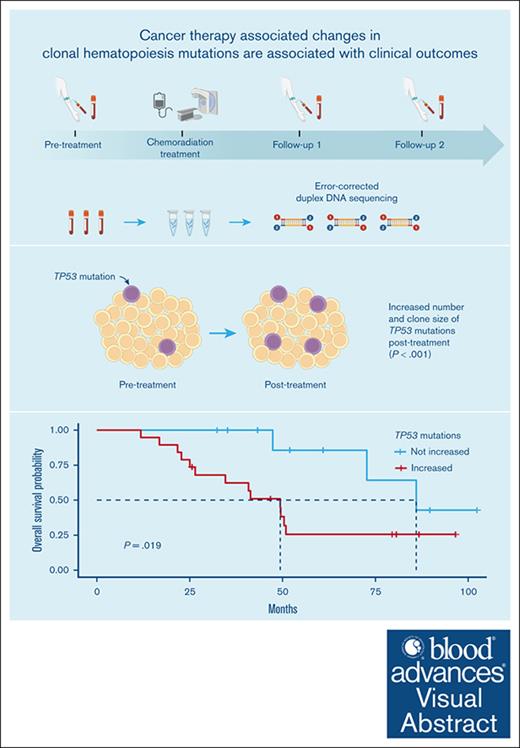
Key Points
We observed an increase in the number and clone size of TP53 CH mutations after chemoradiation therapy.
We found an association between increased TP53 mutations after chemoradiation therapy and shorter overall survival.
Visual Abstract
Exposure to cancer therapies is associated with an increased risk of clonal hematopoiesis (CH). The objective of our study was to investigate the genesis and evolution of CH after cancer therapy. In this prospective study, we undertook error-corrected duplex DNA sequencing in blood samples collected before and at 2 time points after chemoradiation in patients with esophageal or lung cancer recruited from 2013 to 2018. We applied a customized workflow to identify the earliest changes in CH mutation count and clone size and determine their association with clinical outcomes. Our study included 29 patients (87 samples). Their median age was 67 years, and 76% (n = 22) were male; the median follow-up period was 3.9 years. The most mutated genes were DNMT3A, TET2, TP53, and ASXL1. We observed a twofold increase in the number of mutations from before to after treatment in TP53, which differed from all other genes examined (P < .001). Among mutations detected before and after treatment, we observed an increased clone size in 38% and a decreased clone size in 5% of TP53 mutations (odds ratio, 3.7; 95% confidence interval [CI], 1.75-7.84; P < .001). Changes in mutation count and clone size were not observed in other genes. Individuals with an increase in the number of TP53 mutations after chemoradiation experienced shorter overall survival (hazard ratio, 7.07; 95% CI, 1.50-33.46; P = .014). In summary, we found an increase in the number and size of TP53 CH clones after chemoradiation that were associated with adverse clinical outcomes.
Introduction
Cancers frequently arise from a multistage successive acquisition of somatic (ie, acquired) mutations that increase cell fitness. A limited number of these mutations may lead to a clonal expansion of the mutated cell population, without malignant transformation. Clonal hematopoiesis (CH) refers to somatic mutations that lead to clonal expansion in the hematopoietic system and has been identified from sequencing data as a common age-associated process.1,2 Individuals with CH have a substantially higher risk of hematologic malignancy and shorter overall survival (OS) than those without CH.3 Additionally, CH is associated with an increased risk of numerous noncancer adverse health outcomes, including cardiovascular, cerebrovascular, pulmonary, endocrine, renal, and liver disease.3-5 Therefore, CH may represent a common, readily detectable, and potentially actionable biomarker that meaningfully increases the risk of numerous future adverse health outcomes.
Although increasing age has been identified as the strongest predictor of CH, environmental exposures are emerging as key risk factors.6-9 Among these, prior exposure to cancer therapies, including chemotherapy and radiation therapy, is associated with an increased risk of carrying detectable CH mutations, particularly in DNA damage response (DDR) genes.10-13 In fact, CH mutations have been shown to be common in patients with cancer, detectable in ∼1 in 3 individuals, and are associated with a high increased risk of therapy–related myeloid neoplasm.13 However, limited studies have investigated the genesis and evolution of CH after exposure to cancer therapy, largely secondary to the need for serial blood samples from before and after therapy as well as sequencing approaches that are sufficient to examine small clone sizes (eg, low variant allele fraction [VAF] mutations ≤2%).
In this prospective study, we determined the impact of cancer treatment on CH mutations by conducting error-corrected duplex DNA sequencing in patients with solid malignancies using serially obtained blood samples from before any treatment and after chemoradiation therapy. We also determined the association between observable changes in CH after cancer therapy and clinical outcomes. Cancer treatment may induce or select for CH, and CH is associated with adverse health outcomes across multiple organ systems. Therefore, understanding the impact of cancer therapy on CH may have important public health implications.
