Key Points
cGVHD declined by ∼19% with each 5-year increment in HCT date even after adjusting for the typically expected cause-associated factors.
cGVHD declines were not fully explained by demographic shifts or greater use of HCT approaches generally associated with lower cGVHD rates.
Visual Abstract
Since 2005, there has been a steady decline in chronic graft-versus-host disease (cGVHD) at the Fred Hutchinson Cancer Center. To better understand this phenomenon, we studied the risk of cGVHD requiring systemic immunosuppression (cGVHD-IS) as a function of hematopoietic cell transplantation (HCT) date in 3066 survivors from 2005 through 2019. Cox regression models were fit to assess associations of HCT date (as a continuous linear variable) with cause-specific hazards of cGVHD using unadjusted and adjusted models. Median follow-up for study subjects was 7.0 years (range, 1.0-17.2). Two-year probabilities of cGVHD-IS declined among all survivors from 45% to 52% (2005-2007) to ∼40% (2008-2012) and then further to ∼26% by 2017. A decline was also observed when the analysis was restricted to 502 pediatric survivors, with cGVHD-IS probabilities <10% since 2013. Among 305 adult and pediatric survivors who underwent transplantation for nonmalignant diseases, cGVHD rates showed greater fluctuation but remained <20% after 2016. Each 5-year increase in HCT date was associated with a 27% decrease in the cause-specific hazard of cGVHD (unadjusted hazard ratio [HR], 0.73; 95% confidence interval [CI], 0.68-0.78; P < .0001); the HR was 0.81 (95% CI, 0.75-0.87; P < .0001) even after adjusting for various factors (age, donor/stem-cell source, race, sex, conditioning intensity, GVHD prophylaxis, among others) that could lead to cGVHD reduction. The decline in cGVHD was not fully explained by demographic shifts and greater use of HCT approaches that are generally associated with lower cGVHD rates. This observation underscores that single-cohort cGVHD prevention studies should use contemporaneous and not historical controls for comparison.
Introduction
Prospective data capture for chronic graft-versus-host disease (cGVHD) according to the National Institutes of Health (NIH) Consensus Criteria began at the Fred Hutchinson Cancer Center (FHCC) in 2002. Since 2005, we have observed a steady decline in cGVHD requiring systemic immunosuppressive therapy (cGVHD-IS). To better understand this phenomenon, we evaluated the incidence of NIH cGVHD requiring systemic IS therapy among all FHCC patients who survived without relapse to day 100 and underwent allogeneic hematopoietic cell transplantation (HCT) between January 2005 and December 2019. We postulated that newer HCT approaches and demographic shifts might explain most of the reduction in cGVHD. For example, increased ex vivo naïve T-cell depletion, cord blood transplantation, posttransplant cyclophosphamide, advances in HLA matching, and a greater proportion of transplants for children and for nonmalignant diseases (NMD) which incorporate augmented GVHD prophylaxis with serotherapy.
Methods
This retrospective study identified 3066 survivors who survived ≥100 days without relapse after receiving their first allogeneic HCT at FHCC between 1 January 2005 and 31 December 2019. All patients signed the FHCC Institutional Review Board–approved consent for the use of their data for research.
Patients were evaluated between 80 and 100 days, 1 year after HCT, and whenever clinically indicated, to establish a cGVHD diagnosis according to NIH criteria.1,2 Medical records were reviewed annually to collect data on cGVHD and treatment with immunosuppression.
The primary goal of this study was to assess the risk of cGVHD-IS as a function of HCT date. To avoid arbitrary categorization, the cGVHD date of diagnosis was modeled as a continuous nonlinear variable using a restricted 5-knot cubic spline,3 with knots at the 5th, 25th, 50th, 75th, and 95th percentiles of the date of HCT. Cox regression was used to assess the association of HCT date with the cause-specific hazard of cGVHD-IS, using an unadjusted model as well as a model adjusted for recipient age, donor type/stem-cell source (Table 1), patient/donor sex (male/female [MF] vs others), patient/donor cytomegalovirus (CMV) serostatus (+/+ vs +/– vs –/+ vs –/–), disease severity (low vs intermediate vs high),4 Total body irradiation (TBI) dose (as a continuous linear variable), conditioning intensity (nonmyeloablative vs reduced intensity vs ablative), self-reported race, and GVHD prophylaxis. The cause-specific hazard of relapse (restricted to 2756 patients who underwent transplantion for malignancy) was modeled in the same way as described above. Among those who developed cGVHD-IS, overall mortality was examined with regression model fit using the same approach to examine whether the risk of mortality among those with cGVHD-IS changed over time. For descriptive purposes, HCT dates were also categorized into 3 windows: 2005 to 2009, 2010 to 2014, or 2015 to 2019. For visual purposes, 2-year point estimates of cGVHD-IS, relapse, nonrelapse mortality (NRM), grade 2 to 4 acute GVHD (all using cumulative incidence), and overall survival (Kaplan-Meier method) were estimated by year of HCT. Formal statistical analyses were not conducted for these descriptive categories.
