TO THE EDITOR:
Systemic light chain (AL) amyloidosis results from the misfolding of immunoglobulin light chains produced by clonal plasma cells in the bone marrow, leading to aggregation and fibril formation that deposit in organs and tissues.1 This condition can affect multiple organ systems, causing significant morbidity and mortality if not diagnosed promptly. Despite its rarity, AL amyloidosis has led to many advances in diagnosis and treatment, particularly through clinical trials.2
Clinical trials are essential for evaluating the effectiveness and safety of new therapies. The number of AL amyloidosis trials has grown considerably in recent decades, improving patient outcomes.2 Current standards of care are built upon trials that evaluate novel interventions and refine existing therapies. Selected patients with AL amyloidosis can be treated with high-dose melphalan and autologous stem cell transplantation (SCT), leading to deep and durable responses.3 For ineligible patients, an increasing number of non-SCT options are available, including proteasome inhibitors, immunomodulatory agents, and monoclonal anti-CD38 antibodies, by virtue of clinical trials.4,5
The success of clinical trials depends on innovative treatments and the effective and equitable recruitment and retention of participants.6 Researchers in the field of amyloidosis have encountered challenges, including patient-related factors, protocol-specific requirements, logistical constraints, and disparities in access to care.7,8 Addressing these barriers is crucial for ensuring the representativeness and generalizability of trial findings and for maximizing the impact of research on clinical practice.
This article provides an overview of the clinical trial landscape in AL amyloidosis at a large center of excellence, focusing on challenges in patient enrollment. By examining the spectrum of trials over 3 decades and identifying recent recruitment impediments, we aim to uncover opportunities to enhance the inclusivity and success of future clinical trials in this rare disease.
We conducted a retrospective analysis of clinical trials involving patients with AL amyloidosis enrolled at our institution from July 1994 to December 2023. Data were extracted from institutional databases, research registries, and clinical trial records. All participants were diagnosed with systemic AL amyloidosis based on established criteria.
Our analysis was stratified by intervention type, including SCT and non-SCT therapies. For each trial, we collected information on study design, sponsor type (industry, investigator initiated, and cooperative group), enrollment criteria, and participant demographics. Baseline disease characteristics, such as organ involvement, disease stage, and laboratory parameters (serum and urine protein electrophoresis, serum free light chain levels, and cardiac biomarkers such as troponin and N-terminal prohormone of brain natriuretic peptide), were gathered for all prescreened and enrolled participants. These clinical data were compared against each trial’s eligibility criteria to identify factors affecting recruitment success or failure. We also examined the racial and ethnic composition of trial cohorts to explore potential disparities in minority group participation.
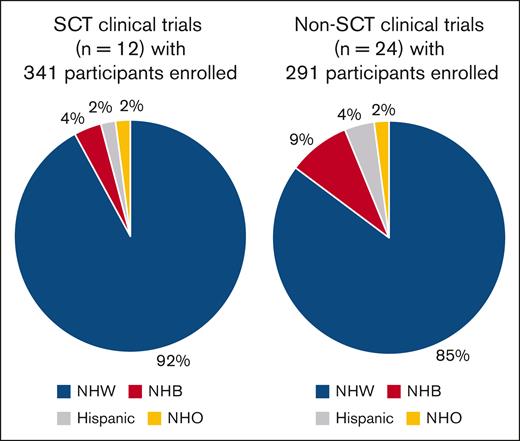
Between 1994 and 2023, there were 632 enrollments in various clinical trials at our center, although this number does not represent unique patients, because some individuals participated in multiple different clinical trials. There were 341 enrollments in 12 SCT trials and 291 enrollments in 24 non-SCT trials. SCT trials were predominantly investigator initiated (n = 11), with 1 cooperative group trial. Non-SCT trials included industry-sponsored (n = 10), investigator-initiated (n = 8), multicenter investigator-initiated (n = 4), and cooperative group trials (n = 2).
Among all participants (n = 632), most were Non-Hispanic White, with minority representation (Non-Hispanic Black and Hispanic) in only 11%. Specifically, 6% were Non-Hispanic Black and 3% were Hispanic, indicating potential disparities in trial participation (Figure 1).
Racial/ethnic composition of participants in clinical trials for AL amyloidosis. NHB, non-Hispanic Black; NHO, non-Hispanic others; NHW, non-Hispanic White.
Racial/ethnic composition of participants in clinical trials for AL amyloidosis. NHB, non-Hispanic Black; NHO, non-Hispanic others; NHW, non-Hispanic White.