Methods
Patients
We recruited patients aged ≥18 years from 2013 to 2018 with locally advanced esophageal or non–small cell lung cancer who were undergoing treatment at The University of Texas MD Anderson Cancer Center. Patients’ demographic and clinical data were collected at enrollment, and their detailed treatment information was recorded. Patients were followed for cancer recurrence and vital status. The study was approved by the institutional review board of MD Anderson Cancer Center, with informed consent obtained from all participants.
Blood samples were collected before the initiation of cancer treatment, midway through chemoradiation therapy, at the conclusion of chemoradiation therapy, and at interval clinical follow-up appointments. DNA was derived from buffy coat. Consistent with the standard of care, some patients underwent surgical resection during the interval between the pretreatment and follow-up blood draws.
Identification of CH mutations
We undertook duplex DNA sequencing14-16 using the TwinStrand Duplex Sequencing AML-29 assay, which incorporates a targeted panel of 29 genes (supplemental Table 1) to detect single nucleotide variants, insertions and deletions, and structural variants that are recurrently mutated in adults with acute myeloid leukemia, CH, and myelodysplastic syndromes. Per sample, our average read depth was 15 564 (interquartile range, 14 893-16 308). We applied a series of postprocessing filters to remove possible false-positive variants and putative germ line polymorphisms adapted from published methods.17,18 Detailed methods are available in the supplemental Material.
Selection analysis
We quantified selection for nonsynonymous mutations using the ratio of nonsynonymous to synonymous substitutions.19 We used the dNdScv package (https://github.com/im3sanger/dndscv) to estimate the ratio at the gene level.13 Statistical significance was prespecified as a multiple testing corrected q-value of <0.10 for this analysis.
Mutational signatures
We used the identified somatic singleton mutations in our data set to generate mutational signatures, as previously described.20 We compared the signatures identified in our data set with those in the Catalogue of Somatic Mutations in Cancer (COSMIC) Mutational Signatures v3.2 using the R package MutationalPatterns.20
mCAs
We investigated acquired structural chromosomal events or mosaic chromosomal alterations (mCAs) in the collected serial blood samples. Specifically, we conducted genome-wide genotyping using the Illumina Infinium Global Screening Array, which encompasses ∼660 000 single nucleotide polymorphisms. We then applied haplotype-based methods (hapLOH) to identify mCAs.21
Statistical analysis
We examined the association between the absolute number of mutations per individual with age at baseline using the Pearson correlation coefficient. The proportion of mutations from before treatment to last follow-up by gene was calculated using a χ2 test. When examining changes in VAF from baseline to last follow-up, we defined an increase as a VAF ratio of last follow-up VAF to pretreatment VAF ≥2.0 and a decrease as last follow-up VAF to pretreatment VAF ≤0.5.22 Changes in mutation count per individual from before treatment to last follow-up were examined using a paired Wilcoxon signed-rank test.
We tested the association between an increase in CH mutations from before treatment to last follow-up with clinical outcomes, including treatment toxicity, 5-year cancer relapse, and OS. Specifically, we examined the absolute increase in CH mutations, defined as “total CH mutations at last follow-up – total CH mutations before treatment,” as a binary variable comparing increased (≥1) vs not increased (<1). We defined treatment toxicity, including esophagitis, dermatitis, and pneumonitis, per the Common Terminology Criteria for Adverse Events. We examined the association between changes in CH mutations with any grade ≥2 treatment toxicity using a logistic regression analysis adjusted for age (continuous) and cancer type (categorical). We examined the association between changes in CH mutations with 5-year cancer relapse and OS using Cox proportional hazards regression analysis, adjusted for age and cancer type. Survival curves were compared using the Peto-Peto method. We calculated OS from the date of cancer diagnosis until death or until censorship at last follow-up. We did not analyze the cause of death because we were unable to reliably determine the cause of death in this cohort.
We accounted for multiple testing by calculating q-values using a false discovery rate of 5%. Tests were considered statistically significant with a 2-sided alpha of 0.05 unless otherwise stated. Statistical analyses were conducted in R (4.1.0). K.T.N., T.B., and L.J. were responsible for the analysis of data.
Results
Cohort characteristics
Twenty-nine patients with esophageal or non–small cell lung cancer were identified and included in this study. Their demographic and cancer diagnosis and treatment data are presented in Table 1. All individuals underwent concurrent chemoradiation therapy for esophageal or non-small cell lung cancer. The median cohort age was 66.9 years; 22 patients (76%) were male, and 22 (76%) were ever smokers. The median cohort follow-up period was 3.9 years (range, 1.0-8.5). We analyzed DNA sequencing data from 87 unique blood samples across 29 individuals from before treatment and at 2 separate time points after the completion of chemoradiation therapy. The median time from the start of chemoradiation therapy to the first analyzed blood draw was 5 months (range, 2-11) and to the last analyzed blood draw was 17 months (range, 7-37).