Study populations
| Characteristic . | All patients N (%) . | Adults n (%) . | Children n (%) . |
|---|---|---|---|
| Total | 3066 (100) | 2564 (84) | 502 (16) |
| Sex, female | 1294 (42) | 1080 (42) | 214 (43) |
| Donor recipient: female/male∗ | 749 (26) | 629 (26) | 120 (25) |
| Race/ethnicity | |||
| American Indian or Alaska native | 54 (2) | 35 (1) | 19 (4) |
| Asian | 217 (7) | 161 (6) | 56 (11) |
| Black or African American | 76 (2) | 51 (2) | 25 (5) |
| Native Hawaiian/other Pacific Islander | 44 (1) | 34 (1) | 10 (2) |
| White (non-Hispanic) | 2382 (78) | 2106 (82) | 276 (55) |
| White (Hispanic) | 178 (6) | 103 (4) | 75 (15) |
| White (unknown) | 5 (<1) | 5 (<1) | 0 |
| Multiple/unknown | 110 (4) | 69 (3) | 41 (8) |
| Median age (range), y | 49.0 (0.1-78.9) | 52.8 (18.1-78.9) | 8.2 (0.1-17.9) |
| Malignant diseases | 2761 (90) | 2476 (97) | 285 (57) |
| Acute myeloid leukemia/MDS | 1355 (44) | 1256 (49) | 99 (20) |
| Myeloproliferative diseases | 213 (7) | 205 (8) | 8 (2) |
| Acute lymphoblastic leukemia | 476 (16) | 330 (13) | 146 (29) |
| Chronic leukemia (CML/CLL) | 231 (8) | 222 (9) | 9 (2) |
| Lymphoma | 308 (10) | 290 (11) | 18 (4) |
| Plasma cell disorders | 144 (5) | 144 (6) | 0 (0) |
| Biphenotypic leukemias | 30 (1) | 25 (1) | 5 (1) |
| Other | 4 (<1) | 4 (<1) | 0 (0) |
| NMD | 305 (10) | 88 (3) | 217 (43) |
| Aplastic anemia | 115 (4) | 55 (2) | 60 (12) |
| Inherited BM failure syndrome | 41 (1) | 8 (<1) | 33 (7) |
| Primary immunodeficiency disease | 80 (3) | 11 (<1) | 69 (14) |
| Primary immunodysregulatory disease | 26 (<1) | 3 (<1) | 23 (5) |
| Hemoglobinopathy (thalassemia, SCD) | 26 (<1) | 6 (<1) | 20 (4) |
| Other | 17 (<1) | 5 (<1) | 12 (2) |
| Conditioning intensity† | |||
| MA-TBI | 680 (22) | 462 (18) | 218 (43) |
| NMA | 1101 (36) | 1018 (40) | 83 (17) |
| RIC | 1285 (42) | 1084 (42) | 201 (40) |
| Donor type/graft source | |||
| HLA-identical sibling, BM | 167 (5) | 45 (2) | 122 (24) |
| HLA-identical sibling, PBSC | 731 (24) | 719 (28) | 12 (2) |
| Mismatched related | 30 (1) | 26 (1) | 4 (1) |
| Unrelated BM | 298 (10) | 158 (6) | 140 (28) |
| 10/10 HLA-matched | 243 (8) | 123 (5) | 120 (24) |
| ≤9/10 HLA-matched | 55 (2) | 35 (1) | 20 (4) |
| Unrelated PBSC | 1350 (44) | 1273 (50) | 77 (15) |
| 10/10 HLA-matched | 1102 (36) | 1041 (41) | 61 (12) |
| ≤9/10 HLA-matched | 248 (8) | 232 (9) | 16 (3) |
| Haploidentical, BM | 83 (3) | 62 (2) | 21 (4) |
| Haploidentical, PBSC | 60 (2) | 52 (2) | 8 (2) |
| Cord blood | 347 (11) | 229 (9) | 118 (24) |
| GVHD prophylaxis approaches | |||
| T-cell depletion | 134 (4) | 100 (4) | 34 (7) |
| ATG or alemtuzumab | 252 (8) | 90 (4) | 162 (32) |
| PTCY-based | 246 (8) | 219 (9) | 27 (5) |
| Methotrexate-based | 1072 (35) | 927 (36) | 145 (29) |
| MMF or sirolimus-based | 1338 (44) | 1215 (47) | 123 (25) |
| CNI only | 15 (<1) | 11 (<1) | 4 (1) |
| Other | 9 (<1) | 2 (<1) | 7 (1) |
| Characteristic . | All patients N (%) . | Adults n (%) . | Children n (%) . |
|---|---|---|---|
