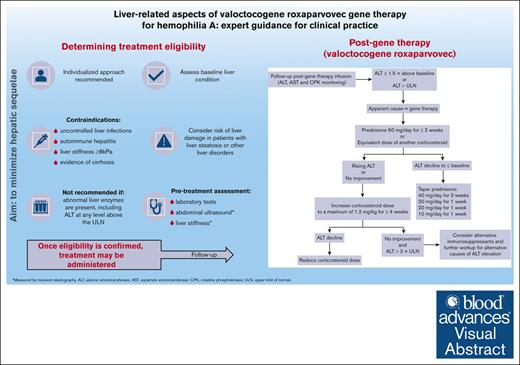
Visual Abstract
Adeno-associated virus–based gene therapy (valoctocogene roxaparvovec) is an attractive treatment for hemophilia A. Careful clinical management is required to minimize the risk of hepatotoxicity, including assessment of baseline liver condition to determine treatment eligibility and monitoring liver function after gene therapy. This article describes recommendations (developed by a group of hemophilia experts) on hepatic function monitoring before and after gene therapy. To prevent harmful liver-related effects, gene therapy is contraindicated in patients with uncontrolled liver infections, autoimmune hepatitis, liver stiffness ≥8 kPa, or cirrhosis. Before using gene therapy in patients with liver steatosis or other liver disorders, the risk of liver damage should be considered using a highly individualized approach. Treatment is not recommended in patients with abnormal liver enzymes, including alanine aminotransferase (ALT) at any level above the upper limit of normal (ULN). Therefore, pretreatment assessment of liver health should include laboratory tests, abdominal ultrasound, and liver stiffness measurements by transient elastography (TE). In the first year after therapy, ALT levels should be monitored 1 to 2 times per week to detect elevations ≥1.5× ULN, which may require immunosuppressant therapy. Patients with ALT elevation should receive prednisone 60 mg/d for 2 weeks, followed by stepwise tapering when ALT returns to baseline. ALT monitoring should continue long term (every 3-6 months), along with abdominal ultrasound (every 6 months) and TE (yearly) evaluations. When patients with good liver health are selected for treatment and closely monitored thereafter, ALT elevations can be promptly treated and are expected to resolve without long-term hepatic sequelae.
Introduction
Hemophilia A (HA) is an X-linked congenital bleeding disorder resulting in deficiency of coagulation factor VIII (FVIII), which is encoded by the F8 gene and produced primarily by liver sinusoidal endothelial cells.1-3 The severe form of the disease is characterized by spontaneous and trauma-induced recurrent bleeding, especially in joints and muscles, eventually leading to painful and disabling hemophilic arthropathy.1 Life-threatening bleeding in the brain and other internal organs can also occur.1 Prophylaxis with FVIII replacement products is the standard of care for severely affected patients and requires regular administration by IV infusion (at least once per week), thereby imposing a high treatment burden on patients.1,2,4 However, breakthrough bleeding episodes may still occur during prophylaxis, particularly when levels of FVIII are at their lowest or for trauma-related reasons.
In addition to FVIII replacement products, emicizumab, a subcutaneously administered, FVIII-mimetic bispecific monoclonal antibody, is now approved for HA prophylaxis. Although less frequent dosing regimens are possible with emicizumab, maintenance therapy must be administered regularly (every 1-2 weeks), and administration of FVIII replacement is still required for breakthrough bleeding or surgery.2,4-6
Being a well-characterized monogenic disease, HA is ideal for gene therapy in which correction of a single defective gene (F8) can provide symptomatic relief.2 Authorized gene therapy for HA uses recombinant adeno-associated virus (AAV) vectors to transfer functional F8 genetic information into the patient’s hepatocytes, with the goal of enabling consistent long-term endogenous production of FVIII after a single IV infusion.2,4 Although the hepatocyte is not the natural site for endogenous FVIII synthesis (which occurs in liver sinusoidal endothelial cells), expression of FVIII in hepatocytes results in the synthesis of a functional protein.2 AAV vectors are generally considered nonpathogenic, have high liver specificity, and are predominantly nonintegrating, whereby the genome of the AAV vector is largely episomal in the nucleus of transduced cells, with comparatively less functional integration rates into genomic DNA.2,3,7-10 However, immune responses to AAV gene transfer have the potential to induce a degree of hepatotoxicity, with an increase in serum levels of alanine aminotransferase (ALT), which is usually transient and asymptomatic.2 This may contribute to a reduction or loss of transgene expression, thereby reducing the curative efficacy of gene therapy.2,3,11-15
