Key Points
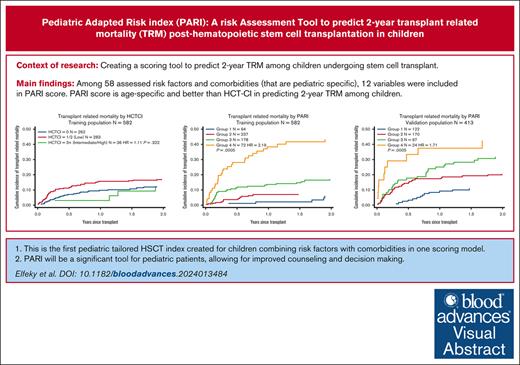
To our knowledge, this is the first pediatric tailored HSCT index combining RFs with CoMs in 1 scoring model.
PARI will be a significant tool for pediatric patients, allowing for improved counseling and decision-making.
Visual Abstract
Several attempts have been made to optimize pretransplant risk assessment to improve hematopoietic stem cell transplantation (HSCT) decision-making and to predict post-HSCT outcomes. However, the relevance of pretransplant risk assessment to the pediatric population remains unclear. We report the results of revalidation of the hematopoietic cell transplantation comorbidity index (HCT-CI) in 874 children who received 944 HSCTs for malignant or nonmalignant diseases at a single center. After finding the HCT-CI invalid in our patient population, we proposed a modified pediatric adapted scoring system that captures risk factors (RFs) and comorbidities (CoMs) relevant to pediatrics. Each RF/CoM was assigned an integer weight based on its hazard ratio (HR) for transplant-related mortality (TRM): 0 (HR < 1.2), 1 (1.2 ≥ HR < 1.75), 2 (1.75 ≥ HR < 2.5), and 3 (HR ≥ 2.5). Using these weights, the pediatric adapted risk index (PARI) for HSCT was devised, and patients were divided into 4 risk groups (group 1: without RF/CoM; group 2: score 1-2; group 3: score 3-4; and group 4: score ≥5). There was a linear increase in 2-year TRM from group 1 to 4 (TRM, 6.2% in group 1, 50.9% in group 4). PARI was successfully validated on an internal and external cohort of pediatric patients. Comparing models using c-statistics, PARI was found to have better performance than HCT-CI in predicting 2-year TRM in children, with Akaike and Schwarz Bayesian information criteria values of 1069.245 and 1073.269, respectively, using PARI, vs 1223.158 and 1227.051, respectively, using HCT-CI. We believe that PARI will be a valuable tool enabling better counseling and decision-making for pediatric patients with HSCT.
Introduction
Accurate estimation of the potential risks of hematopoietic stem cell transplantation (HSCT) is important to provide better counseling,1-3 especially when nontransplant treatment options exist. Although several risk-assessment models have been proposed to assess the effects of pre-HSCT comorbidities (CoMs) on outcome, such models have had a gross under-representation of the pediatric population.4
The hematopoietic cell transplantation comorbidity index (HCT-CI) was designed to identify the impact of CoMs contributing to nonrelapse mortality (NRM) and transplant-related mortality (TRM) in patients undergoing HSCT. HCT-CI has since been validated with variable degrees of predictability in multiple independent retrospective and prospective, predominantly adult studies.5-11 Validation in children and adolescents is scarce; some of the HCT-CI CoMs have a low prevalence in this age group, limiting its value.12
Hence, the primary objective of our study was to evaluate the validity of the HCT-CI in predicting 2-year TRM in children undergoing HSCT at a single center over a period of 16 years. The second objective was to identify risk factors (RFs)–influencing 2-year TRM and to use this information to devise a scoring system relevant to the pediatric cohort.
Methods
Patient characteristics
The study cohort consisted of 874 patients who received 1 (92.3%) or more HSCTs at the Great Ormond Street Hospital (GOSH) for Children between January 2000 and December 2016 for malignant or nonmalignant diseases. All treatment protocols were approved by the institutional review board, and all patients/guardians provided signed informed consent in accordance with the Declaration of Helsinki. Patient demographics, disease category, and HSCT characteristics are described in Tables 1 and 2. Main disease categories included inborn errors of immunity (IEI) (40%) followed by hematologic or nonhematologic malignancies (30%) and an almost equal proportion of both metabolic and bone marrow failures (8% each).