| Total | 3066 (100) | 2564 (84) | 502 (16) |
| Sex, female | 1294 (42) | 1080 (42) | 214 (43) |
| Donor recipient: female/male∗ | 749 (26) | 629 (26) | 120 (25) |
| Race/ethnicity | |||
| American Indian or Alaska native | 54 (2) | 35 (1) | 19 (4) |
| Asian | 217 (7) | 161 (6) | 56 (11) |
| Black or African American | 76 (2) | 51 (2) | 25 (5) |
| Native Hawaiian/other Pacific Islander | 44 (1) | 34 (1) | 10 (2) |
| White (non-Hispanic) | 2382 (78) | 2106 (82) | 276 (55) |
| White (Hispanic) | 178 (6) | 103 (4) | 75 (15) |
| White (unknown) | 5 (<1) | 5 (<1) | 0 |
| Multiple/unknown | 110 (4) | 69 (3) | 41 (8) |
| Median age (range), y | 49.0 (0.1-78.9) | 52.8 (18.1-78.9) | 8.2 (0.1-17.9) |
| Malignant diseases | 2761 (90) | 2476 (97) | 285 (57) |
| Acute myeloid leukemia/MDS | 1355 (44) | 1256 (49) | 99 (20) |
| Myeloproliferative diseases | 213 (7) | 205 (8) | 8 (2) |
| Acute lymphoblastic leukemia | 476 (16) | 330 (13) | 146 (29) |
| Chronic leukemia (CML/CLL) | 231 (8) | 222 (9) | 9 (2) |
| Lymphoma | 308 (10) | 290 (11) | 18 (4) |
| Plasma cell disorders | 144 (5) | 144 (6) | 0 (0) |
| Biphenotypic leukemias | 30 (1) | 25 (1) | 5 (1) |
| Other | 4 (<1) | 4 (<1) | 0 (0) |
| NMD | 305 (10) | 88 (3) | 217 (43) |
| Aplastic anemia | 115 (4) | 55 (2) | 60 (12) |
| Inherited BM failure syndrome | 41 (1) | 8 (<1) | 33 (7) |
| Primary immunodeficiency disease | 80 (3) | 11 (<1) | 69 (14) |
| Primary immunodysregulatory disease | 26 (<1) | 3 (<1) | 23 (5) |
| Hemoglobinopathy (thalassemia, SCD) | 26 (<1) | 6 (<1) | 20 (4) |
| Other | 17 (<1) | 5 (<1) | 12 (2) |
| Conditioning intensity† | |||
| MA-TBI | 680 (22) | 462 (18) | 218 (43) |
| NMA | 1101 (36) | 1018 (40) | 83 (17) |
| RIC | 1285 (42) | 1084 (42) | 201 (40) |
| Donor type/graft source | |||
| HLA-identical sibling, BM | 167 (5) | 45 (2) | 122 (24) |
| HLA-identical sibling, PBSC | 731 (24) | 719 (28) | 12 (2) |
| Mismatched related | 30 (1) | 26 (1) | 4 (1) |
| Unrelated BM | 298 (10) | 158 (6) | 140 (28) |
| 10/10 HLA-matched | 243 (8) | 123 (5) | 120 (24) |
| ≤9/10 HLA-matched | 55 (2) | 35 (1) | 20 (4) |
| Unrelated PBSC | 1350 (44) | 1273 (50) | 77 (15) |
| 10/10 HLA-matched | 1102 (36) | 1041 (41) | 61 (12) |
| ≤9/10 HLA-matched | 248 (8) | 232 (9) | 16 (3) |
| Haploidentical, BM | 83 (3) | 62 (2) | 21 (4) |
| Haploidentical, PBSC | 60 (2) | 52 (2) | 8 (2) |
| Cord blood | 347 (11) | 229 (9) | 118 (24) |
| GVHD prophylaxis approaches | |||
| T-cell depletion | 134 (4) | 100 (4) | 34 (7) |
| ATG or alemtuzumab | 252 (8) | 90 (4) | 162 (32) |
| PTCY-based | 246 (8) | 219 (9) | 27 (5) |
| Methotrexate-based | 1072 (35) | 927 (36) | 145 (29) |
| MMF or sirolimus-based | 1338 (44) | 1215 (47) | 123 (25) |
| CNI only | 15 (<1) | 11 (<1) | 4 (1) |
| Other | 9 (<1) | 2 (<1) | 7 (1) |
ATG, antithymocyte globulin; BM, bone marrow; CLL, chronic lymphocytic leukemia; CML, chronic myelogenous leukemia; CNI, calcineurin inhibitor; MA-TBI, myeloablative Total body irradiation; MDS, myelodysplastic syndrome; MMF, mycophenolate mofetil; NMA, nonmyeloablative; PBSC, peripheral blood stem cells; PTCY, posttransplant cyclophosphamide; RIC, reduced-intensity conditioning; SCD, sickle cell disease.