One AAV-based gene therapy (valoctocogene roxaparvovec; ROCTAVIAN) has thus far been approved for use in adult patients with severe HA without a history of FVIII inhibitors and without detectable antibodies to AAV5.4,16 Valoctocogene roxaparvovec includes an AAV5 vector containing a B-domain–deleted human F8 gene (hFVIII-SQ) controlled by a liver-selective promoter.17-19 In clinical studies, valoctocogene roxaparvovec resulted in significant and sustained improvements in FVIII activity (for ≥2 years) and bleeding rates, compared with standard prophylaxis with FVIII replacement therapy before gene therapy, albeit with declining FVIII activity levels over time.14-16,19-21 Despite clinical studies revealing that elevated ALT levels was the most common adverse event in patients with HA receiving valoctocogene roxaparvovec, no patients had developed liver dysfunction.14,16,20,21
Clinical guidelines for the management of hemophilia have yet to be updated to address best use of gene therapy to minimize hepatotoxicity and optimize the long-term success of treatment.22 To bridge this gap, an interdisciplinary group of hemophilia and hepatology experts has developed recommendations for the management of liver-related aspects of gene therapy for HA.
Methods
A meeting with an expert panel of 13 hemophilia experts and hepatologists from the main national hemophilia centers was convened to develop guidance on the management of liver-related aspects of gene therapy for HA. In Italy, gene therapy is available at designated tertiary centers equipped with the expertise to deliver this treatment, including the relevant hepatology services. At the meeting, the panel was split into 2 groups, which were assigned to deal with 1 of 2 topics: assessment of liver health to determine patient eligibility for gene therapy for HA; and monitoring liver health after gene therapy for HA. To develop guidance and recommendations related to these topics, the groups started from the discussion of liver-related information from the European Union summary of product characteristics (SmPC) and the US prescribing information (PI) for valoctocogene roxaparvovec (ROCTAVIAN; BioMarin Pharmaceutical),23,24 as well as of the phase 3 pivotal GENEr8-1 studies that led to the approval of valoctocogene roxaparvovec.14,16 The panel had discussed the emergent recommendations of each subgroup during the meeting and by subsequent virtual meetings and email correspondence until consensus was reached, but no formal voting process was used.
During the development of this article, additional literature to support the expert panel’s opinions and recommendations on liver-related aspects of gene therapy was identified by searching the PubMed database using the following combination of search terms: “genetic therapy,” “HA,” and “liver.” Recent literature on general liver health and monitoring practices was also consulted.
Determining liver health before gene therapy
Given the potential of HA gene therapy for hepatotoxicity, it is extremely important to carefully assess liver health when determining whether a patient with HA is eligible for gene therapy.3,4 Collaboration with a hepatology specialist is a prerequisite for the assessment of liver health before gene therapy, ideally within a “hub and spoke” model, in which the spoke is the local hemophilia treatment center attended by the patient, and the hub is a multidisciplinary hemophilia treatment center experienced in gene therapy.3,25,26 Hubs should manage all aspects of therapy delivery and data collection, whereas spokes are responsible for prescreening and patient selection.2
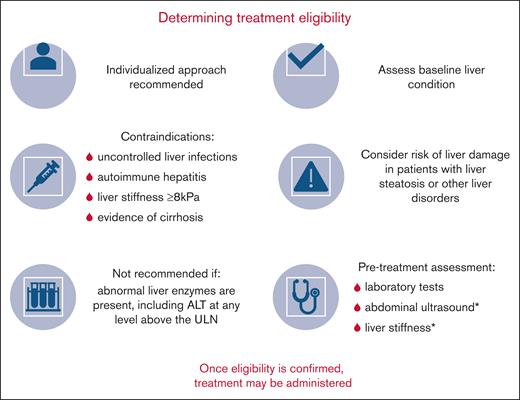
Inclusion and exclusion criteria of clinical gene therapy studies provide useful information to help identify gene therapy candidates and avoid gene therapy–induced liver reactions.3 As outlined in Table 1 and Figure 1, liver-related precautions and contraindications for valoctocogene roxaparvovec listed in the SmPC/PI are consistent with the criteria for patient exclusion in clinical studies of valoctocogene roxaparvovec.23,24
Phase 3 clinical study liver-related exclusion criteria and SmPC/PI-listed liver-related contraindications for valoctocogene roxaparvovec gene therapy for HA
| Phase 3 study liver-related exclusion criteria14 . | European SmPC-listed liver-related contraindications and precautions23 . | US PI-listed liver-related contraindications and precautions24 . |
|---|---|---|
|
|
|
| Phase 3 study liver-related exclusion criteria14 . | European SmPC-listed liver-related contraindications and precautions23 . | US PI-listed liver-related contraindications and precautions24 . |
|---|---|---|
|
|
|
ALP, alkaline phosphatase; GGT, gamma-glutamyl transferase; HBsAb, hepatitis B surface antibody; HBsAg, hepatitis B surface antigen; INR, international normalized ratio.