Patient characteristics
| Characteristics . | n (%) . |
|---|---|
| No. of transplants | 944 |
| No. of patients | 874 |
| No. of transplants per patient | |
| 1 | 807 |
| 2 | 64 |
| 3 | 3 |
| Median age (range) at first transplant, y | 3.11 (0.04-17.73) |
| Recipient’s sex | |
| Male | 534 (61.10) |
| Diagnoses | |
| Acute leukemia | 206 (23.57) |
| Acute lymphoblastic leukemia | 119 |
| Acute myeloid leukemia | 82 |
| Mixed lineage leukemia | 2 |
| Acute promyelocytic leukemia | 1 |
| CML in blast crisis | 1 |
| Other malignant hematological diseases | 58 (6.64) |
| Juvenile myelomonocytic leukemia | 23 |
| Myelodysplastic syndrome | 21 |
| Chronic myeloid leukemia | 4 |
| Myeloproliferative disorder | 1 |
| Lymphoma | 8 |
| Nonfocal histiocytic sarcoma | 1 |
| Primary immune deficiencies | 355 (40.62) |
| SCID | 137 |
| Non-SCID | 219 |
| Histiocytic disease | 67 (7.67) |
| Metabolic disease | 72 (8.24) |
| MPS I-II-VI | 32 |
| Osteopetrosis | 19 |
| ALD | 10 |
| Alpha-mannosidosis | 5 |
| Others | 6 |
| Autoimmunity disorders | 7 (0.80) |
| Aplastic anemias | 75 (8.58) |
| Acquired aplastic anemia | 32 |
| BM failure syndromes | 43 |
| Fanconi anemia | 17 |
| Severe congenital neutropenia | 9 |
| Dyskeratosis congenita | 2 |
| Diamond-Blackfan anemia | 2 |
| Congenital amegakaryocytic thrombocytopenia | 2 |
| Shwachman-Diamond syndrome | 1 |
| Paroxysmal nocturnal hemoglobinuria | 1 |
| Congenital BM failure (other/unspecified) | 10 |
| Hemoglobinopathies | 9 (1.03) |
| Glanzmann thrombasthenia | 6 (0.69) |
| Gastroenterology | 19 (2.17) |
| Characteristics . | n (%) . |
|---|---|
| No. of transplants | 944 |
| No. of patients | 874 |
| No. of transplants per patient | |
| 1 | 807 |
| 2 | 64 |
| 3 | 3 |
| Median age (range) at first transplant, y | 3.11 (0.04-17.73) |
| Recipient’s sex | |
| Male | 534 (61.10) |
| Diagnoses | |
| Acute leukemia | 206 (23.57) |
| Acute lymphoblastic leukemia | 119 |
| Acute myeloid leukemia | 82 |
| Mixed lineage leukemia | 2 |
| Acute promyelocytic leukemia | 1 |
| CML in blast crisis | 1 |
| Other malignant hematological diseases | 58 (6.64) |
| Juvenile myelomonocytic leukemia | 23 |
| Myelodysplastic syndrome | 21 |
| Chronic myeloid leukemia | 4 |
| Myeloproliferative disorder | 1 |
| Lymphoma | 8 |
| Nonfocal histiocytic sarcoma | 1 |
| Primary immune deficiencies | 355 (40.62) |
| SCID | 137 |
| Non-SCID | 219 |
| Histiocytic disease | 67 (7.67) |
| Metabolic disease | 72 (8.24) |
| MPS I-II-VI | 32 |
| Osteopetrosis | 19 |
| ALD | 10 |
| Alpha-mannosidosis | 5 |
| Others | 6 |
| Autoimmunity disorders | 7 (0.80) |
| Aplastic anemias | 75 (8.58) |
| Acquired aplastic anemia | 32 |
| BM failure syndromes | 43 |
| Fanconi anemia | 17 |
| Severe congenital neutropenia | 9 |
| Dyskeratosis congenita | 2 |
| Diamond-Blackfan anemia | 2 |
| Congenital amegakaryocytic thrombocytopenia | 2 |
| Shwachman-Diamond syndrome | 1 |
| Paroxysmal nocturnal hemoglobinuria | 1 |
| Congenital BM failure (other/unspecified) | 10 |
| Hemoglobinopathies | 9 (1.03) |
| Glanzmann thrombasthenia | 6 (0.69) |
| Gastroenterology | 19 (2.17) |
ALD, adrenoleukodystrophy; BM, bone marrow; MPS, mucopolysaccharidosis; SCID, severe combined immunodeficiency.
Transplant characteristics
| . | n (%) . |
|---|---|
| No. of transplants | 944 |
| No. of patients | 874 |
| Donor type | |
| Matched donors | 623 (65.89) |
| MSD | 232 |
| MFD | 65 |
| MUD | 325 |
| Mismatched donors | 322 (34.11) |
| MMUD | 239 |
| MMSD and MMFD | 16 |
| Haploidentical | 67 |
| Stem cell source | |
| BM | 526 (55.72) |
| PBSCs | 302 (31.99) |
| Cord | 116 (12.29) |
| Preparative conditioning regimens | |
| MAC | 416 (44.07) |
| Bu-based conditioning | 220 |
| Bu/Flu ± Cyc/Mel | 58 |
| Bu/Cyc ± Mel | 162 |
| Treo-based conditioning | 56 |
| Treo/Flu ± TT | 39 |
| Treo/Cy/Mel | 17 |
| TBI conditioning | 121 |