Baseline cohort participant characteristics
| Characteristic . | Esophageal cancer . | Lung cancer . | All . |
|---|---|---|---|
| (n = 14) . | (n = 15) . | (N = 29) . | |
| Age, y | |||
| Mean (SD) | 65.3 (9.4) | 66.2 (6.5) | 65.8 (7.9) |
| Median (Q1, Q3) | 66.5 (64.0, 71.9) | 67.0 (64.7, 70.2) | 66.9 (63.5, 71.7) |
| Race and ethnicity | |||
| Black | 1 (7%) | 1 (7%) | 2 (7%) |
| Non-Hispanic White | 13 (93%) | 13 (87%) | 26 (90%) |
| Hispanic | 0 (0%) | 1 (7%) | 1 (3%) |
| Sex | |||
| Female | 1 (7%) | 6 (40%) | 7 (24%) |
| Male | 13 (93%) | 9 (60%) | 22 (76%) |
| Ever smoked | |||
| No | 5 (36%) | 2 (13%) | 7 (24%) |
| Yes | 9 (64%) | 13 (87%) | 22 (76%) |
| Histology | |||
| Adenocarcinoma | 10 (71%) | 9 (60%) | 19 (66%) |
| SCC | 3 (21%) | 5 (33%) | 8 (28%) |
| Other | 1 (7%) | 1 (7%) | 2 (7%) |
| T stage | |||
| T1 | 1 (7%) | 4 (27%) | 5 (17%) |
| T2 | 1 (7%) | 4 (27%) | 5 (17%) |
| T3 | 11 (79%) | 3 (20%) | 14 (48%) |
| T4 | 1 (7%) | 4 (27%) | 5 (17%) |
| N stage | |||
| N0 | 6 (43%) | 2 (13%) | 8 (28%) |
| N1 | 7 (50%) | 1 (7%) | 8 (28%) |
| N2 | 0 (0%) | 7 (47%) | 7 (24%) |
| N3 | 0 (0%) | 5 (33%) | 5 (17%) |
| Nx | 1 (7%) | 0 (0%) | 1 (3%) |
| Stage group | |||
| I | 1 (7%) | 0 (0%) | 1 (3%) |
| IIb | 4 (29%) | 1 (7%) | 5 (17%) |
| IIIa | 8 (57%) | 5 (33%) | 13 (45%) |
| IIIb | 1 (7%) | 9 (60%) | 10 (35%) |
| Chemotherapy | |||
| Platinum and taxane | 7 (50%) | 12 (80%) | 19 (66%) |
| Platinum only | 3 (21%) | 3 (20%) | 6 (21%) |
| Taxane only | 4 (29%) | 0 (0%) | 4 (14%) |
| Radiation modality | |||
| Photon | 11 (79%) | 10 (67%) | 21 (72%) |
| Proton | 3 (21%) | 5 (33%) | 8 (28%) |
| Radiation dose, median (Q1, Q3), Gy | 50.4 (50.4, 50.4) | 70.0 (66.0, 70.0) | 60.0 (50.4, 70.0) |
| Radiation fractions, median (Q1, Q3) | 28 (28, 28) | 33 (30-35) | 30 (28, 33) |
| Radiation volume, planning target volume (Q1, Q3), cm3 | 532 (349, 809) | 483 (319, 580) | 519 (328, 689) |
| Surgery | |||
| No | 8 (57%) | 15 (100%) | 23 (79%) |
| Yes | 6 (43%) | 0 (0%) | 6 (21%) |
| Characteristic . | Esophageal cancer . | Lung cancer . | All . |
|---|---|---|---|
| (n = 14) . | (n = 15) . | (N = 29) . | |
| Age, y | |||
| Mean (SD) | 65.3 (9.4) | 66.2 (6.5) | 65.8 (7.9) |
| Median (Q1, Q3) | 66.5 (64.0, 71.9) | 67.0 (64.7, 70.2) | 66.9 (63.5, 71.7) |
| Race and ethnicity | |||
| Black | 1 (7%) | 1 (7%) | 2 (7%) |
| Non-Hispanic White | 13 (93%) | 13 (87%) | 26 (90%) |
| Hispanic | 0 (0%) | 1 (7%) | 1 (3%) |
| Sex | |||
| Female | 1 (7%) | 6 (40%) | 7 (24%) |
| Male | 13 (93%) | 9 (60%) | 22 (76%) |
| Ever smoked | |||
| No | 5 (36%) | 2 (13%) | 7 (24%) |
| Yes | 9 (64%) | 13 (87%) | 22 (76%) |
| Histology | |||
| Adenocarcinoma | 10 (71%) | 9 (60%) | 19 (66%) |
| SCC | 3 (21%) | 5 (33%) | 8 (28%) |
| Other | 1 (7%) | 1 (7%) | 2 (7%) |
| T stage | |||
| T1 | 1 (7%) | 4 (27%) | 5 (17%) |
| T2 | 1 (7%) | 4 (27%) | 5 (17%) |
| T3 | 11 (79%) | 3 (20%) | 14 (48%) |
| T4 | 1 (7%) | 4 (27%) | 5 (17%) |
| N stage | |||
| N0 | 6 (43%) | 2 (13%) | 8 (28%) |
| N1 | 7 (50%) | 1 (7%) | 8 (28%) |
| N2 | 0 (0%) | 7 (47%) | 7 (24%) |
| N3 | 0 (0%) | 5 (33%) | 5 (17%) |
| Nx | 1 (7%) | 0 (0%) | 1 (3%) |
| Stage group | |||
| I | 1 (7%) | 0 (0%) | 1 (3%) |
| IIb | 4 (29%) | 1 (7%) | 5 (17%) |
| IIIa | 8 (57%) | 5 (33%) | 13 (45%) |
| IIIb | 1 (7%) | 9 (60%) | 10 (35%) |
| Chemotherapy | |||
| Platinum and taxane | 7 (50%) | 12 (80%) | 19 (66%) |
| Platinum only | 3 (21%) | 3 (20%) | 6 (21%) |
