 7 (4.3) | 5 (5.9) | 2 (2.5) |
| ≥1 comorbidity | 149 (95.7) | 76 (94.0) | 73 (97.5) |
| Total comorbidities | 147 | 87 | 102 |
| Frequent comorbidities (>10% in patient population) | |||
| Neuropathy | 80 (51.2) | 36 (44.4) | 44 (58.6) |
| Hypertension | 56 (35.8) | 23 (28.3) | 33 (44.0) |
| Pain | 53 (33.9) | 23 (28.3) | 30 (40.0) |
| Diabetes | 28 (17.9) | 13 (16.0) | 15 (20.0) |
| Hyperlipidemia | 26 (16.6) | 10 (12.3) | 16 (21.3) |
| Immunodeficiency | 25 (16.0) | 10 (12.3) | 15 (20.0) |
| Renal dysfunction∗ | 22 (14.1) | 9 (11.1) | 13 (17.3) |
| GERD | 18 (11.5) | 10 (12.3) | 8 (10.6) |
| Anxiety | 18 (11.5) | 11 (13.5) | <10% |
| Depression | <10% | 11 (13.5) | <10% |
| Arthritis | <10% | 9 (11.3) | <10% |
| Anemia | <10% | <10% | 9 (12.0) |
| Hypothyroidism | <10% | <10% | 8 (10.6) |
| OSA | <10% | <10% | 8 (10.6) |
| Did not meet KarMMa trial criteria | 111 (71.1) | 53 (65.4) | 58 (77.3) |
| Individual KarMMa trial exclusion criteria | |||
| Laboratory abnormalities | 46 (41.4) | 28 (52.8) | 18 (31.0) |
| Prior cellular therapies | 41 (36.9) | 21 (39.6) | 20 (34.4) |
| ECOG PS score of ≥2 | 17 (15.3) | 9 (16.9) | 8 (13.7) |
| CNS pathology | 13 (11.7) | 6 (11.3) | 7 (12.0) |
| Cardiac dysfunction | 16 (14.4) | 4 (7.5) | 12 (20.6) |
| Anti-MM therapy within 2 wk of leukapheresis | 14 (12.6) | 7 (13.2) | 7 (12.0) |
| Active or history of PCL, POEMS, malignancy, etc | 20 (18.0) | 10 (18.8) | 10 (17.2) |
| RI | 18 (16.2) | 6 (11.3) | 12 (20.6) |
| Infections | 7 (6.3) | 5 (9.4) | 2 (3.4) |
| Significant medical, laboratory, or psychiatric events | 11 (9.9) | 8 (15.0) | 3 (5.1) |
| Nonsecretory MM | 6 (5.4) | 5 (9.4) | 1 (1.7) |
| Others | 1 (0.9) | 0 (0.0) | 1 (1.7) |
| . | Total . | Age of <65 y . | Age of ≥65 y . |
|---|---|---|---|
| Number of patients, n (%) | 156 | 81 (51.9) | 75 (48.0) |
| Comorbidity burden | |||
| No comorbidities | 7 (4.3) | 5 (5.9) | 2 (2.5) |
| ≥1 comorbidity | 149 (95.7) | 76 (94.0) | 73 (97.5) |
| Total comorbidities | 147 | 87 | 102 |
| Frequent comorbidities (>10% in patient population) | |||
| Neuropathy | 80 (51.2) | 36 (44.4) | 44 (58.6) |
| Hypertension | 56 (35.8) | 23 (28.3) | 33 (44.0) |
| Pain | 53 (33.9) | 23 (28.3) | 30 (40.0) |
| Diabetes | 28 (17.9) | 13 (16.0) | 15 (20.0) |
| Hyperlipidemia | 26 (16.6) | 10 (12.3) | 16 (21.3) |
| Immunodeficiency | 25 (16.0) | 10 (12.3) | 15 (20.0) |
| Renal dysfunction∗ | 22 (14.1) | 9 (11.1) | 13 (17.3) |
| GERD | 18 (11.5) | 10 (12.3) | 8 (10.6) |
| Anxiety | 18 (11.5) | 11 (13.5) | <10% |
| Depression | <10% | 11 (13.5) | <10% |
| Arthritis | <10% | 9 (11.3) | <10% |
| Anemia | <10% | <10% | 9 (12.0) |
| Hypothyroidism | <10% | <10% | 8 (10.6) |
| OSA | <10% | <10% | 8 (10.6) |
| Did not meet KarMMa trial criteria | 111 (71.1) | 53 (65.4) | 58 (77.3) |
| Individual KarMMa trial exclusion criteria | |||
| Laboratory abnormalities | 46 (41.4) | 28 (52.8) | 18 (31.0) |
| Prior cellular therapies | 41 (36.9) | 21 (39.6) | 20 (34.4) |
| ECOG PS score of ≥2 | 17 (15.3) | 9 (16.9) | 8 (13.7) |
| CNS pathology | 13 (11.7) | 6 (11.3) | 7 (12.0) |
| Cardiac dysfunction | 16 (14.4) | 4 (7.5) | 12 (20.6) |
| Anti-MM therapy within 2 wk of leukapheresis | 14 (12.6) | 7 (13.2) | 7 (12.0) |
| Active or history of PCL, POEMS, malignancy, etc | 20 (18.0) | 10 (18.8) | 10 (17.2) |
| RI | 18 (16.2) | 6 (11.3) | 12 (20.6) |
| Infections | 7 (6.3) | 5 (9.4) | 2 (3.4) |
| Significant medical, laboratory, or psychiatric events | 11 (9.9) | 8 (15.0) | 3 (5.1) |
| Nonsecretory MM | 6 (5.4) | 5 (9.4) | 1 (1.7) |
| Others | 1 (0.9) | 0 (0.0) | 1 (1.7) |
KarMMa trial inclusion and exclusion criteria detailed in supplemental Materials.