Based on ≥2 measurements.
Determination of treatment eligibility for gene therapy with valoctocogene roxaparvovec for HA, with the aim of minimizing hepatic sequelae. ∗Measured by TE.
Determination of treatment eligibility for gene therapy with valoctocogene roxaparvovec for HA, with the aim of minimizing hepatic sequelae. ∗Measured by TE.
In the GENEr8-1 phase 3 clinical study, which is, to our knowledge, the largest reported trial of AAV gene therapy to date, 134 men (mean age, 30 years) with severe HA who had been receiving regular prophylaxis with exogenous FVIII for ≥6 months received 1 dose of valoctocogene roxaparvovec 6 × 1013 vector genomes per kg.14,16 Patients were excluded from the study if they had substantial liver dysfunction, significant liver fibrosis, cirrhosis, or active/chronic viral infections with hepatitis B virus (HBV) or hepatitis C virus (HCV; Table 1).14 Overall, the risk-benefit profile appeared favorable in this population.14,16 Two years after the infusion (n = 132), it was reported that ALT had increased in 88.8% of the study population.16 Most ALT elevation adverse events were grade 1 or 2 and had occurred within 26 weeks of infusion. None of the events met Hy’s law criteria for drug-induced liver injury.27 ALT elevations (median duration, 21.0 days) were treated with immunosuppressants (mainly corticosteroids; median duration of therapy, 32.9 weeks) according to the study protocol. After 2 years of follow-up, valoctocogene roxaparvovec conferred a median FVIII activity in the range of mild HA (6%-39% of the normal value), which facilitated an 84.5% mean reduction in the annualized rate of treated bleeding events without routine prophylaxis and a 98.2% reduction in the annualized rate of exogenous FVIII use.16,28
As stated in the SmPC/PI for valoctocogene roxaparvovec, therapy is contraindicated in patients with acute or uncontrolled chronic infection with hepatotropic viruses and in patients with significant liver fibrosis or cirrhosis.23,24 Valoctocogene roxaparvovec is not recommended in patients with other liver disorders (including cholestatic and vascular diseases and inherited metabolic liver diseases), abnormal liver chemistry, or a history of hepatic malignancy (Table 1).23,24 In addition to the contraindications listed in the SmPC/PI, the panel recommends that valoctocogene roxaparvovec should be contraindicated in patients with autoimmune hepatitis because of the potential risk of major liver-related complications after exposure to AAV5 antigens.29
The GENEr8-1 phase 3 clinical study originally included patients with HIV, but the protocol was later amended to exclude these individuals after a patient receiving efavirenz had developed transaminase elevations in a separate study of valoctocogene roxaparvovec.14 Therefore, given the current lack of evidence, we suggest that gene therapy with valoctocogene roxaparvovec is contraindicated in patients with HIV infection.23,24 If gene therapy is to be considered in these patients, antiretroviral therapy regimens must be carefully evaluated to ensure that the least hepatotoxic regimen available is administered.23
Steatosis is increasingly found in the general population,18,30 and FVIII deficiency may increase the propensity for hepatic steatosis, as suggested by experiments in FVIII-deficient mice fed with a high-fat high-fructose diet.31 If steatosis is detected in the absence of alcohol use and other causes of chronic liver disease in patients with normal liver biochemistry and a liver stiffness measurement (LSM) <8 kPa, the final decision to administer gene therapy should be made on a case-by-case basis in joint agreement with the hematologist and hepatologist and considering patient preference. Before using gene therapy in patients with steatosis or any other liver disorder, the potential for reduced therapeutic effect and more serious hepatic reactions should be considered.23 Patients with steatosis must be informed that it is currently unknown whether steatosis has an effect on transgene expression and that, if they go ahead with gene therapy, strict safety monitoring will be required.