| Taxane only | 4 (29%) | 0 (0%) | 4 (14%) |
| Radiation modality | |||
| Photon | 11 (79%) | 10 (67%) | 21 (72%) |
| Proton | 3 (21%) | 5 (33%) | 8 (28%) |
| Radiation dose, median (Q1, Q3), Gy | 50.4 (50.4, 50.4) | 70.0 (66.0, 70.0) | 60.0 (50.4, 70.0) |
| Radiation fractions, median (Q1, Q3) | 28 (28, 28) | 33 (30-35) | 30 (28, 33) |
| Radiation volume, planning target volume (Q1, Q3), cm3 | 532 (349, 809) | 483 (319, 580) | 519 (328, 689) |
| Surgery | |||
| No | 8 (57%) | 15 (100%) | 23 (79%) |
| Yes | 6 (43%) | 0 (0%) | 6 (21%) |
Data are presented as count (%) unless otherwise indicated.
N, number; Q, quartile; SCC, squamous cell carcinoma; SD, standard deviation.
CH mutations at baseline
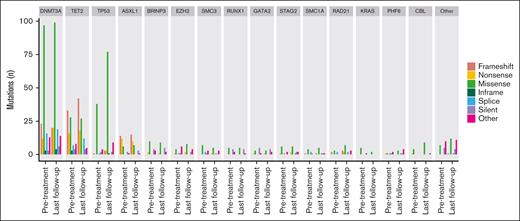
The overall number of mutations detected before treatment and at posttreatment follow-up for the full cohort are presented in Figure 1. Mutation counts by individual at each time point are presented in supplemental Figure 1. Individual CH mutations identified are summarized in supplemental Table 2. Consistent with the results of prior studies,13,23 the most mutated genes in our analysis at baseline were DNMT3A, TET2, TP53, and ASXL1. When considering all CH mutations identified at baseline, the median VAF was 0.04% (range 0.01%-4.56%). Consistent with the detection of ultralow VAF mutations, we identified ≥1 CH mutations in all individuals at baseline with a median of 15 CH mutations per individual (range, 4-36). When considering CH mutations with a VAF of ≥2%, we detected ≥1 CH mutations in 8 individuals (28%) at baseline. The VAF for each mutation identified at all time points is summarized in supplemental Figure 2.
Total CH mutations detected before treatment and at posttreatment follow-up. Bar plot of the number of total mutations in each gene detected before chemoradiation therapy and at last follow-up, stratified by gene.
Total CH mutations detected before treatment and at posttreatment follow-up. Bar plot of the number of total mutations in each gene detected before chemoradiation therapy and at last follow-up, stratified by gene.
CH mutation changes after chemoradiation therapy
We found a more than twofold increase in the number of mutations detected from before treatment to last follow-up in TP53 (pretreatment, n = 46; last follow-up, n = 95) and RAD21 (pretreatment, n = 7; last follow-up, n = 17). The observed increase in the proportion of TP53 mutations statistically significantly differed from those observed in DNMT3A, TET2, ASXL1, and all other genes combined, including when accounting for multiple comparisons (supplemental Table 3). RAD21 was not analyzed against all other genes owing to low counts but did not statistically differ from TP53 when compared directly (P = .921). We observed a statistically significantly positive association between the number of CH mutations per individual and patient age (Pearson R = 0.47; P = .010; supplemental Figure 3), consistent with published data.1,2 Notably, the observed increase in TP53 mutations at last follow-up was similar to that observed at the first follow-up, which occurred at a median of 4 months after the completion of chemoradiation (Figure 1; supplemental Table 3). We continued to observe a large relative increase in TP53 mutations from before treatment to last follow-up compared with other genes when examining only CH mutations with VAFs ≥0.2% (supplemental Figure 4).