CNS, central nervous system; GERD, gastroesophageal reflux disease; OSA, obstructive sleep apnea; PCL, plasma cell leukemia; POEMS, polyneuropathy, organomegaly, endocrinopathy, monoclonal protein, skin changes.
Renal dysfunction: acute kidney disease, or chronic kidney disease, or renal insufficiency.
Various physical and/or physiological characteristics, especially in older patients, could diminish their chances to participate in clinical trials. Therefore, we assessed these patients to evaluate whether they would meet KarMMa trial eligibility. As shown in Table 5, 65.4% of the younger patients did not meet eligibility criteria for KarMMa trial participation compared with 77.3% of the older patients. Exclusion because of cardiac dysfunction and renal insufficiency were more prevalent in older patients. In contrast, laboratory abnormalities were more frequent exclusion events in younger patients.
All 156 patients were also reanalyzed with a different age cutoff (aged <70 years, n = 120 vs aged ≥70 years, n = 36) for frailty, response, PFS, and OS (supplemental Figure 1). A notable discrepancy was observed in the prevalence of patients who are frail (46.7% of those aged <70 years vs 87.5% of those aged ≥70 years, P < .001). The rate of ORR (84.2% for those aged <70 years vs 88.9% for those aged ≥70 years) and CR or better (54.2% for those aged <70 years vs 58.3% for those aged ≥70 years) were similar across the age groups. There was no significant difference in median PFS (7.5 months for those aged <70 years vs 9.8 months for those aged ≥70 years), as well as OS (24.2 months for those aged <70 years vs >28 months for those aged ≥70 years).
Discussion
Because the median age of diagnosis for MM is 69 years, the clinical outcomes in older patients along with frailty and geriatric characteristics are an intense focus of interest because of its importance in identifying and selecting an appropriate patient who could potentially benefit from CART therapy. To the best of our knowledge, this is the first multicenter study that evaluated safety and efficacy along with frailty and geriatric characteristics in real-world older patients with MM who received ide-cel.
In contrast to the inferior outcomes often observed in older patients compared with younger counterparts receiving anti-MM therapies,15 our findings indicate that ide-cel demonstrates comparable efficacy and safety profiles across age groups. This suggests that ide-cel treatment mitigates the disparity in outcomes typically seen with other therapies and provides equitable benefits. Similarly, our results were also comparable with the results of pivotal KarMMa study population as well as the real-world US MM Immunotherapy Consortium population, the former received investigational ide-cel whereas the later received commercial ide-cel.10,16 A recently published single-center study also found globally comparable safety and efficacy outcomes between younger (aged <70 years) and older (aged ≥70 years) patients with MM who received BCMA-directed CART therapy.17 In terms of ORR and PFS in younger and older patients, our findings are comparable as well to the aforementioned recently published study. Although the study by Reyes et al17 had all patients aged ≥70 years with an ECOG PS score of either 0 or 1, our subcohort of older patients (aged ≥70 years) had 17% individuals with an ECOG PS score of ≥ 2, which might potentially be representative of a real-world older MM population. Our findings are also comparable with recently presented Center for International Blood and Marrow Transplant Research (CIBMTR) data that showed significant improvements in PFS in those aged ≥70 years and no differences in PFS based on frailty in a larger cohort of patients (n = 686).18 We also observed similar numerical differences in PFS (7.5 vs 9.8 months favoring older patients aged ≥70 years) and OS (24 vs 28 months favoring older patients aged ≥70 years) but lack of statistical significance could be because of limited sample size. Although our univariate analysis suggested association between ECOG PS score of ≥2 with risk of poor survival in older patients (aged ≥65 years; P < .05), this was not significant on multivariable analysis. There was no association observed between OS and selected patient characteristics such as high-risk cytogenetics, extramedullary disease, prior BCMA therapies, penta-refractory status, polypharmacy, or comorbidities, either with univariate or multivariable analysis. Moreover, the younger patient cohort observed to be equipoise with the older patient cohort in several baseline characteristics such as sex, ethnicity, myeloma subtypes, extramedullary disease, high marrow burden, disease stage, and cytogenetics abnormalities. It could be argued that the improved patient outcomes observed in our older cohort may be attributed to presence of indolent disease, which often requires no treatment or less intensive therapies compared with individuals with overt MM.19 We assessed time between MM diagnosis to CART infusion and number of prior lines of therapies received by both the age groups but did not identify any significant difference. This finding effectively dismisses the possibility of an indolent disease driving the comparable efficacy outcomes observed in older patients. Nevertheless, provider decision-making in selecting an older patient for CART therapy could favor those with better functional, mental, and physiological status, and may play role in equitable outcomes in older patients. However, a validated patient selection tool would be instrumental in avoiding such potential biases in patient selection.