Missing donor sex information for 129 of 3066 patients (106 adults and 23 children).
MA-TBI (TBI ≥12 Gy–containing regimens); NMA (fludarabine 90 mg/m2 and 2-3 Gy TBI or fludarabine ≤150 mg/m2; cyclophosphamide 29 mg/kg and 2-3 Gy TBI); and RIC (all other conditioning regimens including 4 Gy TBI–containing regimens).
Results
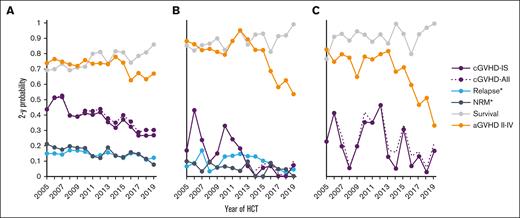
The median follow-up among study subjects alive at last contact was 7.0 years (range, 1.0-17.2). The median age at HCT was 49 years (range, <1-78); 58% were male. Two-year point estimates of pertinent outcomes per year for HCT are summarized in Figure 1. The probability of 2-year cGVHD-IS among all survivors ranged from 45% to 52% earlier in the window, to ∼40% in later years, and then ∼26% by the later years in the window (Figure 1A). These estimates were only minimally higher when all cGVHD was considered by also including cGVHD cases not treated with systemic immunosuppression. A similar decline was observed when the analysis was restricted to 502 pediatric survivors, with cGVHD probabilities being <10% in the latter 7 years (Figure 1B). Among 305 adult and pediatric survivors who underwent transplantation for NMD, cGVHD rates showed greater fluctuation but remained <20% after 2016 (Figure 1C). Acute GVHD probabilities declined more substantially over the last decade in children and patients who underwent transplantation for NMDs, whereas 2-year point estimates of survival steadily improved across all 3 windows. The yearly 2-year point estimates of NRM among all patients showed an overall downward trend over time (Figure 1A). Among patients who underwent transplantation for malignancy, the yearly 2-year point estimates of relapse were reasonably steady across time, with a slight numerical increase in earlier years, followed by a slight numerical decrease in later years (Figure 1A). Because patients selected for this analysis had to survive ≥100 days without relapse after their first allogeneic HCT, the possibility that higher 100-day mortality in later years could artificially reduce the frequency of cGVHD is not an issue because such patients would not be in the “denominator” for the current analyses. Moreover, the day-100 mortality was lower, on average, across time, with rates in the 3 eras of 13% for 2005 to 2009, 8% for 2010 to 2014, and 8% for 2015 to 2019. Figures 2 and 3 summarize pertinent demographics and treatments hypothesized to influence the risk of cGVHD across the 3 defined HCT date windows.
Two-year point estimates of various outcomes by calendar year of HCT. Shown for all patients (A), age <18 years (B), and all patients who underwent transplantation for NMD (C). ∗Relapse in patients who underwent transplantation for malignancy; aGVHD, acute GVHD; cGVHD-All, cGVHD whether treated with systemic IS (cGVHD-IS) or not; NRM∗, NRM in all patients.