| TBI (12 Gy)/Cyc ± Flu | 84 |
| TBI (12 Gy)/etoposide | 33 |
| TBI (12 Gy)/Flu/TT | 4 |
| Others | 19 |
| Flu/Mel/TT | 6 |
| Carmustine/etoposide/cytarabine/Mel | 3 |
| RIC | 408 (43.22) |
| Treo/Flu | 125 |
| Bu/Flu | 41 |
| Flu/Mel ± anti-CD66 | 187 |
| Flu/cytarabine ± low dose TBI | 3 |
| Other | 10 |
| MIC | 96 (10.28) |
| Flu/Cyc ± low dose TBI or ± anti-CD45 | 67 |
| Cyc alone | 14 |
| Flu/low dose TBI | 8 |
| Cyc/low dose TBI | 7 |
| No conditioning | 66 (6.99) |
| GVHD prophylaxis regimens | 884 (93.65) |
| CSA based | 869 (92.06) |
| CSA | 234 |
| CSA/MMF ± MP | 422 |
| CSA/MTX | 212 |
| CSA/OKT3 | 1 |
| Tacrolimus-based | 5 (0.53) |
| Tac | 2 |
| Tac/MMF | 2 |
| Tac/MTX | 1 |
| Sirolimus based | 4 (0.42) |
| Sirolimus/MMF | 4 |
| Others | 6 (0.64) |
| MMF | 2 |
| MMF/OKT3 | 3 |
| MP | 1 |
| In vivo T-cell depletion | 620 (64.83) |
| Alemtuzumab (0.3-1 mg/kg) | 491 |
| rATG (4-60 mg/kg) | 121 |
| . | n (%) . |
|---|---|
| No. of transplants | 944 |
| No. of patients | 874 |
| Donor type | |
| Matched donors | 623 (65.89) |
| MSD | 232 |
| MFD | 65 |
| MUD | 325 |
| Mismatched donors | 322 (34.11) |
| MMUD | 239 |
| MMSD and MMFD | 16 |
| Haploidentical | 67 |
| Stem cell source | |
| BM | 526 (55.72) |
| PBSCs | 302 (31.99) |
| Cord | 116 (12.29) |
| Preparative conditioning regimens | |
| MAC | 416 (44.07) |
| Bu-based conditioning | 220 |
| Bu/Flu ± Cyc/Mel | 58 |
| Bu/Cyc ± Mel | 162 |
| Treo-based conditioning | 56 |
| Treo/Flu ± TT | 39 |
| Treo/Cy/Mel | 17 |
| TBI conditioning | 121 |
| TBI (12 Gy)/Cyc ± Flu | 84 |
| TBI (12 Gy)/etoposide | 33 |
| TBI (12 Gy)/Flu/TT | 4 |
| Others | 19 |
| Flu/Mel/TT | 6 |
| Carmustine/etoposide/cytarabine/Mel | 3 |
| RIC | 408 (43.22) |
| Treo/Flu | 125 |
| Bu/Flu | 41 |
| Flu/Mel ± anti-CD66 | 187 |
| Flu/cytarabine ± low dose TBI | 3 |
| Other | 10 |
| MIC | 96 (10.28) |
| Flu/Cyc ± low dose TBI or ± anti-CD45 | 67 |
| Cyc alone | 14 |
| Flu/low dose TBI | 8 |
| Cyc/low dose TBI | 7 |
| No conditioning | 66 (6.99) |
| GVHD prophylaxis regimens | 884 (93.65) |
| CSA based | 869 (92.06) |
| CSA | 234 |
| CSA/MMF ± MP | 422 |
| CSA/MTX | 212 |
| CSA/OKT3 | 1 |
| Tacrolimus-based | 5 (0.53) |
| Tac | 2 |
| Tac/MMF | 2 |
| Tac/MTX | 1 |
| Sirolimus based | 4 (0.42) |
| Sirolimus/MMF | 4 |
| Others | 6 (0.64) |
| MMF | 2 |
| MMF/OKT3 | 3 |
| MP | 1 |
| In vivo T-cell depletion | 620 (64.83) |
| Alemtuzumab (0.3-1 mg/kg) | 491 |
| rATG (4-60 mg/kg) | 121 |
Patients receiving Bu-based conditioning had either RIC conditioning with Bu AUC levels of 45 to 65 mg × h/L or MAC conditioning with Bu AUC ≥70 mg × h/L.
AUC, area under the curve; Bu, busulfan; Cyc, cyclophosphamide; CSA, cyclosporine; Flu, fludarabine; MAC, myeloablative conditioning; Mel, melphalan; MFD, matched family donor; MIC, minimal-intensity conditioning; MP, methylprednisolone; MSD, matched sibling donor; MMF, mycophenolate mofetil; MMFD, mismatched family donor; MMSD, mismatched sibling donor; MMUD, mismatched unrelated donor; MTX, methotrexate; MUD, matched unrelated donor; PBSC, peripheral blood stem cell; OKT3, anti-CD3; rATG, rabbit antithymocyte globulin; RIC, reduced-intensity conditioning; Tac, tacrolimus Treo, treosulfan; TT, thiotepa.
Patient-, transplant-, treatment-, and organ-related RF and CoM variables influencing 2-year TRM
| Variable . | Total cases (%) . | 2-y TRM (95% CI) . | HR (95% CI) . | P value . |
|---|---|---|---|---|
| All patients | 582 | 14.8% (12-17.9) | ||
| Age, y | ||||
| <2 | 242 (42) | 14.8% (10.6-19.7) | ||
| 2-5 | 160 (27) | 12.9% (8.2-18.7) | 1.09 (0.86-1.38) | .471 |
| 6+ | 180 (31) | 16.0% (10.9-21.9) | ||
| Continuous | 1.01 (0.96-1.06) | .811 | ||
| Patient sex | ||||
| Male | 360 (62) | 15.9% (11.4-21.2) | 1.01 (0.96-1.06) | .349 |
| Female | 222 (38) | 13.8% (10.4-17.7) | ||
| Weight | ||||
| Continuous | 1.00 (0.99-1.02) | .733 | ||