Physical and physiological decline with age and presence of multiple comorbidities render the older patients with a higher risk of frailty and vulnerability to increased safety concerns with various anti-MM therapies. In contrast, despite the significant number of older patients who are frail, we observed comparable safety-related outcomes in these patients. The rates of any grade and grade ≥3 CRS and ICANS were similar without a significant difference between younger and older patients. Recently, a review was conducted to understand the complex problem of CART therapy–associated cytopenias, which could negatively affect patient outcomes with increased infections, use of blood component transfusions, and increased use of medical resources.20 In our study population, the rates of grade ≥3 anemia, thrombocytopenia, and neutropenia at days 30, 60, and 90 after CART therapy were the same for younger and older patients. A gradual decline was observed in the number of patients requiring supportive care measures for cytopenias from month 1 until end of month 3 after CART infusion. We observed that <20% of patients with unresolved cytopenias compared with a recently published study that reported >20% of patients (age range, 36-78 years) with grade 3/4 cytopenias requiring supportive care at the 3- to 6-month mark.21 Thus, resolution of cytopenias in ∼80% of our patient population could be attributed to marrow recovery after CART therapy. Despite a notably higher prevalence of patients who are frail and the presence of geriatric characteristics such as comorbidities, polypharmacy, and organ dysfunction, it is noteworthy that older patients did not experience any severe adverse events in this study. Our findings align with the results of the study by Reyes et al17 and Akhtar et al18 that reported comparable safety-related outcomes in younger and older patients.
It is recently reported that <3% of older adults with MM participate in clinical trials.22,23 A recent systematic review of literature, to identify barriers in enrollment of older patients in cancer clinical trials, found multifaceted barriers categorized as system barriers, provider barriers, patient barriers, and caregiver barriers.22,23 Stringent eligibility criteria has been reported as 1 of the major barriers for older patients’ participation in clinical trials.22,23 The use of certain eligibility criteria is often used to ensure older patients’ safety and wellbeing. However, such criteria should be based on scientific evidence rather than exaggerated concerns for safety. We noted that 77.3% of older patients (aged ≥65 years) did not meet eligibility criteria for KarMMa study and, despite this fact, the efficacy as well as safety results were comparable. Such observation provides scientific evidence rather for consideration of relaxing clinical trial eligibility criteria to pave the way for more participation by older patients. Based on our observation of the older patient group, we are proposing some of the modifications to amend eligibility criteria (please refer to supplemental Table 5) for future clinical trials, which could pave the way for increased geriatric patient participation.
The high prevalence of multiple geriatric characteristics such as polypharmacy, comorbidities, neuropathy, fall, and organ dysfunction in older patients poses significant risk for frailty and poorer overall outcomes. We found significant differences in frailty and geriatric characteristics such as comorbidities, polypharmacy, and organ dysfunction between younger and older patients, but none of these characteristics had an adverse impact on safety and efficacy outcomes. Our findings suggest that ide-cel could be a safe treatment option for older patients with multiple geriatric characteristics. Polypharmacy is a consequence of multiple comorbidities in older adults and poses higher risk for both negative health outcomes and mortality.24-26 In patients with MM, autonomic neuropathy and fall risk were reported to be significantly associated with inappropriate polypharmacy.27 In fact, in our study population, neuropathy is the most common comorbidity with prevalence rate of 44.4% in younger vs 58.9% older patients. It is unclear whether such high prevalence of neuropathy in these patients is because of polypharmacy or is MM related or associated with anti-MM therapies.