Two-year point estimates of various outcomes by calendar year of HCT. Shown for all patients (A), age <18 years (B), and all patients who underwent transplantation for NMD (C). ∗Relapse in patients who underwent transplantation for malignancy; aGVHD, acute GVHD; cGVHD-All, cGVHD whether treated with systemic IS (cGVHD-IS) or not; NRM∗, NRM in all patients.
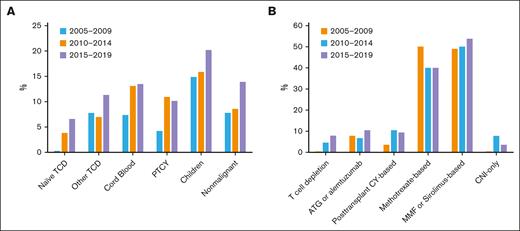
HCT approaches and demographic shifts that might lower cGVHD-IS rates. Proportions shown by the 5-year eras in HCT date (A) and nonmutually exclusive GVHD prophylaxis categories (B). ATG, antithymocyte globulin; CNI, calcineurin inhibitor; CY, cyclophosphamide; MMF, mycophenolate mofetil; PTCY, posttransplant cyclophosphamide; TCD, T-cell depletion.
HCT approaches and demographic shifts that might lower cGVHD-IS rates. Proportions shown by the 5-year eras in HCT date (A) and nonmutually exclusive GVHD prophylaxis categories (B). ATG, antithymocyte globulin; CNI, calcineurin inhibitor; CY, cyclophosphamide; MMF, mycophenolate mofetil; PTCY, posttransplant cyclophosphamide; TCD, T-cell depletion.
Donor-recipient stem-cell source relationships, disease, and conditioning intensity. Proportions show 5-year eras in HCT date for donor type and stem-cell source (A); male recipients with female donors, children vs adult recipients, transplants performed for NMD, and myeloablative vs nonmyeloablative conditioning (B). BM, bone marrow; F, female; M, male; PB, peripheral blood.
Donor-recipient stem-cell source relationships, disease, and conditioning intensity. Proportions show 5-year eras in HCT date for donor type and stem-cell source (A); male recipients with female donors, children vs adult recipients, transplants performed for NMD, and myeloablative vs nonmyeloablative conditioning (B). BM, bone marrow; F, female; M, male; PB, peripheral blood.
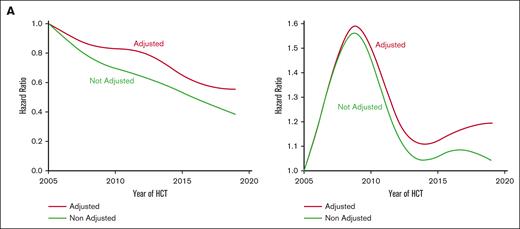
To formally assess cGVHD risk as a function of the HCT date and to avoid arbitrary categorization, the HCT date was modeled as a continuous nonlinear variable, as described above. Figure 4A shows the unadjusted and adjusted hazard ratio (HR) for cGVHD as a function of HCT date relative to 1 January 2005. A statistical test of the null hypothesis that all coefficients of the cubic spline are 0 (ie, the curve is flat) yielded P < .0001, indicating an association between the HCT date and cGVHD risk. Additionally, there was little evidence to suggest a nonlinear association (P = .64 for the null hypothesis that nonlinear coefficients of the cubic spline are equal to 0). Therefore, the HCT date was modeled as a continuous linear variable (Table 2).
Association between time and cause-specific hazard of cGVHD. (A) Estimate of HR of NIH cGVHD-IS as a function of HCT date modeled as a continuous nonlinear variable (N = 3066), with HR relative to 1 January 2005; green line, unadjusted HR; red line, adjusted HR. (B) HR of relapse as a function of HCT date modeled as a continuous nonlinear variable among patients with malignant disease (n = 2756), with HR relative to 1 January 2005; green line, unadjusted HR; red line, adjusted HR.
Association between time and cause-specific hazard of cGVHD. (A) Estimate of HR of NIH cGVHD-IS as a function of HCT date modeled as a continuous nonlinear variable (N = 3066), with HR relative to 1 January 2005; green line, unadjusted HR; red line, adjusted HR. (B) HR of relapse as a function of HCT date modeled as a continuous nonlinear variable among patients with malignant disease (n = 2756), with HR relative to 1 January 2005; green line, unadjusted HR; red line, adjusted HR.