| Disease category | ||||
| Malignant diseases | 165 (28) | 15.0% (10.0-21.0) | 1.14 (0.75-1.74) | .535 |
| Nonmalignant diseases | 417 (72) | 14.5% (11.2-18.1) | ||
| Presence of sex mismatch (yes) | 264 (46) | 14.4% (10.4-19.1) | 1.08 (0.73-1.61) | .696 |
| CMV mismatch (yes) | 188 (33) | 15.3% (10.5-20.9) | 1.08 (0.71-1.65) | .89 |
| Stem cell source | ||||
| BM | 339 (58) | 11.4% (8.2-15.2) | 1.53 (1.01-2.34) | .047 |
| PBSC | 171 (29) | 20.7% (14.9-27.2) | 1.14 (0.60-2.18) | .692 |
| Cord | 72 (12) | 15.5% (8.2-24.8) | ||
| Period of transplantation (y) | ||||
| 2000-2005 | 167 | 17.4% (12-23) | 0.98 (0.75-1.27) | .853 |
| 2006-2011 | 205 | 13.1% (14.6-27.5) | ||
| 2012-2016 | 210 | 14% (9.6-19.2) | ||
| Donor type | ||||
| Mismatched donor | 190 (33) | 24.1% (18.2-30.5) | 2.15 (1.45-3.20) | .0005 |
| Matched donor | 392 (67) | 10.0% (7.2-13.3) | ||
| Transplant | ||||
| >1 transplant | 47 (8) | 22% (11-33) | 1.40 (0.71-2.76) | .328 |
| No previous transplant | 535 (92) | 14% (11-17) | ||
| Conditioning | ||||
| MAC | 263 (45) | 13% (9-17) | 0.82 (0.60-1.12) | .21 |
| RIC/MIC | 278 (48) | 16% (12-21) | ||
| None | 41 (7) | 18% (8-31) | ||
| TBI | ||||
| TBI-based conditioning | 85 (15%) | 18% (11-27) | 1.41 (0.86-2.32) | .168 |
| Non-TBI based | 496 (85) | 14% (11-17) | ||
| Use of serotherapy | ||||
| Yes | 382 (66) | 16% (13-20) | 1.16 (0.76-1.78) | .487 |
| No | 200 (34) | 11% (7-16) | ||
| Use of any GVHD prophylaxis | ||||
| Yes | 549 (94) | 14.4% (11.5-17.5) | 1.47 (0.72-2.97) | .288 |
| No | 33 (6) | 18.8% (7.6-33.7) | ||
| Pre-HSCT infections | ||||
| Presence of probable/confirmed fungal infection | ||||
| Yes | 50 (9) | 12.3% (5-23.1) | 0.81 (0.38-1.74) | .591 |
| No | 531 (91) | 14.8% (11.9-18.1) | ||
| Presence of disseminated BCGiosis | ||||
| Yes | 12 (2) | 16.7% (2.7-41.3) | 0.95 (0.23-3.90) | .944 |
| No | 569 (98) | 14.6% (11.8-17.7) | ||
| Presence of EBV Day -10 to day 0 | ||||
| Yes | 9 (2) | 12.5% (0.7-42.3) | 1.34 (0.37-4.82) | .658 |
| No | 572 (98) | 14.7% (11.9-17.8) | ||
| Presence of AdV Day -10 to day 0 | ||||
| Yes | 6 (1) | 0% | Dropped | |
| No | 575 (99) | 14.8% (12.0-17.9) | ||
| Presence of CMV Day -10 to day 0 | ||||
| Yes | 18 (3) | 28% (10-49) | 1.74 (0.68-4.41) | .246 |
| No | 564 (97) | 14% (11-17) | ||
| Presence of respiratory viral infection Day -10 to day 0 | ||||
| Yes | 26 (4) | 31% (15-49) | 2.26 (1.04-4.91) | .039 |
| No | 556 (96) | 14% (11-17) | ||
| Need to continue antimicrobials at D0 | ||||
| Yes | 104 (18) | 19% (12-28) | 1.32 (0.81-2.16) | .263 |
| No | 478 (82) | 13.6% (10.6-16.9) | ||
| Cardiac variables | ||||
| Presence of heart valve disease∗ | ||||
| Yes | 15 (3) | 13.3% (2.2-34.6) | 0.84 (0.42-1.68) | .616 |
| No | 566 (97) | 14.7% (11.8-17.8) | ||
| Presence of a cardiac anomaly | ||||
| Yes | 31 (5) | 19.5% (7.9-34.9) | 1.12 (0.49-2.55) | .79 |
| No | 551 (95) | 14.3% (11.5-17.5) | ||
| Pulmonary variable | ||||
| Presence of dyspnea at slight activity | ||||
| Yes | 3 (1) | 0% | Dropped | |
| No | 578 (99) | 14.7% (11.9-17.8) | ||
| Presence of dyspnea at rest or requiring oxygen | ||||
| Yes | 21 (4) | 9.5% (1.6-26.1) | 0.50 (0.12-2.00) | .325 |
| No | 560 (96) | 14.8% (12.0-18.0) | ||
| Presence of pulmonary hypertension | ||||
| Yes | 2 (<1) | 0% | Dropped | |
| No | 579 (99) | 14.7% (11.9-17.8) | ||
| Presence of obstructive sleep apnea | ||||
| Yes | 9 (2) | 33.3% (7.8-6.2) | 2.12 (0.66-6.79) | .208 |
| No | 572 (98) | 14.3% (11.5-17.4) | ||
| Being on assisted ventilation at D0 | ||||
| Yes | 4 (1) | 25.0% (0.9-66.5) | 1.63 (0.17-5.4) | .670 |
| No | 578 (99) | 14.6% (11.8-17.6) | ||
| Requiring assisted ventilation at any time (including D0) | ||||
| Yes | 74 (13) | 22% (13-32) | 1.48 (0.87-2.50) | .146 |
| No | 508 (87) | 14% (11-17) | ||
| Presence of any respiratory structural abnormality† | ||||