Liver health assessment
To determine whether a patient with HA has adequate liver health to be eligible for gene therapy, liver health assessments should include clinical and laboratory tests to detect the most common risk factors of chronic liver damage, such as active hepatitis infection (Figure 1).3,23,24 Liver imaging (ultrasound) and LSM by vibration-controlled transient elastography (VCTE; FibroScan; Echosens, Paris, France) must also be performed to detect liver fibrosis, cirrhosis, steatosis, and malignancy.3,23,24,32 On the occasion of an uncertain diagnosis, a liver biopsy may be required.33
Specific risk estimation is needed for patients who have recovered from HBV or HCV infection because these patients have a relatively high risk of residual liver damage and incidental/prevalent risk factors that cause progression of liver disease despite successful etiological therapy.3,34 Special care also needs to be taken in aging patients with hemophilia, who are often affected by multiple chronic diseases associated with aging, as well as comorbidities of hemophilia.35 Previous HCV infection is common in older generations of patients with HA, a subset of whom were also HIV positive and have liver disease as the primary cause of morbidity.36 Patients with type 2 diabetes mellitus also need to be carefully assessed because it itself increases the risk of progressive liver fibrosis.37,38 This is also the case for patients with metabolic syndrome, which is a common cause of chronic liver damage and increased transaminase levels.39 Metabolic syndrome is present if >3 of the following 5 criteria are met: waist circumference >102 cm in men or 88 cm in women, blood pressure >130/85 mm Hg, fasting triglyceride level >150 mg/dL, fasting high-density lipoprotein cholesterol level <40 mg/dL (men) or 50 mg/dL (women), and fasting blood sugar >100 mg/dL.40
Blood tests
A panel of tests is required to identify current or past hepatitis infection. Patients should be tested for HCV antibody and, if positive, should undergo HCV RNA testing for confirmation; the presence of HCV RNA indicates active infection.41,42 The following tests are recommended for HBV: hepatitis B surface antigen, which is indicative of HBV infection; hepatitis B surface antibody, which reflects immunity to infection; hepatitis B core antibody (HBcAb) immunoglobulin G (IgG), which may indicate current or previous HBV infection; and HBcAb immunoglobulin M (IgM), which may be the only detectable indicator of HBV during the period of acute infection.42,43 In hepatitis B surface antigen–positive patients, it is necessary to test for HBV DNA (which confirms chronic infection if present) and hepatitis D virus antibody, which, if positive, implies the need to assay hepatitis D virus (HDV) RNA to confirm coinfection/superinfection with HDV causing a higher rate of cirrhosis and its complications than HBV monoinfection.43 Hepatitis A virus (HAV) infection is confirmed by the detection of IgM (active infection) and IgG (past infection) antibodies for HAV.44 HBV and HAV vaccinations are recommended for unexposed and unvaccinated patients.
As specified in the valoctocogene roxaparvovec SmPC/PI, liver chemistry tests should include ALT, aspartate aminotransferase (AST), gamma-glutamyl transferase, alkaline phosphatase, total bilirubin and international normalized ratio. Gene therapy is not recommended in patients with ALT, AST, gamma-glutamyl transferase, alkaline phosphatase, or total bilirubin >1.25 × the upper limit of normal (ULN) or international normalized ratio ≥1.4.23,24 We also recommend testing for platelet count, albumin, pseudocholinesterase, gamma globulin, and direct bilirubin to provide a more comprehensive assessment of liver health and to exclude a possible case of liver disease or alteration in liver biochemistry.45-48
Transaminase levels should be consistently within the normal range before therapy.3 It is recommended in the SmPC/PI that ≥2 measurements should be obtained within 4 months before treatment with valoctocogene roxaparvovec23,24; however, we believe that ≥3 measurements should be obtained in the 6 months before treatment to increase the probability of capturing fluctuating enzyme levels. Because the threshold of ULN for ALT varies according to characteristics (eg, body mass index) of the local population,49 measurements should ideally always be performed at the patient’s center of care. Although valoctocogene roxaparvovec is officially not recommended in patients with transaminases >1.25 × ULN,23,24 it is important to note that ALT levels may be normal even in advanced liver disease.42 Therefore, a finding of transaminase levels <1.25 × ULN may not be sufficient and cannot be considered an exhaustive evaluation of liver health before considering implementing gene therapy.