Cox regression models for cGVHD and mortality among patients with cGVHD
| Parameter . | Unadjusted models . | Adjusted models . |
|---|---|---|
| NIH chronic GVHD | ||
| HCT date | HR, 0.73; 95% CI, 0.69-0.78; P < .0001 | HR, 0.81; 95% 0.75-0.87; P < .0001 |
| Overall mortality after cGVHD | - | - |
| HCT date | HR, 0.94; 95% CI, 0.83-1.07; P = .375 | HR, 0.88; 95% CI, 0.76-1.01; P = .067 |
| Parameter . | Unadjusted models . | Adjusted models . |
|---|---|---|
| NIH chronic GVHD | ||
| HCT date | HR, 0.73; 95% CI, 0.69-0.78; P < .0001 | HR, 0.81; 95% 0.75-0.87; P < .0001 |
| Overall mortality after cGVHD | - | - |
| HCT date | HR, 0.94; 95% CI, 0.83-1.07; P = .375 | HR, 0.88; 95% CI, 0.76-1.01; P = .067 |
HCT dates were modeled as continuous linear variables, and HR presented in terms of 5-year increments for date of transplantation. Multivariable models were adjusted for recipient age, donor/stem-cell source, patient/donor sex, patient/donor CMV serostatus, the severity of disease (low vs intermediate vs high), TBI dose (modeled as a continuous linear variable), conditioning intensity (NMA vs reduced intensity vs ablative), self-reported race, and GVHD prophylaxis.
The unadjusted HR for each 5-year increase in HCT date was 0.73 (95% confidence interval [CI], 0.69-0.78; P < .0001). The adjusted HR was closer to the null of 1.0 but still showed a reduction as the HCT date increased (HR, 0.81 for each 5-year increase, 95% CI, 0.75-0.87; P < .0001). These results suggest that, even after adjusting for factors previously shown to be associated with the risk of cGVHD, the risk of cGVHD still decreased over time.
Among patients who developed cGVHD-IS, the risk of subsequent mortality was modeled as above (day 0 was the day of cGVHD diagnosis). Statistical testing of the null hypothesis that the curve is flat yielded P = .07, and a test of linearity yielded P = .16. Although there was some evidence of nonlinearity, with HCT date modeled as a continuous linear variable, the adjusted HR of death (for each 5-year increase after cGVHD onset) was 0.88 (95% CI, 0.76-1.01; P = .067), suggesting that the detrimental impact of cGVHD on mortality was somewhat lessened in more recent years (Table 2).
Given the reduction in cGVHD risk in recent years and the recognized association of cGVHD with lower relapse rates, we examined relapse risk among patients who underwent HCT for malignancy, again modeling HCT date as a continuous nonlinear variable (Figure 4B). Statistical testing of the null hypothesis that the curve is flat yielded P = .073, and a test of linearity yielded P = .064, together suggesting a nonlinear association. The risk of relapse increased early in the window from 2005 to 2019 but then began to decrease in later years, eventually reaching a risk that was similar to that in earlier years, albeit numerically higher.
Changes in patient and donor characteristics and HCT procedures (Figures 2 and 3) did not fully explain the decrease in cGVHD as evidenced by the multivariable model presented above. To further explore this, we conducted a subset analysis of patients receiving methotrexate-based acute GVHD prophylaxis for malignant disease from a matched related or matched unrelated donor, which also showed a numerical decline in the risk of cGVHD, although lower in magnitude relative to the decline observed in the entire population (adjusted HR 0.88 for each 5-year increase; 95% CI, 0.79-1.00; P = .042).
Discussion
We have shown that the cause-specific hazard of cGVHD requiring IS therapy declined by ∼27% on average, with each 5-year increment in HCT date after 2005. The cause-specific hazard of cGVHD still declined by ∼19% on average, with each 5-year increment in HCT date, even after adjusting for the typically expected cause-associated factors. The decline in the risk of cGVHD was still evident even after adjustment for demographic shifts and greater use of HCT approaches that are generally associated with lower cGVHD rates.