| Yes | 13 (2) | 23% (6-47) | 1.91 (0.72-5.06) | .19 |
| No | 13 (2) | 14% (12-18) | ||
| Cerebrovascular CoMs | ||||
| Encephalopathy | ||||
| Yes | 4 (1) | 14.6% (11.8-17.6) | 1.52 (0.22-10.3) | .669 |
| No | 577 (99) | 25% (0.9-66.5) | ||
| Hydrocephalus | ||||
| Yes | 7 (1) | 14.3% (0.7-46.5) | 1.63 (0.43-6.13) | .472 |
| No | 574 (99) | 14.6% (11.8-17.7) | ||
| Sinus thrombosis | ||||
| Yes | 2 (<1) | 0% | Dropped | |
| No | 579 (99) | 14.7% (11.9-17.8) | ||
| PRES | ||||
| Yes | 3 (1) | 67% (5-95) | 5.21 (1.56-17.3) | .007 |
| No | 569 (99) | 14% (12-17) | ||
| Epilepsy requiring medical intervention | ||||
| Yes | 6 (1) | 50% (11-80) | 3.90 (1.03-14.8) | .045 |
| No | 576 (99) | 14% (11-17) | ||
| Presence of any CNS structural abnormality‡ | ||||
| Yes | 11 (2) | 45% (17-71) | 2.95 (1.26-6.92) | .013 |
| No | 571 (98) | 14% (11-17) | ||
| Presence of any of the following: CNS infection, PRES, epilepsy, or CNS structural abnormality | ||||
| Yes | 32 (6) | 47% (29-63) | 3.85 (2.17-6.83) | .0005 |
| No | 550 (94) | 13% (10-16) | ||
| Gastrointestinal disease variables | ||||
| Presence of chronic diarrhea | ||||
| Yes | 44 (8) | 20.9% (10.3-33.9) | 1.35 (0.71-2.54) | .361 |
| No | 538 (92) | 14.1% (11.3-17.2) | ||
| Active infectious diarrhea at D0 | ||||
| Yes | 12 (2) | 42% (15-67) | 3.59 (1.27-10.1) | .016 |
| No | 540 (98) | 14% (11-17) | ||
| Continuation on TPN at D0 | ||||
| Yes | 46 (8) | 26.5% (14.7-39.8) | 1.74 (0.93-3.25) | .08 |
| No | 46 (8) | 13.6% (10.8-6.7) | ||
| Perianal inflammation/fistula | ||||
| Yes | 5 (1) | 0% | Dropped | |
| No | 576 (99) | 14.7% (11.9-17.8) | ||
| Endocrine disease variables | ||||
| Diabetes requiring treatment | ||||
| Yes | 3 (1) | 0% | Dropped | |
| No | 578 (99) | 14.7% (11.9-17.8) | ||
| Presence of any other endocrine disease on treatment§ | ||||
| Yes | 21 (4) | 22.0% (6.7-42.7) | 1.49 (0.63-3.48) | .362 |
| No | 561 (96) | 14.4% (11.6-17.5) | ||
| Hepatobiliary disease, renal and biochemical variables | ||||
| Moderate/severe hepatobiliary | 153 | 16.6% (11.1-23.2) | 1.33 (1.06-1.67) | .014 |
| Mild hepatobiliary | 198 | 18% (12.9-23.7) | ||
| No abnormality | 231 | 10.5% (6.9-15) | ||
| Serum creatinine | ||||
| >1.5 ULN | 29 | 14.3% (8.3-22.0) | 1.1 (0.9-1.27) | .693 |
| Above ULN to 1.5 ULN | 72 | 13.2% (10.2-16.8) | ||
| Normal | 481 | 13.5% (10.3-17.1) | ||
| Presence of renal malformation | ||||
| Yes | 5 (1) | 20% (1-58) | 1.29 (0.18-9.48) | .801 |
| No | 577 (99) | 15% (12-18) | ||
| Serum albumin level | ||||
| <25 g/L | 10 (2) | 50.0% (18.4-75.3) | 1.80 (1.23-2.65) | .003 |
| ≥25-35 g/L | 155 (27) | 20.7% (14.6-27.5) | ||
| >35 g/L | 416 (52) | 11.5% (8.6-14.9) | ||
| Other comorbidities | ||||
| Having a prior surgery | ||||
| Yes | 56 (10) | 13.0% (5.7-23.4) | 0.93 (0.46-1.86) | .838 |
| No | 525 (90) | 14.8% (11.9-18.1) | ||
| Having a prior malignancy | ||||
| Yes | 15 (3) | 18.3% (2.9-44.4) | 1.83 (0.76-4.40) | .180 |
| No | 566 (97) | 14.6% (11.8-17.7) | ||
| Prior autoimmunity or immune dysregulation | ||||
| Yes | 63 | 14.4% (11.5-17.6) | 1.02 | .949 |
| No | 519 | 16.45% (8.4-26.8) | ||
| Being on prednisolone ≥0.3 mg/kg per day at D0 | ||||
| Yes | 44 (8) | 27.5% (15.3-21.1) | 1.98 (1.08-3.61) | .026 |
| No | 537 (92) | 13.6% (10.8- 16.4) |
| Variable . | Total cases (%) . | 2-y TRM (95% CI) . | HR (95% CI) . | P value . |
|---|---|---|---|---|
| All patients | 582 | 14.8% (12-17.9) | ||
| Age, y | ||||
| <2 | 242 (42) | 14.8% (10.6-19.7) | ||
| 2-5 | 160 (27) | 12.9% (8.2-18.7) | 1.09 (0.86-1.38) | .471 |
| 6+ | 180 (31) | 16.0% (10.9-21.9) | ||
| Continuous | 1.01 (0.96-1.06) | .811 | ||
| Patient sex | ||||
| Male | 360 (62) | 15.9% (11.4-21.2) | 1.01 (0.96-1.06) | .349 |
| Female | 222 (38) | 13.8% (10.4-17.7) | ||
| Weight | ||||
| Continuous | 1.00 (0.99-1.02) | .733 | ||
| Disease category | ||||