To determine whether cases of elevated total bilirubin can be attributed to Gilbert syndrome, a benign condition present in ∼15% of the population,42,50 the direct bilirubin fraction should be measured. Although direct bilirubin is typically <20% of total bilirubin in patients with Gilbert syndrome,50 these patients usually have otherwise normal liver chemistries.42,50
Alpha-fetoprotein tumor marker testing can be performed to help rule out the possible presence of hepatocellular carcinoma (HCC).46,51
In the case of unexplained alterations of the liver profile, patients should be referred to their local hepatologist.
Liver imaging
It is recommended that all candidates receive a LSM by VCTE before considering gene therapy (Figure 1). In addition, all patients should undergo multiparametric ultrasound imaging, which can be used to assess and characterize focal liver lesions and detect signs of liver steatosis, biliary diseases, cirrhosis, and portal hypertension.52 Combining hepatic Doppler ultrasound with the abdominal ultrasound assessment will allow for the visualization of hepatic vessels without markedly increasing the time required until diagnosis and treatment.53 Any solid focal liver lesion detected on ultrasound that are not typical of simple cysts or hepatic hemangiomas must be further investigated by the hepatologist before the patient can be considered a candidate for gene therapy. We recommend that the LSM and ultrasound results should be carefully evaluated by an experienced hepatologist.
VCTE is available widely at specialized hepatology centers.54 To rule out advanced fibrosis and cirrhosis, it has previously been suggested, even for LSM values between 8.0 kPa and 11.5 kPa, a highly individualized approach to gene therapy should be taken.3 We recommend a more conservative approach, permitting gene therapy only in patients with LSM <8 kPa, which is the threshold defined by the European Association for the Study of the Liver guidelines to rule out advanced fibrosis in patients with nonalcoholic fatty liver disease, now termed metabolic dysfunction–associated steatotic liver disease.32 Other easily obtainable tests, including the fibrosis-4 (FIB-4) index, AST/ALT ratio, AST-to-platelet ratio index, enhanced liver fibrosis panel, and nonalcoholic fatty liver disease fibrosis score, may be useful to support the TE result.55 A number of these tests can be used to stratify patient risk, with high-risk patients likely to have advanced fibrosis or cirrhosis (Table 2).56
Noninvasive tests to assess hepatic fibrosis in fatty liver disease
| Test . | Fibrosis risk . | ||
|---|---|---|---|
| Low . | Intermediate . | High . | |
| LSM measured by TE (FibroScan) | <8 kPa | 8-13 kPa | >13 kPa |
| FIB4 Index∗ | <1.3 | 1.3-2.67 | >2.67 |
| AAR | <0.8 | 0.8-1 | >1 |
| NFS† | Less than –1.455 | –1.455 to 0.676 | >0.676 |
| MRE | <2.5 kPa | 2.5-4 kPa | >4 kPa |
| Test . | Fibrosis risk . | ||
|---|---|---|---|
| Low . | Intermediate . | High . | |
| LSM measured by TE (FibroScan) | <8 kPa | 8-13 kPa | >13 kPa |
| FIB4 Index∗ | <1.3 | 1.3-2.67 | >2.67 |
| AAR | <0.8 | 0.8-1 | >1 |
| NFS† | Less than –1.455 | –1.455 to 0.676 | >0.676 |
| MRE | <2.5 kPa | 2.5-4 kPa | >4 kPa |
Adapted from Di Minno et al56 with permission.
AAR, AST/ALT ratio; BMI, body mass index; FIB4, fibrosis 4; MRE, magnetic resonance elastography; NFS, nonalcoholic fatty liver disease fibrosis score.
Calculated as age × AST (IU/L)/platelet count (×109/L) × √ALT (IU/L).57
Calculated as 1.675 + 0.0373 × age (years) + 0.0943 BMI (kg/m2) + 1.133 × impaired fasting glycaemia or diabetes (yes = 1; no = 0) + 0.993 × AST/ALT ratio – 0.0133 × platelet (×109/L) – 0.663 × albumin (g/dL).58
Controlled attenuation parameter measurement should be performed by TE to detect liver steatosis.52,59 If steatosis is detected (eg, controlled attenuation parameter >275 dB/m) in patients with normal transaminases and LSM <8 kPa, and in the absence of alcohol use and other active causes of chronic liver disease, the final decision to administer gene therapy should be made on a case-by-case basis.