From 2018 to 2023, a total of 110 patients were prescreened for 9 non-SCT trials, with 79 (71%) failing recruitment. Patients were considered “prescreened” if they and their physicians demonstrated interest in a particular trial and if available local laboratory values and imaging parameters met the criteria for participation in the study before signing informed consent. The main barriers were as follows: patient preference due to trial logistics (27%), difference in involved and uninvolved light chain levels not meeting criteria (18%), cardiac biomarkers not meeting criteria (16%), and other inclusion criteria (20%; Table 1). This study was approved by the institutional review board of Boston Medical Center and Boston University Medical Campus.
Barriers to recruitment on clinical trials for AL amyloidosis from 2018 to 2023 at a referral center
| Clinical trials . | AQUARIUS∗ . | ANDROMEDA† . | CAEL 301‡ . | CAEL 302§ . | AFFIRM-AL|| . | ETCTN10440¶ . | STI6129# . | DPd∗∗ . | OP201†† . | Total . |
|---|---|---|---|---|---|---|---|---|---|---|
| No. of enrolled patients | 4 | 8 | 2 | 8 | 1 | 2 | 3 | 3 | 0 | 31 |
| No. of prescreen failures (before informed consent) | 11 | 9 | 7 | 17 | 13 | 5 | 5 | 9 | 3 | 79 |
| Reasons for prescreen failure | ||||||||||
| Patient preference due to logistics of the trial | 7 | 3 | 1 | 4 | 4 | 0 | 0 | 2 | 0 | 21 |
| dFLC not meeting eligibility | 1 | 3 | 0 | 1 | 2 | 3 | 2 | 2 | 0 | 14 |
| eGFR not meeting eligibility | 0 | 0 | 0 | 1 | 1 | 2 | 1 | 0 | 1 | 6 |
| Cardiac biomarkers not meeting eligibility | 1 | 1 | 1 | 3 | 3 | 0 | 0 | 3 | 1 | 13 |
| Orthostatic hypotension | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 2 |
| Myeloma by SLiM CRAB criteria | 2 | 0 | 2 | 2 | 1 | 0 | 0 | 0 | 0 | 7 |
| Other reasons‡‡ | 0 | 1 | 3 | 5 | 2 | 0 | 2 | 2 | 1 | 16 |
| Clinical trials . | AQUARIUS∗ . | ANDROMEDA† . | CAEL 301‡ . | CAEL 302§ . | AFFIRM-AL|| . | ETCTN10440¶ . | STI6129# . | DPd∗∗ . | OP201†† . | Total . |
|---|---|---|---|---|---|---|---|---|---|---|
| No. of enrolled patients | 4 | 8 | 2 | 8 | 1 | 2 | 3 | 3 | 0 | 31 |
| No. of prescreen failures (before informed consent) | 11 | 9 | 7 | 17 | 13 | 5 | 5 | 9 | 3 | 79 |
| Reasons for prescreen failure | ||||||||||
| Patient preference due to logistics of the trial | 7 | 3 | 1 | 4 | 4 | 0 | 0 | 2 | 0 | 21 |
| dFLC not meeting eligibility | 1 | 3 | 0 | 1 | 2 | 3 | 2 | 2 | 0 | 14 |
| eGFR not meeting eligibility | 0 | 0 | 0 | 1 | 1 | 2 | 1 | 0 | 1 | 6 |
| Cardiac biomarkers not meeting eligibility | 1 | 1 | 1 | 3 | 3 | 0 | 0 | 3 | 1 | 13 |
| Orthostatic hypotension | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 2 |
| Myeloma by SLiM CRAB criteria | 2 | 0 | 2 | 2 | 1 | 0 | 0 | 0 | 0 | 7 |
| Other reasons‡‡ | 0 | 1 | 3 | 5 | 2 | 0 | 2 | 2 | 1 | 16 |
CRAB, hypercalcemia, renal failure, anemia and bone lesion; CrCl, creatinine clearance; dFLC, difference in involved and uninvolved light chain levels; eGFR, estimated glomerular filtration rate; IVSd, interventricular septal diameter; LVEF: left ventricular ejection fraction; NTproBNP, N-terminal prohormone of brain natriuretic peptide; PR, partial hematologic response; QTcF, corrected QT interval, SLiM, >60% bone marrow plasmacytosis, serum light chain ratio >100, MRI with 3 focal lesions.
AQUARIUS (NCT05250973): newly diagnosed, Mayo Clinic 2004 stage II and IIIA cardiac with or without other organ involvement, and dFLC ≥40 mg/L with an abnormal ratio.