| Malignant diseases | 165 (28) | 15.0% (10.0-21.0) | 1.14 (0.75-1.74) | .535 |
| Nonmalignant diseases | 417 (72) | 14.5% (11.2-18.1) | ||
| Presence of sex mismatch (yes) | 264 (46) | 14.4% (10.4-19.1) | 1.08 (0.73-1.61) | .696 |
| CMV mismatch (yes) | 188 (33) | 15.3% (10.5-20.9) | 1.08 (0.71-1.65) | .89 |
| Stem cell source | ||||
| BM | 339 (58) | 11.4% (8.2-15.2) | 1.53 (1.01-2.34) | .047 |
| PBSC | 171 (29) | 20.7% (14.9-27.2) | 1.14 (0.60-2.18) | .692 |
| Cord | 72 (12) | 15.5% (8.2-24.8) | ||
| Period of transplantation (y) | ||||
| 2000-2005 | 167 | 17.4% (12-23) | 0.98 (0.75-1.27) | .853 |
| 2006-2011 | 205 | 13.1% (14.6-27.5) | ||
| 2012-2016 | 210 | 14% (9.6-19.2) | ||
| Donor type | ||||
| Mismatched donor | 190 (33) | 24.1% (18.2-30.5) | 2.15 (1.45-3.20) | .0005 |
| Matched donor | 392 (67) | 10.0% (7.2-13.3) | ||
| Transplant | ||||
| >1 transplant | 47 (8) | 22% (11-33) | 1.40 (0.71-2.76) | .328 |
| No previous transplant | 535 (92) | 14% (11-17) | ||
| Conditioning | ||||
| MAC | 263 (45) | 13% (9-17) | 0.82 (0.60-1.12) | .21 |
| RIC/MIC | 278 (48) | 16% (12-21) | ||
| None | 41 (7) | 18% (8-31) | ||
| TBI | ||||
| TBI-based conditioning | 85 (15%) | 18% (11-27) | 1.41 (0.86-2.32) | .168 |
| Non-TBI based | 496 (85) | 14% (11-17) | ||
| Use of serotherapy | ||||
| Yes | 382 (66) | 16% (13-20) | 1.16 (0.76-1.78) | .487 |
| No | 200 (34) | 11% (7-16) | ||
| Use of any GVHD prophylaxis | ||||
| Yes | 549 (94) | 14.4% (11.5-17.5) | 1.47 (0.72-2.97) | .288 |
| No | 33 (6) | 18.8% (7.6-33.7) | ||
| Pre-HSCT infections | ||||
| Presence of probable/confirmed fungal infection | ||||
| Yes | 50 (9) | 12.3% (5-23.1) | 0.81 (0.38-1.74) | .591 |
| No | 531 (91) | 14.8% (11.9-18.1) | ||
| Presence of disseminated BCGiosis | ||||
| Yes | 12 (2) | 16.7% (2.7-41.3) | 0.95 (0.23-3.90) | .944 |
| No | 569 (98) | 14.6% (11.8-17.7) | ||
| Presence of EBV Day -10 to day 0 | ||||
| Yes | 9 (2) | 12.5% (0.7-42.3) | 1.34 (0.37-4.82) | .658 |
| No | 572 (98) | 14.7% (11.9-17.8) | ||
| Presence of AdV Day -10 to day 0 | ||||
| Yes | 6 (1) | 0% | Dropped | |
| No | 575 (99) | 14.8% (12.0-17.9) | ||
| Presence of CMV Day -10 to day 0 | ||||
| Yes | 18 (3) | 28% (10-49) | 1.74 (0.68-4.41) | .246 |
| No | 564 (97) | 14% (11-17) | ||
| Presence of respiratory viral infection Day -10 to day 0 | ||||
| Yes | 26 (4) | 31% (15-49) | 2.26 (1.04-4.91) | .039 |
| No | 556 (96) | 14% (11-17) | ||
| Need to continue antimicrobials at D0 | ||||
| Yes | 104 (18) | 19% (12-28) | 1.32 (0.81-2.16) | .263 |
| No | 478 (82) | 13.6% (10.6-16.9) | ||
| Cardiac variables | ||||
| Presence of heart valve disease∗ | ||||
| Yes | 15 (3) | 13.3% (2.2-34.6) | 0.84 (0.42-1.68) | .616 |
| No | 566 (97) | 14.7% (11.8-17.8) | ||
| Presence of a cardiac anomaly | ||||
| Yes | 31 (5) | 19.5% (7.9-34.9) | 1.12 (0.49-2.55) | .79 |
| No | 551 (95) | 14.3% (11.5-17.5) | ||
| Pulmonary variable | ||||
| Presence of dyspnea at slight activity | ||||
| Yes | 3 (1) | 0% | Dropped | |
| No | 578 (99) | 14.7% (11.9-17.8) | ||
| Presence of dyspnea at rest or requiring oxygen | ||||
| Yes | 21 (4) | 9.5% (1.6-26.1) | 0.50 (0.12-2.00) | .325 |
| No | 560 (96) | 14.8% (12.0-18.0) | ||
| Presence of pulmonary hypertension | ||||
| Yes | 2 (<1) | 0% | Dropped | |
| No | 579 (99) | 14.7% (11.9-17.8) | ||
| Presence of obstructive sleep apnea | ||||
| Yes | 9 (2) | 33.3% (7.8-6.2) | 2.12 (0.66-6.79) | .208 |
| No | 572 (98) | 14.3% (11.5-17.4) | ||
| Being on assisted ventilation at D0 | ||||
| Yes | 4 (1) | 25.0% (0.9-66.5) | 1.63 (0.17-5.4) | .670 |
| No | 578 (99) | 14.6% (11.8-17.6) | ||
| Requiring assisted ventilation at any time (including D0) | ||||
| Yes | 74 (13) | 22% (13-32) | 1.48 (0.87-2.50) | .146 |
| No | 508 (87) | 14% (11-17) | ||
| Presence of any respiratory structural abnormality† | ||||