First, comparing our results with others, we note that before the implementation of NIH cGVHD consensus definitions, a large registry analysis of 100-day survivors of HCT for malignant disease indications showed increased cGVHD incidence between 1995 and 2007 that persisted after adjusting for donor type, stem-cell source, and conditioning intensity.5 Although NRM declined, the increase in long-term survivors did not explain the rise in cGVHD incidence, which was instead attributed to older patients, more peripheral blood grafts, and alternative donors.
One obvious explanation for the declining cGVHD rates is changing definitions. We purposely limited our analysis to ≥2005, 3 years after we began using NIH cGVHD definitions, to avoid reporting a “spurious” decline in cGVHD incidence that was simply due to the reclassification of isolated late acute GVHD cases per the NIH definition. In a prospective 2011 to 2014 cGVHD Consortium cohort, the probability of NIH cGVHD was 47% at a median of 7.4 months,6 similar to the 42% 3-year probability from a large 2016 to 2017 US claims analysis cohort7 and the 45% probability that we report here for our 2005 to 2009 cohort.
Therefore, the question remains, what led to the decline in the risk of cGVHD-IS during our 15-year study timespan? We speculate that universal clinical practice advancements played a combinatory role beyond the variables included in our analyses. We discuss below several avenues for future studies, acknowledging that some may be difficult to pursue without very large, detailed patient cohorts.
First, although the role of microbiota dysbiosis in cGVHD remains open for investigation it can clearly impact acute GVHD independent of genetic disparity8 because of its broad immunomodulatory roles, which are a focus of ongoing investigation.9 It is plausible that newer, less toxic, yet more effective, anticancer, or nonmalignant disease therapeutics, together with better supportive care, might lead to more patients entering HCT with less inflammation and less dysbiosis. For example, 1 group interested in why adult acute myelogenous leukemia (AML) outcomes were superior for dual-drug liposomal encapsulation of cytarabine and daunorubicin compared with traditional “7+3,”10,11 showed in a murine model that unlike 7 + 3, the liposomal version protected against gut dysbiosis, mucosal damage, and gut morbidity while increasing antifungal resistance.12 Another example is how specifically targeting interferon-gamma with emapalumab provided an effective bridge to HCT in patients with primary HLH,13 whereas prior standard etoposide plus dexamethasone was associated with 20% pre-HCT mortality.14
Second, since acute GVHD is the most powerful predictor of subsequent cGVHD,15 we evaluated whether the after 2012 decline in acute GVHD observed in children might explain the cGVHD decline in that subgroup (Figure 1B). Including acute GVHD in the regression model had little impact on the risk of cGVHD (data not shown), and we did not observe a similar decline in acute GVHD rates for the total population (Figure 1A). Regardless of any alterations in the acute GVHD rates, better overall acute GVHD management might have contributed to the decline in cGVHD. For example, less cumulative prednisone use (starting at 0.5-1 mg/kg vs 2 mg/kg), particularly since 2009,16 more emphasis on enteral feeding vs parenteral nutrition,17 and more microbiome-friendly antibiotic practices, particularly with well-developed antibiotic stewardship programs.18 Also unaccounted for in our analysis were the potential impacts of CMV reactivation. There is a bidirectional relationship between CMV replication and acute GVHD19; 1 study showed more cGVHD when there was prior CMV infection,20 and another showed less cGVHD when there was polymerase chain reaction-based pre-emptive CMV therapy.21 Therefore, although declining rates and/or levels of CMV viremia over time could help explain our main finding, this would need to be explored with a different analysis. It is worth mentioning that letermovir prophylaxis for adults was not standard for FHCC before 2018, and is still not standard for children.
We currently do not understand how novel agents used to prevent acute GVHD or treat steroid-refractory acute GVHD might later impact the rates of cGVHD, whether by their proposed mechanisms of action and/or via modulation of the composition and function of the gut microbiome, including immune responses.22,23 Our analysis included patients who underwent transplantation from 2005 to 2019, so it preceded the US Food and Drug Administration approval of ruxolitinib for steroid-refractory acute GVHD in 2019. Only a small percentage of patients with myelofibrosis received ruxolitinib early after transplantation on an FHCC research protocol that was not open for accrual until 2020.