The observed increase in the total number of mutations in TP53 and RAD21 from before treatment to last follow-up were primarily driven by a more than twofold change in missense mutations (Figure 2). To explore this finding, we quantified the strength of positive selection for missense mutations detected before treatment and at last follow-up using the dN/dS method (supplemental Figure 5). We observed evidence of selection that met our prespecified significance threshold for missense mutations in TP53, DNMT3A, TET2, ASXL1, and RAD21. The strength of positive selection for TP53 missense mutations was higher after chemoradiation therapy than before treatment, whereas an inverse relationship was observed for all other genes examined.
CH mutation types detected before treatment and at posttreatment follow-up in each gene. “Other” includes variants in 3′ and 5′ untranslated regions, upstream and downstream gene variants, and intronic variants.
CH mutation types detected before treatment and at posttreatment follow-up in each gene. “Other” includes variants in 3′ and 5′ untranslated regions, upstream and downstream gene variants, and intronic variants.
We conducted a mutational signature analysis and observed enrichment for C>T mutations at baseline that was numerically increased at last follow-up, particularly in non-CpG sites (supplemental Figure 6A-B). The largest magnitude of change from before treatment to last follow-up was for signatures that were correlated with immunosuppression and defective DNA mismatch repair (supplemental Figure 6C).
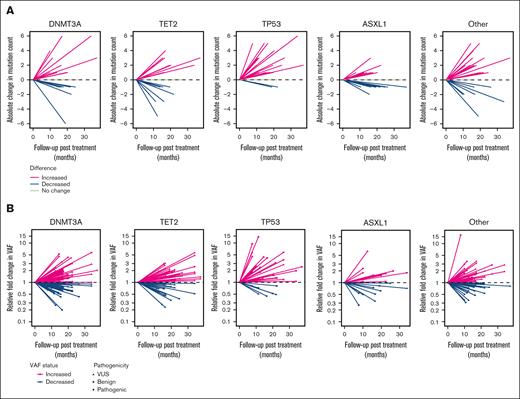
We next examined changes in mutation count from before treatment to last follow-up by gene within each patient (Figure 3A; supplemental Figure 7A). We observed a statistically significant increase in the number of TP53 mutations from before treatment to last follow-up (P < .001), including after adjusting for multiple testing (supplemental Table 4). We observed no statistically significant changes in mutation count from baseline to last follow-up in any other genes examined, although a marginal significance threshold for increased mutation count was observed for RAD21 (P = .074).
Changes in variant count and VAF before treatment to last follow-up by gene. (A) Line plot in which each line represents an individual study participant and shows the absolute change in the number of mutations before treatment to last follow-up. (B) Line plot in which each line represents an individual mutation and the relative fold change in VAF before treatment to last follow-up. VUS, variant of uncertain significance.
Changes in variant count and VAF before treatment to last follow-up by gene. (A) Line plot in which each line represents an individual study participant and shows the absolute change in the number of mutations before treatment to last follow-up. (B) Line plot in which each line represents an individual mutation and the relative fold change in VAF before treatment to last follow-up. VUS, variant of uncertain significance.
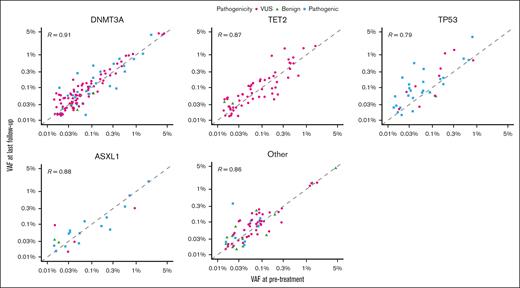
We next assessed relative changes in VAF from before treatment to last follow-up for variants detected at both time points (Figure 3B; supplemental Figure 7B). After chemoradiation therapy, there was an increased clone size in 38% of TP53 mutations and a decreased clone size in 5%. This difference corresponded to greater odds of observing an increase in clone size in TP53 after chemoradiation therapy than in all other genes combined (odds ratio, 3.7; 95% confidence interval [CI], 1.75-7.84; P < .001). We observed similar findings for changes in mutation count and clone size when examining TP53 in lung and esophageal cancers separately (supplemental Figure 8). Data on absolute changes in VAF from before treatment to last follow-up by gene are presented in Figure 4.
Correlation between VAF before and after treatment. Mutations present at both before treatment and last follow-up are shown. Dashed line represents a reference R value of 1.0.
Correlation between VAF before and after treatment. Mutations present at both before treatment and last follow-up are shown. Dashed line represents a reference R value of 1.0.
mCAs
We identified mCAs in 3 of the 29 participants (10%) before treatment and 4 (14%) at last follow-up (supplemental Table 5). All mCAs detected at baseline were identified at both posttreatment time points. In 1 participant, we identified a mosaic gain on chromosome 9 at both posttreatment time points that was not detected before treatment. Individuals with mCAs detected before treatment had a nonstatistically significant increased mean number of TP53 mutations before treatment (1.4 vs 2.3 mutations; P = .378). Similarly, individuals with mCAs detected at last follow-up had a numerically increased mean number of TP53 mutations at last follow-up (3.0 vs 4.5 mutations; P = .281). No directional effects were observed for any other genes or overall mutation counts.