Alcohol consumption
Given the damaging effects of alcohol on the liver,60 we recommend that patients be advised to avoid alcohol intake for 6 months before the valoctocogene roxaparvovec infusion. Patients should also be educated on the importance of avoiding/limiting alcohol consumption for at least 1 year (and preferably 2 years) after the infusion.23 The experts agreed that pretreatment avoidance enables patients to get used to posttreatment abstinence from alcohol and provides physicians with a good indication of the patient’s likeliness for adherence to this recommendation after gene therapy.
Suitability for corticosteroids and other immunosuppressive therapies
Given that 88.8% of patients treated with valoctocogene roxaparvovec in the GENEr8-1 study had ALT elevations, which were mainly treated with corticosteroids for a median duration of therapy of 32.9 weeks,16 it is likely that corticosteroids will be indicated for ALT elevation for prolonged periods after gene therapy in most patients.3,4,14,16 Therefore, the patient’s ability to receive corticosteroid therapy and/or other immunosuppressive therapy should be assessed before gene therapy to ensure that the risks associated with such regimens are acceptable at the individual level.23,24 Corticosteroids may, for example, lead to increased fat deposition in the liver,61 which is a concern in patients with steatosis, and should be considered in the decision-making process. In general, patients who are not good candidates for immunosuppression may also not be good candidates for gene therapy. Particular attention should be paid to HBcAb-positive candidates in whom occult HBV infection could be reactivated after immunosuppression.62
Postinfusion ALT and FVIII monitoring
In the GENEr8-1 study, ALT elevations usually occurred within 26 weeks after valoctocogene roxaparvovec infusion.16 In a less selected, real-life patient population, including those with advanced age and previous HCV infection, ALT elevation is expected to be even more frequent and persistent than that reported in the GENEr8-1 study.16 After gene therapy, ALT levels should be monitored according to SmPC/PI recommendations (every 1-2 weeks in the first year; Table 3). To minimize the impact of interlaboratory variability, we recommend that the same laboratory used for hepatic testing before gene therapy is used for liver function monitoring after treatment. The relationship between ALT elevation and FVIII expression is currently unclear,3,14-16 and FVIII levels should be monitored in conjunction with ALT. Pharmacokinetic modeling has indicated that, although declining over time,15 FVIII activity levels will remain in the mild hemophilia range for ≥5 years after valoctocogene roxaparvovec infusion.16 Many questions remain as to why FVIII expression decreases after gene therapy,2,15,28 but it is possible that the production of FVIII in hepatocytes, which are not the natural site of FVIII synthesis, activates the endoplasmic reticulum (ER) stress response, potentially contributing to a reduction in protein expression over time, although evidence for this hypothesis is contradictory.4,18,63,64 Preliminary data presented at the European Association for Haemophilia and Allied Disorders conference in 2024 suggest that the decline in FVIII expression may also occur due to reduced transcription of episomal vector DNA to RNA in hepatocytes65 or from epigenetic silencing mechanisms linked to the activity of histone deacetylase.66 However, further research is needed to confirm these mechanisms.