| Yes | 13 (2) | 23% (6-47) | 1.91 (0.72-5.06) | .19 |
| No | 13 (2) | 14% (12-18) | ||
| Cerebrovascular CoMs | ||||
| Encephalopathy | ||||
| Yes | 4 (1) | 14.6% (11.8-17.6) | 1.52 (0.22-10.3) | .669 |
| No | 577 (99) | 25% (0.9-66.5) | ||
| Hydrocephalus | ||||
| Yes | 7 (1) | 14.3% (0.7-46.5) | 1.63 (0.43-6.13) | .472 |
| No | 574 (99) | 14.6% (11.8-17.7) | ||
| Sinus thrombosis | ||||
| Yes | 2 (<1) | 0% | Dropped | |
| No | 579 (99) | 14.7% (11.9-17.8) | ||
| PRES | ||||
| Yes | 3 (1) | 67% (5-95) | 5.21 (1.56-17.3) | .007 |
| No | 569 (99) | 14% (12-17) | ||
| Epilepsy requiring medical intervention | ||||
| Yes | 6 (1) | 50% (11-80) | 3.90 (1.03-14.8) | .045 |
| No | 576 (99) | 14% (11-17) | ||
| Presence of any CNS structural abnormality‡ | ||||
| Yes | 11 (2) | 45% (17-71) | 2.95 (1.26-6.92) | .013 |
| No | 571 (98) | 14% (11-17) | ||
| Presence of any of the following: CNS infection, PRES, epilepsy, or CNS structural abnormality | ||||
| Yes | 32 (6) | 47% (29-63) | 3.85 (2.17-6.83) | .0005 |
| No | 550 (94) | 13% (10-16) | ||
| Gastrointestinal disease variables | ||||
| Presence of chronic diarrhea | ||||
| Yes | 44 (8) | 20.9% (10.3-33.9) | 1.35 (0.71-2.54) | .361 |
| No | 538 (92) | 14.1% (11.3-17.2) | ||
| Active infectious diarrhea at D0 | ||||
| Yes | 12 (2) | 42% (15-67) | 3.59 (1.27-10.1) | .016 |
| No | 540 (98) | 14% (11-17) | ||
| Continuation on TPN at D0 | ||||
| Yes | 46 (8) | 26.5% (14.7-39.8) | 1.74 (0.93-3.25) | .08 |
| No | 46 (8) | 13.6% (10.8-6.7) | ||
| Perianal inflammation/fistula | ||||
| Yes | 5 (1) | 0% | Dropped | |
| No | 576 (99) | 14.7% (11.9-17.8) | ||
| Endocrine disease variables | ||||
| Diabetes requiring treatment | ||||
| Yes | 3 (1) | 0% | Dropped | |
| No | 578 (99) | 14.7% (11.9-17.8) | ||
| Presence of any other endocrine disease on treatment§ | ||||
| Yes | 21 (4) | 22.0% (6.7-42.7) | 1.49 (0.63-3.48) | .362 |
| No | 561 (96) | 14.4% (11.6-17.5) | ||
| Hepatobiliary disease, renal and biochemical variables | ||||
| Moderate/severe hepatobiliary | 153 | 16.6% (11.1-23.2) | 1.33 (1.06-1.67) | .014 |
| Mild hepatobiliary | 198 | 18% (12.9-23.7) | ||
| No abnormality | 231 | 10.5% (6.9-15) | ||
| Serum creatinine | ||||
| >1.5 ULN | 29 | 14.3% (8.3-22.0) | 1.1 (0.9-1.27) | .693 |
| Above ULN to 1.5 ULN | 72 | 13.2% (10.2-16.8) | ||
| Normal | 481 | 13.5% (10.3-17.1) | ||
| Presence of renal malformation | ||||
| Yes | 5 (1) | 20% (1-58) | 1.29 (0.18-9.48) | .801 |
| No | 577 (99) | 15% (12-18) | ||
| Serum albumin level | ||||
| <25 g/L | 10 (2) | 50.0% (18.4-75.3) | 1.80 (1.23-2.65) | .003 |
| ≥25-35 g/L | 155 (27) | 20.7% (14.6-27.5) | ||
| >35 g/L | 416 (52) | 11.5% (8.6-14.9) | ||
| Other comorbidities | ||||
| Having a prior surgery | ||||
| Yes | 56 (10) | 13.0% (5.7-23.4) | 0.93 (0.46-1.86) | .838 |
| No | 525 (90) | 14.8% (11.9-18.1) | ||
| Having a prior malignancy | ||||
| Yes | 15 (3) | 18.3% (2.9-44.4) | 1.83 (0.76-4.40) | .180 |
| No | 566 (97) | 14.6% (11.8-17.7) | ||
| Prior autoimmunity or immune dysregulation | ||||
| Yes | 63 | 14.4% (11.5-17.6) | 1.02 | .949 |
| No | 519 | 16.45% (8.4-26.8) | ||
| Being on prednisolone ≥0.3 mg/kg per day at D0 | ||||
| Yes | 44 (8) | 27.5% (15.3-21.1) | 1.98 (1.08-3.61) | .026 |
| No | 537 (92) | 13.6% (10.8- 16.4) |
95% CI, 95% confidence interval; AdV, adenovirus; CMV, cytomegalovirus infection; CSA, cyclosporine; EBV, Epstein-Barr virus; TPN, total parenteral nutrition.
Apart from trivial regurgitant lesions.
This includes bronchiectasis, chronic lung disease, cystic fibrosis, bronchopulmonary fistula, and laryngomalacia.
Macrocephaly/microcephaly/computed tomography changes showing cerebral abnormalities.
Includes insulin-dependent diabetes mellitus, Addison’s disease, syndrome of inappropriate antidiuretic hormone secretion, hypoparathyroidism, recurrent hypoglycemia, hypercalcemia, and hypothyroidism.