Clinical outcomes
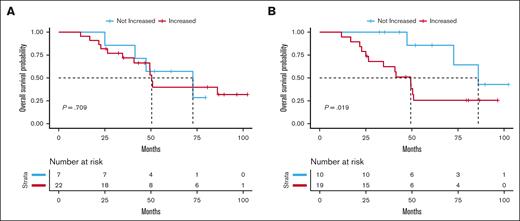
Finally, we tested the association between the increase in CH mutations from before treatment to last follow-up and clinical outcomes. We did not observe an association between an increase in the number of CH mutations, when considering all genes together, and grade ≥2 treatment toxicity, relapse, or OS (supplemental Table 6). No therapy–related myeloid neoplasms were recorded in our cohort. We next examined the association between TP53 mutations and clinical outcomes, given that TP53 showed a large magnitude, statistically significant change from before treatment to last follow-up, with positive selection for mutations that potentially alter function. We did not observe an association between changes in TP53 and toxicity or relapse. However, we did observe a statistically significant association between an increase in the number of TP53 mutations after chemoradiation therapy and shorter OS (Figure 5), including after adjusting for age and cancer type (hazard ratio [HR], 7.07; 95% CI, 1.50-33.46; P = .014). This association was similar in age-adjusted analyses when analyzing lung (HR, 5.56; 95% CI, 0.81-38.08; P = .081) and esophageal cancer (HR, 4.81; 95% CI, 0.45-51.19; P = .193) separately and when examining only variants with a VAF of ≥0.2% (supplemental Figure 9). A summary of all variants and outcomes by individual can be found in supplemental Figure 10.
OS curves according to the absolute change in the number of CH mutations from before treatment to last follow-up. (A) OS probability curve comparing individuals with increased vs not increased total mutation count in all genes from before treatment to last follow-up. (B) OS probability curve comparing individuals with increased vs not increased total mutation count in TP53 from before treatment to last follow-up.
OS curves according to the absolute change in the number of CH mutations from before treatment to last follow-up. (A) OS probability curve comparing individuals with increased vs not increased total mutation count in all genes from before treatment to last follow-up. (B) OS probability curve comparing individuals with increased vs not increased total mutation count in TP53 from before treatment to last follow-up.
Discussion
In this study, we analyzed serial samples in a cohort of patients undergoing chemoradiation therapy for solid malignancies to better understand the impact of cancer treatment on CH mutations. We observed an increase in the number of TP53 mutations after treatment, both at population and individual levels, compared with before treatment, which occurred within months of the completion of chemoradiation therapy and remained durable over time. A similar finding was observed for RAD21, which, similar to TP53, is also a DDR pathway gene. Additionally, among mutations that were present before treatment, we found an increased clone size among TP53 mutations after chemoradiation therapy, further supporting a therapy–related selection gradient. Finally, we observed an association between an increase in the number of TP53 mutations before treatment to last follow-up and shorter OS, including in adjusted models. Our study reinforces the impact of cancer treatment on somatic DDR pathway mutations in the hematopoietic system and suggests that these changes have clinical implications that deserve further investigation.
The impact of cancer therapy on CH mutations, particularly in DDR genes, has been observed in prior studies. An initial analysis of the MSK-IMPACT sequencing data among 8810 individuals with nonhematologic malignancies identified CH mutations in 25% of patients.10 They found that patients who had undergone prior radiation therapy and chemotherapy had a higher probability of carrying at least 1 CH mutation in the DDR genes TP53 and PPM1D. In an expanded analysis of 24 146 patients, prior treatment with radiation therapy, platinum agents, and topoisomerase II inhibitors was associated with CH mutations in the DDR genes TP53, PPM1D, and CHEK2.13 The largest magnitude of effect for the risk of carrying a CH putative driver mutation across all agents and genes was observed for individuals receiving external-beam radiation and radionuclide therapy. Importantly, therapy–associated TP53 mutations have been shown to contribute to the development of therapy–related myeloid neoplasms and treatment toxicity.24,25
In addition to cancer therapy, exposure to environmental mutagens has been associated with CH mutations. Numerous studies have demonstrated an increased risk of CH mutations among smokers, particularly in ASXL1.13,26 An analysis of 481 World Trade Center disaster first responders found that they had greater than threefold increased odds of CH mutations than 255 nonexposed firefighters.8 The increased CH mutations were most commonly observed in DNMT3A and TET2, which are more consistent with inflammation-mediated CH.6 Potential exposure to nontherapeutic forms of radiation have shown similar enrichment for CH mutations in DDR genes as that observed for radiation therapy. An analysis of blood samples from 14 astronauts collected 3 days after returning from space flight identified 34 nonsynonymous somatic variants at low VAF (range, 0.10%-0.95%), most commonly in TP53.27 A similar analysis demonstrated that twin astronauts had mutational profiles and high-risk CH clones that were consistent with those of individuals nearly 2 decades older.28 Overall, additional studies are needed to better understand the impact of environmental exposures, particularly nontherapeutic exposures, on CH mutations.