Recommended monitoring frequency of ALT after valoctocogene roxaparvovec gene therapy for HA
| Time frame . | Monitoring frequency . |
|---|---|
| ALT levels | |
| First 26 wk | Weekly |
| Week 26 to 52 (year 1) | Every 1-2 wk |
| Year 2 | Every 3 mo |
| After year 2 | Every 6 mo |
| Imaging | |
| Ultrasound | At least once a year |
| TE | At least once a year |
| Time frame . | Monitoring frequency . |
|---|---|
| ALT levels | |
| First 26 wk | Weekly |
| Week 26 to 52 (year 1) | Every 1-2 wk |
| Year 2 | Every 3 mo |
| After year 2 | Every 6 mo |
| Imaging | |
| Ultrasound | At least once a year |
| TE | At least once a year |
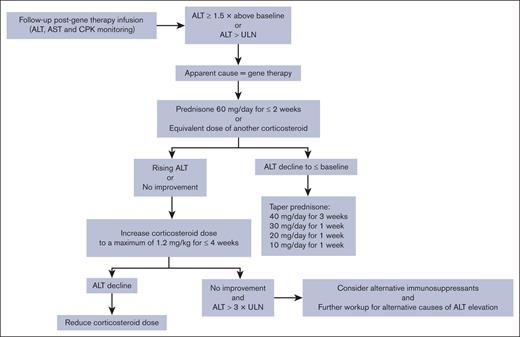
In the first year after gene therapy, ALT and FVIII monitoring should be conducted to detect immune-mediated increases in ALT that should be treated with immunosuppressant therapy and that may be accompanied by decreased FVIII activity as a potential consequence of immune-mediated cell damage.23,67 However, there is currently no evidence-based threshold for FVIII or ALT levels in this setting. To help rule out alternative causes for ALT elevations (eg, drug-induced hepatotoxicity and alcohol consumption), AST and creatine phosphokinase monitoring is also recommended.23,24 After the first year of monitoring, ALT and FVIII testing should be continued to routinely assess liver health and bleeding risk.23,67
Managing ALT elevations with corticosteroids
The exact causes of ALT elevation are still unclear, but an immune response to high-dose AAV5 may be a contributing factor.3,14 Therefore, immunosuppression has been widely investigated to diminish the T-cell response to adenovirus and thus maintain transgene expression.68 As demonstrated in the GENEr8-1 study,14,16 corticosteroids can be used to modify immune reactivity and manage ALT elevations, with the goal of helping to preserve therapeutic response.2,25 However, no data currently support a role of corticosteroids in the preservation of transgene expression when they are used to treat ALT elevations.16 A phase 3 study evaluating the efficacy and safety of valoctocogene roxaparvovec with prophylactic corticosteroids in patients with HA is currently underway (GENEr8-3; clinicaltrials.gov identifier: NCT04323098).
To be consistent with the GENEr8-1 study,16 an increase in ALT of at least 1.5-fold from baseline or above ULN is the threshold at which immunosuppression should currently be initiated.3 Based on clinical experience, it is recommended that patients with valoctocogene roxaparvovec–associated ALT elevation should receive prednisone 60 mg per day for 2 weeks, followed by stepwise downward tapering when ALT levels return to baseline (Figure 2).3,23,24 However, caution is advised with this approach because methylprednisolone itself has been associated with drug-induced liver autoimmune-like hepatitis.69 Prolonged administration of high corticosteroid doses is not considered appropriate, so in addition to further workup for alternative causes of ALT elevations, other immunosuppressants may be considered if high-dose prednisone proves to be ineffective.23,24
Recommended corticosteroid regimen (according to the SmPC23,24) in response to ALT elevations after valoctocogene roxaparvovec gene therapy for HA. CPK, creatine phosphokinase.
Long-term considerations
Data on the long-term effects of gene therapy on the liver are currently limited, although recent data from liver biopsies revealed no liver toxicity.18 Histopathological analysis of liver biopsy samples collected 2.6 to 4.1 years after gene transfer from 5 participants in the phase 1 to 2 trial of valoctocogene roxaparvovec revealed no dysplasia, architectural distortion, fibrosis, or chronic inflammation, and no ER stress was detected in hepatocytes expressing hFVIII-SQ protein.18 However, because of the potential for the integration of the vector into the genome, there is a theoretical long-term risk of HCC.3,8,14,67 Furthermore, ER stress may sustain HCC development independent of viral vector integration.70,71 This is in line with the observation that, after in vivo DNA vector delivery to hepatocytes, expression of B-domain–deleted FVIII, which is prone to misfolding in the ER, caused HCC in mice fed a high-fat diet, whereas expression of well-folded proteins did not.72 The suggestion that protein misfolding may trigger cellular stress and HCC in the context of a high-fat diet is of particular concern for patients with steatosis. Thorough examination of any future malignancies in patients treated with valoctocogene roxaparvovec, including histopathology, transcriptomics, and/or genetic mutations, will be required to determine whether there is any connection with gene therapy.3,70,71