Third, at study inception, FHCC donor selection already required 10/10 HLA-typing data (HLA-A, -B, -C, -DRB1, and -DQB1) and had recently begun limiting the total number of HLA-mismatches based on Petersdorf et al.24 In 2015, after our demonstration that mismatches at HLA-DPB1 were added to the GVHD risk, we began HLA-12/12 matching.25,26 In 2020, Petersdorf et al showed in a huge HCT cohort (N = 33 982), the additive effects of multilocus mismatching on the risk for developing cGVHD (odds ratio [OR]: 1.16 for 8/10 match, P < .0001; 1.32 for 7/10; P < .0001) and among 9/10 matches, increased cGVHD rates for locus-specific mismatches at HLA-A (OR, 1.13; P = .02) and HLA-C (OR, 1.14; P = .006) relative to DQB1.27 However, our model did adjust for donor/stem-cell source, and patients with an unrelated donor were categorized as 10/10 vs mismatched for at least 1 allele at HLA-A, -B, -C, -DRB1, and -DQB1.
Lastly, regarding donor selection, it is interesting to consider clonal hematopoiesis (CH) mutations that can be transferred from the donor to the recipient during HCT and persist over time. After HCT outcomes among recipients of donor-derived CH and no-CH have been compared. One study showed that donor-derived CH was associated with lower relapse and a 1.7-fold higher rate of cGVHD.28 A similar study in the context of calcineurin inhibitor–based GVHD prophylaxis showed that the most common CH mutation (DNMT3A) was associated with reduced relapse and increased cGVHD (HR, 1.36).29 A third study additionally showed that donor-derived CH was associated with higher rates of extensive-moderate/severe GVHD and less likelihood of discontinuing immunosuppression than controls (P = .03).30 Given that CH is age-related,31 it is conceivable that our center’s preference over time for selecting younger donors contributed to our declining cGVHD rates.
Our study had additional limitations. Although cGVHD global severity scores were associated with NRM,32 we were unable to analyze severity trends because these data were not captured for all eras, particularly for patients diagnosed outside our clinics. The proportion of study patients who were treated without IS therapy (dotted purple line, Figure 1) was smaller than the expected 10% to 25%,32,33 possibly because a new cGVHD diagnosis is most frequently triggered at our center when a patient begins IS therapy; some cGVHD cases, either asymptomatic or mild enough to only be prescribed topicals, might have been missed, especially when diagnosed outside of our clinics. Our analysis did not include HCTs conducted during the COVID-19 pandemic, but in-person visits and cGVHD diagnoses might have been affected by the pandemic. Our analysis was conducted at a single large center, which has the advantage of consistent medical management and data collection. However, the results may not apply to other centers.
In conclusion, we demonstrated a decline in the risk of cGVHD since 2005 among a cohort of patients who underwent transplantation over 15 years at FHCC. The <10% rates of cGVHD requiring systemic immunosuppressive therapy (IST) in children after 2013 are particularly encouraging, as was the decline in cGVHD rates in the nonmalignant disease subgroup. Likewise encouraging, this phenomenon was not accompanied by an obvious uniform increase in the risk of relapsed malignancies over time, and overall mortality also declined in the population of patients who developed cGVHD. All populations seemed to benefit as the risk of cGVHD also decreased in patients with malignant diseases who received methotrexate-based acute GVHD prophylaxis and were transplanted from a matched donor. Although cGVHD rates have declined, for patients who develop cGVHD, further studies will be needed to better understand how more recent HCT approaches impact the incidence and/or IS therapy responsiveness of highly morbid cGVHD phenotypes (eg, sclerotic, ocular, or lung). Our data underscores that cGVHD prevention studies need to use contemporaneous and not historical controls as comparators to account for a decline in cGVHD in all populations.
Acknowledgments
Grant funding for this study was provided by the NIH (CA018029, CA15704, and CA118953).
Authorship
Contribution: P.A.C. and S.J.L. developed the research question and study protocol and interpreted the results; P.A.C. and S.J.L. wrote the first and subsequent manuscript drafts; T.A.G. focused on the statistical end points in the study design and performed the analysis; and all authors reviewed the data, contributed input to subsequent manuscript drafts, and gave final approval to submit for publication.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Paul A. Carpenter, Clinical Research Division, Fred Hutchinson Cancer Center, 1100 Fairview Ave N, Seattle WA 98109; email: pcarpent@fredhutch.org.
References
Author notes
Study protocol and individual participant data that underlie the results reported in this article, after deidentification (text, tables, figures, and appendixes) can be shared, if approved by our institutional review board. Data will be available to investigators who submit “approved proposals” beginning 9 months and ending 36 months after article publication. Proposals must be “approved” by an independent review committee (“learned intermediary”) identified for this purpose and if approved by our institutional review board.