The key strengths of our study were the examination of CH mutations in serial samples and the utilization of methods to detect clones approaching a lower VAF limit of 0.01%. This study design facilitated the descriptive analysis of ultrasmall CH mutations from before and after cancer treatment that have not been heretofore described. In addition to providing insight regarding the earliest somatic changes after cancer therapy, we were able to determine their associations with clinical outcomes. Our approach facilitates the detection of small clones that may be missed with standard methods but that may expand over time and become increasingly likely to drive clinical outcomes through described29 and as yet undiscovered mechanisms. Additionally, although prior studies have implicated larger clones as the most likely to be clinically relevant,6 it is unclear how much this finding is driven by the limitations of existing studies in accurately identifying low VAF CH mutations (eg, secondary to limited sequencing depth). Our finding of an association between an increased number of TP53 mutations and shorter OS suggests that changes in CH mutations secondary to oncologic therapy, including ultrasmall clones, are a biomarker for adverse outcomes, causal or otherwise, which has not been previously shown and should be investigated in future studies. Our results, including an impact of posttherapy changes in CH mutations in TP53 on OS, were consistent when using higher VAF thresholds less susceptible to potential technical artifact.
Our work builds on prior studies in serial blood samples from patients undergoing oncologic therapy. Bolton et al examined sequential blood samples from 525 patients with solid tumors, of whom 61% received cytotoxic or radiation therapy, to detect CH mutations with a VAF of ≥2%.13 They found that among mutations detected at both time points, 28% had an increased clone size and 10% had a decreased clone size at the later time point. Among those with exposure to cytotoxic or radiation therapy, but not in its absence, the growth in clone size was most pronounced in TP53, PPM1D, and CHEK2. The authors also observed a dose-response relationship on clone selection, further supporting biologic plausibility. Similarly, we observed an increased clone size in 38% of TP53 mutations compared with a decreased clone size in 5% of TP53 mutations after chemoradiation therapy. Additionally, Bolton et al13 showed a nonstatistically significant, numerically greater increase in the proportion of patients with newly detected mutations who received interval cytotoxic or radiation therapy than those who did not (4% vs 1%). Building on this finding, we conducted a related analysis and showed a multiple testing–adjusted statistically significant increase in the number of TP53 mutations per individual after chemoradiation therapy (P < .001).
The examination of CH mutations in serial samples from a prospective study of older patients undergoing treatment with cytotoxic therapy for breast cancer has also demonstrated somatic changes in DDR genes.30 Specifically, they found that all CH mutations detected in TP53 and PPM1D after therapy either became detectable or expanded in size compared with before therapy. Conversely, an analysis of serial samples in younger women treated for breast cancer in the prospective Young Women’s Breast Cancer Study found no association between oncologic therapy and CH mutations, overall or in DDR genes.31 Ours and others’ findings in patients undergoing cancer therapy, which largely support selection for CH mutations in DDR genes, contrast general population studies of serial samples in which spliceosome (SF3B1/SRSF2/U2AF1) and JAK2-mutated clones demonstrate the highest growth rates.32 Although cancer therapy may affect CH mutations in DDR genes, further research is needed to understand which patient populations are most at risk.
In our analysis, we found that individuals with an increase in the number of TP53 mutations after chemoradiation therapy had shorter OS. Prior studies in patients with cancer have noted both a survival detriment in patients with CH mutations and a strong association between CH mutations and therapy–related myeloid neoplasms.3,10,11,13 In particular, TP53 CH mutations may progress to therapy–related myeloid neoplasms through selection of existing clones or the acquisition of additional drivers on an established CH clone.33 Although CH mutations were associated with a shorter OS in the MSK-IMPACT study and were strongly associated with therapy–related myeloid neoplasms related to DDR gene clones, 98% of all deaths were attributable to progression of the primary nonhematologic cancer.10 One explanation for this finding is that CH may contribute to “bad aging” through its association with myriad adverse health outcomes, including cardiovascular, cerebrovascular, pulmonary, endocrine, renal, and liver disease, which may affect patients’ ability to successfully complete standard cancer therapy.3 CH may also directly compound treatment-limiting toxicities. For example, CH mutations are associated with neutropenia, and individuals with CH mutations have been shown to be more likely to require cytotoxic therapy dose reductions and treatment holds, although this was not observed in our cohort.30,34,35 Additionally, both CH mutations and radiation therapy result in proinflammatory cytokine release and subsequent accelerated atherosclerosis through macrophage activation and recruitment, which may ultimately worsen cardiotoxicity.29,36-38 Finally, the development and progression of CH after cancer therapy may be a marker of T-cell exhaustion, in which immune system dysfunction both allows the development of CH and progression of latent cancer cells. Further studies are needed of CH mutations in patients undergoing cancer therapy that are designed to capture treatment toxicity and long-term health outcomes.