Long-term monitoring should be performed by means of laboratory tests and imaging techniques. Laboratory tests should be performed every 3 to 6 months (Table 3).23,24 An ultrasound is also recommended at least annually for most patients, but more frequent monitoring can be undertaken on a case-by-case basis if the hepatologist considers it prudent, given that 6-monthly ultrasound is often conducted in patients at high risk of HCC.73 Although alpha-fetoprotein measurements can be useful in alerting physicians to the possibility of HCC, it has lower specificity than ultrasound for detecting HCC and should not be used alone as surveillance for HCC development.74 Ultrasound is also helpful in diagnosing steatosis; mild steatosis has been reported in some patients 2 to 6 years after treatment with valoctocogene roxaparvovec.18,75 However, it is not clear whether mild steatosis is a treatment effect, because metabolic dysfunction–associated steatotic liver disease is a relatively common finding in the general population76 and affects 40% to 50% of people with moderate to severe HA.77 To monitor for fibrosis, we recommend performing LSM by TE at least once a year. Patients should be enrolled in registries for long-term safety monitoring.3,4,13,23,25
Conclusion
Although gene therapy for HA may not eliminate bleeding completely, it is a 1-time treatment option that offers a long-lasting period of relatively high and stable levels of FVIII expression with independence from frequent administration of prophylactic agents and, therefore, has the potential to markedly improve patient quality of life.2,67,78,79 Candidates for gene therapy need to be fully informed of the potential benefits and risks of therapy, as well as the need for frequent follow-up visits.2,4,13,25,80
In these initial stages of gene therapy in clinical practice, careful selection should be made to exclude patients with compromised liver health or with risk factors for HCC so as to avoid any potential additional liver damage and maximize treatment efficacy.2,4,78 When patients are selected carefully for treatment and liver function is closely monitored after gene therapy, ALT elevations can be promptly treated with immunosuppressant therapy and are expected to resolve without hepatic sequelae.16,18,79 However, long-term monitoring and data collection is necessary to determine whether gene therapy has any long-term hepatotoxic effects.4,25
Acknowledgments
The authors thank Joanne Dalton, who wrote the outline and first draft on behalf of Springer Healthcare, and Catherine Rees, who provided postsubmission medical writing assistance on behalf of Springer Healthcare.
Medical writing assistance was funded by BioMarin. The expert meeting and funding for authors to attend were sponsored by BioMarin.
Authorship
Contribution: V.L.M. and G.C. conceptualized the study, contributed to the study design, and were responsible for study supervision; F.P. assisted in the study design and read and supervised manuscript preparation; V.L.M. collected the data; M.P. performed data analysis; F. Morisco and M.P. wrote, reviewed, and edited the manuscript; and V.C., R.D.C., A.D.S., G.D.M., L.F., F. Marra, C.S., and E.Z. participated in the panel discussion and manuscript revision.
Conflict-of-interest disclosure: V.L.M. is a member of advisory boards of BioMarin, CSL Behring, and Pfizer; a speaker for Gore and Alfasigma; and has received a travel grant from Sanofi and Takeda. V.C. has received a research grant from Ipsen; honoraria from Albireo Pharma, Incyte, and Revalma for seminars; and honoraria from Ipsen for participation in an advisory board meeting. G.D.M. is a member of speaker bureaus for BioMarin, Bayer, CSL Behring, Roche, Takeda, and Viatris Pharmaceuticals; and is an ad-hoc speaker/consultant for BioMarin, Bayer, Pfizer, Takeda, and Viatris Pharmaceuticals. F. Morisco is a member of advisory board for BioMarin. F.P. is a member of advisory boards for Sobi, Sanofi, Roche, CSL Behring, and BioMarin; and a speaker at education symposia organized by Takeda and Spark. C.S. is an advisory board member for BioMarin. E.Z. received fees as an advisory board member from Bayer, BioMarin, CSL Behring, Novo Nordisk, Sobi, and Takeda. G.C. is a member of advisory boards for Bayer, BioMarin, CSL Behring, LFB, Novo Nordisk, Pfizer, Roche, Sobi, and Takeda; and has been a speaker at education symposia organized by BioMarin, BIOVIIIx, CSL Behring, LFB, Roche, and Takeda. F. Marra has received travel grants from Alfasigma; consultant fees from AstraZeneca; speaker honoraria, consultant fees, and travel grants from Bayer; consultant fees from BioMarin; speaker honoraria and consultant fees from Gilead; consultant fees from Ipsen; speaker honoraria from Intercept; consultant fees from Merck Sharp & Dohme, Eisai, Menarini, Novartis, Novo Nordisk, and Roche. The remaining authors declare no competing financial interests.
Correspondence: Vincenzo La Mura, Fondazione IRCCS Ca' Granda Ospedale Maggiore Policlinico, Angelo Bianchi Bonomi Hemophilia and Thrombosis Center, Via Pace 9, 20122 Milan, Italy; email: vincenzo.lamura@unimi.it.
References
Author notes
Data sharing is not applicable to this article because no data sets were generated or analyzed during this study.