ANDROMEDA (NCT03201965): newly diagnosed, dFLC ≥50 mg/L, NTproBNP ≤8500 pg/mL, and QTcF <500 milliseconds.
CAEL301 (NCT04504825): newly diagnosed, Mayo Clinic 2004 stage IIIB, dFLC >40 mg/L, and NTproBNP >8500 pg/mL.
CAEL 302 (NCT04512235): newly diagnosed, Mayo Clinic 2004 stage IIIA, dFLC >40 mg/L, and NTproBNP >650 and <8500 pg/mL.
AFFIRM-AL (NCT04973137): newly diagnosed, Mayo Clinic 2012 stage IV, and NTproBNP <8500 pg/mL.
ETCTN10440 (NCT04847453): relapsed after 1 line of treatment, Mayo Clinic 2004 stage I-IIIA, dFLC >20 mg/L, NTproBNP <8500 pg/mL, and CrCl ≥15 mL/min.
STI-6129 (NCT05692908): relapsed, refractory, dFLC >40 mg/L, and LVEF >40%.
DPd (NCT04270175): relapsed and/or refractory, eGFR >20 mL/min per 1.73 m2, prior daratumumab treatment, and prior pomalidomide exposure was allowed if PR or better was achieved and no disease progression occurred within 60 days of last dose received.
OP201 (NCT04115956): relapsed after 1 line of treatment, Mayo Clinic 2004 stage I to II.
For example, IVSd <12 mm; QTcF >500 milliseconds; LVEF 40%.
This retrospective analysis offers valuable insights into the successes and challenges of AL amyloidosis clinical trials at our institution over the past 30 years. Despite its rarity, many patients have advanced our understanding of this disease through trial participation. Both SCT and non-SCT treatments have contributed to the evolving treatment paradigm for AL amyloidosis.9,10 Our analysis revealed a diverse portfolio of clinical trials over 3 decades, reflecting the evolving landscape of AL amyloidosis research. Most SCT trials were investigator initiated, highlighting the critical role of academic institutions. Non-SCT trials showed diverse sponsorship, with industry-sponsored and multicenter trials complementing investigator-initiated efforts.
Despite progress, several barriers to patient recruitment persist, especially in recent years. Patient preference, logistical concerns (eg, travel burden, clinic visit frequency, and treatment complexity), and a lack of interest were significant barriers. Addressing these issues requires proactive communication, support services, and logistical assistance.
Diversity, equity, and inclusion are vital in clinical trials because treatments may affect subgroups differently. Racial and ethnic minorities were underrepresented in our trials. Addressing barriers to minority inclusion is crucial for equitable and generalizable results. Strategies include improving access to centers of excellence, community outreach, and increasing the diversity of trial staff.11,12
We observed a high rate of recruitment failure in recent years. Restrictive eligibility criteria based on disease markers such as serum free light chain levels and cardiac biomarkers were major hurdles. Although necessary for safety and validity, overly restrictive criteria may exclude many potential candidates, limiting trial generalizability. Strategies to optimize eligibility criteria while maintaining rigor include refining risk stratification algorithms, incorporating novel biomarkers, and using real-world data.
In conclusion, AL amyloidosis research has seen progress but also faces persistent challenges. Clinical trials have been crucial in advancing treatment, yet barriers to enrollment hinder research efforts. Addressing these barriers, including restrictive eligibility criteria and minority participation disparities, is essential for inclusive and successful future trials. By fostering collaboration and implementing innovative recruitment strategies, we can continue to advance the fight against AL amyloidosis.
Acknowledgments: The authors thank the current and past members of the Amyloidosis Center, Cancer Clinical Trials Office, Stem Cell Transplant Program, and Center for Cancer and Blood Disorders at Boston Medical Center and Boston University Chobanian & Avedisian School of Medicine.
Contribution: V.S. designed the study, performed data analysis, and wrote the manuscript; B.B., E.G., and A. Shelton collected the data; L.M., T.J., N.B., A. Staron, J.M.S., and V.S. performed the research and provided clinical care to the patients; and all authors critically reviewed and approved the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Vaishali Sanchorawala, Boston University Chobanian & Avedisian School of Medicine, Boston Medical Center, 72 East Concord St, K 503, Boston, MA 02118; email: vaishali.sanchorawala@bmc.org.
References
Author notes
Data are available on reasonable request from the corresponding author, Vaishali Sanchorawala (vaishali.sanchorawala@bmc.org).