Limitations
Our study has several limitations. First, no sequencing data were available for the primary tumor; therefore, we cannot directly exclude somatic tumor mutations from our analysis. However, our analysis favors CH as the explanation for increased mutations detected after treatment because (1) we observed similar numbers of mutations and clinical effects in lung and esophageal cancer; (2) we did not observe an association between increased CH mutations and relapse; (3) changes were limited to DDR genes, consistent with prior studies; and (4) we used DNA derived from buffy coat samples, which are less likely to contain tumor-derived DNA. However, we cannot exclude the possibility that our results reflect the detection of circulating tumor DNA, which could explain the observed survival association. A second limitation is that all individuals in our study were treated with chemotherapy and radiation therapy; therefore, we were unable to differentiate the contribution of each modality to the observed somatic changes. Third, we did not have an untreated control group and cannot exclude the impact of time and increasing age on CH mutation changes. However, the observed changes in the DDR genes were more consistent with those from oncologic therapies than with those observed in serial samples among general populations.13,32 Fourth, our limited panel did not include all common CH genes, including DDR genes that have been demonstrated to be affected by oncologic therapy in prior studies, such as CHEK2 and PPM1D. Fifth, we were unable to consistently determine the cause of death in our cohort and therefore did not conduct a cause of death analysis, which limits insights regarding the adverse survival association observed in our study. Finally, we are unable to completely exclude the possible inclusion of germ line genetic variation because both CH and germ line mutations were inferred from the same source.
Conclusion
We identified an increase in the number of CH mutations and the size of clones after chemoradiation therapy in patients with solid malignancies, specifically in DDR genes. Additionally, we found an association between increased TP53 mutations after therapy and shorter survival. Further studies are needed to better understand which patients are most at risk for therapy-associated CH and the mechanisms by which these acquired changes may affect clinical outcomes.
Acknowledgments
The authors thank the patients at MD Anderson Cancer Center who graciously participated in this study. The authors thank Ann Sutton of The University of Texas MD Anderson Cancer Center Research Medical Library for her scientific editing assistance.
K.T.N. is a Cancer Prevention and Research Institute of Texas (CPRIT) scholar in cancer research and is supported by grants from CPRIT (RR1 90077) and the National Cancer Institute (NCI; L30 CA253796 and K08 CA263313). C.L. is supported by a grant from the NCI (R03 CA249562). K.L.B. is supported by a grant from the NCI (K08 CA241318). Z.L. is supported by a grant from the National Heart, Lung, and Blood Institute (R01 HL157273).
Authorship
Contribution: K.T.N. contributed to conception, design, acquisition of data, analysis and interpretation of data, drafting of the manuscript, funding, and resources; T.K. and L.J. contributed to design, analysis and interpretation of data, and critically reviewed the manuscript for important intellectual content; T.L.M. was responsible for the acquisition of data; J.W.W. and I.C.C.C. were involved in the analysis and interpretation of data; E.B. was involved in the acquisition of data and critically reviewing the manuscript for important intellectual content; J.Z. contributed to interpretation of data and critically reviewing the manuscript for important intellectual content; T.X. contributed to acquisition of data; C.T. and J.-i.A. contributed to critically reviewing the manuscript for important intellectual content; K.L.B. contributed to design and analysis and interpretation of data; Z.L. contributed to acquisition of data, interpretation of data, and resources; P.S. contributed to design, analysis and interpretation of data, and critically reviewed the manuscript for important intellectual content; S.H.L. contributed to conception, design, acquisition of data, analysis and interpretation of data, drafting of the manuscript, and resources; and all authors provided approval of the final manuscript.
Conflicts-of-interest disclosure: C.T. recieves royalties from Wolters Kluwer and consulting fees and honoraria from Siemen Healthineer, Lantheus, Telix, Molli Surgical, and Boston Scientific. Z.L. serves on the advisory board of AIQ, Inc and Reheva Biosciences. S.H.L. reports grants from STCube, BeyondSpring, and Nektar Therapeutics; serves on the advisory board of AstraZeneca; reports consultant fees from XRad Therapeutics; and is a cofounder of and has stocks in Seek Diagnostics. The remaining authors declare no competing financial interests.
Correspondence: Kevin T. Nead, Department of Epidemiology, The University of Texas MD Anderson Cancer Center, 1515 Pressler St, Office CPB4.3275, Houston, TX 77030; email: ktnead@mdanderson.org.
References
Author notes
Summary genetic data are available in the supplemental Data.
Additional original data are available on request from the corresponding author, Kevin T. Nead (ktnead@mdanerson.org).
The full-text version of this article contains a data supplement.