A retrospective study design is one of the several limitations of our study. Furthermore, it is important to note that although we collected data on geriatric characteristics through chart review, we did not conduct a focused geriatric assessment using a validated geriatric tool for retrospective data collection in our patient population. A simplified Facon frailty scale based on age, CCI, and ECOG PS, may not be an adequate tool to encompass the full spectrum of heterogenous frailty phenotype of older patient population. Besides, because of heavy weighting of age in Facon frailty scoring system, older patients tend be identified as frail. Selection bias, particularly for older patients, could be a factor in an equitable outcome observed in both age groups. The patient-reported outcomes were also not collected because of the retrospective study design. A small sample size, especially for patients aged ≥70 years, would be a barrier to generalize the findings.
In summary, our findings showed comparable efficacy and safety outcomes in older patients with MM who received ide-cel. Despite several significant geriatric characteristics in older patients, the clinical outcomes remained similar for younger and older patients, indicating no detrimental impact of geriatric characteristics in older patient outcomes. Moreover, the outcomes were not affected despite the majority of older patients being frail and having ≥1 physical or physiological limitations as evidenced by the majority of them not meeting KarMMa eligibility criteria. Therefore, clinical trial criteria should be relaxed to increase participation of older patients to overcome their underrepresentation in clinical trials. Thus, the introduction of novel agents such as CART therapy for MM into clinical practice holds immense potential to transform treatment of older adults globally. Nevertheless, future studies with larger patient cohort would certainly help fill the knowledge gap about how to optimize outcomes for older patients with MM and to identify those who could benefit the most.
Acknowledgments
The US MM Immunotherapy Consortium authors thank all members at each participating institution who participated in data collection and clinical patient care activities. The authors thank all of the patients and their families who participated in this study. The authors also thank Gabe De Avila for help with data.
D.K.H. is supported by the International Myeloma Society Young Investigator Award for Exemplary Abstract, the Pentecost Family Myeloma Research Center, and Moffitt Catchment Area Research Enhancements Award. S.S. is supported by Stanford Clinical and Translational Science KL2 Career Development award program (award no. KL2 TR003143) and a Stanford Cancer Institute/American Cancer Society Pilot Grant 2022. H.H. is supported by SITC-Merck Cancer Immunotherapy Clinical Fellowship.
M.A.T.H. is an Andrew Sabin Family Foundation Fellow at The University of Texas MD Anderson Cancer Center.
Authorship
Contribution: N.M.K., M.A.T.H., D.K.H., S.S., L.O.S., and K.K.P. contributed to study design; N.M.K., M.A.T.H., D.K.H., S.S., and K.K.P. contributed to data analysis and interpretation; N.M.K. and M.A.T.H. wrote the original draft of the manuscript and incorporated the comments by D.K.H., S.S., and K.K.P. in all subsequent drafts; and all authors contributed to data acquisition and provided review and edits and approved the final version of the manuscript.
Conflict-of-interest disclosure: D.K.H. has consulted for Bristol Myers Squibb, Janssen, Pfizer, and Karyopharm, and has received research funding from Bristol Myers Squibb, Karyopharm, and Adaptive Biotechnologies. S.S. reports honoraria from Prothena; consultancy with Oncopetides, Sanofi, Janssen, Magenta Therapeutics, and Bristol Myers Squibb; and received research funding from Janssen, Bristol Myers Squibb, and Allogene. J.K. reports consultancy with Janssen and honoraria from Legend, GPCR Therapeutics, and Prothena. C.J.F. reports honoraria from/consulting for Janssen and ownership of publicly traded stock in Affirmed. C.L.F. reports honoraria from/consulting for Bristol Myers Squibb, Seattle Genetics, Celgene, AbbVie, Sanofi, Incyte, Amgen, ONK Therapeutics, and Janssen, and received research funding from Bristol Myers Squibb, Janssen, and Roche/Genentech. K.K.P. reports honoraria from Bristol Myers Squibb, Celgene, Janssen, Merck, Pfizer, Karyopharm, Takeda, Curio Bioscience, and AbbVie, and research funding from Bristol Myers Squibb, Janssen, AbbVie, Nektar, Allogene, Precision Bio, Cellectis, and Takeda. The remaining authors declare no competing financial interests.
Correspondence: Krina K. Patel, Department of Lymphoma/Myeloma, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd, Unit 0429, Houston, TX 77030-4009; email: kpatel1@mdanderson.org.
References
Author notes
N.M.K. and M.A.T.H. are joint first authors and contributed equally to this study.
L.O.S. and K.K. P. are joint senior authors.
Data are available on request from the corresponding author, Krina K. Patel (kpatel1@mdanderson.org).
The full-text version of this article contains a data supplement.