PARI scoring system
| RF/comorbid condition . | 2-y TRM (95% CI) . | Multivariate analysis (HR) . | Weight . |
|---|---|---|---|
| Donor type | |||
| Mismatched donor | 24.1% (18.2- 30.5) | 2.14 | 2 |
| Conditioning regimen | |||
| TBI | 18.4% (10.9-27.5) | 1.75 | 2 |
| Infection | |||
| Presence of respiratory viral infection D-10-D0 | 31% (15-49) | 2.09 | 2 |
| Lung disease | |||
| Any respiratory structural abnormality | 23% (6-47) | 2.15 | 2 |
| CNS disease | |||
| Any CNS abnormality (CNS infection, PRES, epilepsy, and CNS structural abnormality) | 47% (29-63) | 3.68 | 3 |
| Gut disease | |||
| Active infectious diarrhea | 42% (15-67) | 1.61 | 1 |
| Hepatobiliary | |||
| Mild hepatic | 16.6% (11.1-23.2) | 1.31 | 1 |
| Moderate hepatic | 18% (12.9-23.7) | 1.41 | 1 |
| Biochemical CoMs | |||
| Albumin levels ≥25 to <35 mmol/L | 20.7% (14.6-27.5) | 1.32 | |
| Albumin levels <25 mmol/L | 50.0% (18.4-75.3) | 4.17 | 3 |
| Other CoMs | |||
| Continuation of prednisolone therapy ≥0.3 mg/kg per day beyond D0 | 27.5% (15.3-21.1) | 1.62 | 1 |
| Prior malignancy pre-HSCT | 18.3% (2.9-44.4) | 1.82 | 2 |
| RF/comorbid condition . | 2-y TRM (95% CI) . | Multivariate analysis (HR) . | Weight . |
|---|---|---|---|
| Donor type | |||
| Mismatched donor | 24.1% (18.2- 30.5) | 2.14 | 2 |
| Conditioning regimen | |||
| TBI | 18.4% (10.9-27.5) | 1.75 | 2 |
| Infection | |||
| Presence of respiratory viral infection D-10-D0 | 31% (15-49) | 2.09 | 2 |
| Lung disease | |||
| Any respiratory structural abnormality | 23% (6-47) | 2.15 | 2 |
| CNS disease | |||
| Any CNS abnormality (CNS infection, PRES, epilepsy, and CNS structural abnormality) | 47% (29-63) | 3.68 | 3 |
| Gut disease | |||
| Active infectious diarrhea | 42% (15-67) | 1.61 | 1 |
| Hepatobiliary | |||
| Mild hepatic | 16.6% (11.1-23.2) | 1.31 | 1 |
| Moderate hepatic | 18% (12.9-23.7) | 1.41 | 1 |
| Biochemical CoMs | |||
| Albumin levels ≥25 to <35 mmol/L | 20.7% (14.6-27.5) | 1.32 | |
| Albumin levels <25 mmol/L | 50.0% (18.4-75.3) | 4.17 | 3 |
| Other CoMs | |||
| Continuation of prednisolone therapy ≥0.3 mg/kg per day beyond D0 | 27.5% (15.3-21.1) | 1.62 | 1 |
| Prior malignancy pre-HSCT | 18.3% (2.9-44.4) | 1.82 | 2 |
Statistical analysis
Data collection
Pertinent information and pretransplant comorbidities were extracted from patients’ transplant protocols, medical notes, hospital electronic records, pathology reports, and electronic laboratory databases. The CoMs were initially collected according to their HCT-CI definition.5
Validating the HCT-CI
For the whole data set, we calculated the HCT-CI for each patient according to the existing definition reference.5
Development of PARI
Patients were first randomized into training (n = 582) and validation (n = 292) data sets in a 2:1 ratio. Patient randomization was performed using the dplyr package (version 1.0.2). Cumulative incidence (CI) of 2-year TRM stratified by the risk groups of the pediatric adapted risk index (PARI) was visualized using the survminer package (version 0.4.3). The training data set was used to develop the new scoring system, PARI, by modifying existing HCT-CI definitions (supplemental Table 1) and by examining additional RF/CoM (supplemental Table 2). The validation data set was used to perform internal validation. To derive the new scoring weights, competing risk regression analysis13 was performed on the training data set, with 2-year TRM as the outcome.
Training data set
Using the training data set, we examined a total of 58 variables that were grouped under 4 main categories: patient-related, transplant-related, treatment-related, and organ-specific factors.
Factors associated with increased 2-year TRM in univariate analysis (P < .2)14 were included in multivariate analysis, whereas RF/CoM with P ≥ .2 or those not associated with 2-year TRM were excluded. Each RF/CoM was assigned a new integer weight based on its hazard ratio (HR) as follows: 0 (HR < 1.2), 1 (1.2 ≥ HR < 1.75), 2 (1.75 ≥ HR < 2.5), 3 (HR ≥ 2.5). Using these weights, the PARI was devised, and patients were divided into 5 risk groups (1: without RF/CoM; 2: score 1-2; 3: score 3-4; 4: score ≥5).
Model fitness evaluation using c-statistics
The combination and weights of RFs included in PARI were selected to minimize the Akaike information criterion (AIC) and the Schwarz Bayesian information criterion (BIC) for the best model.
AIC15 is a refined technique based on in-sample fit to estimate the likelihood of a model to predict/estimate future values.
A good model is one that has the minimum AIC among all the other models. The AIC can be used to select between the additive and multiplicative Holt-Winters models.
BIC16 is another criterion for model selection that measures the trade-off between model fit and complexity of the model. A lower AIC or BIC value indicates a better fit.
Model validation
To test the ability of PARI to predict the 2-year TRM in previously unseen patient data, it was applied to an internal validation data set from GOSH and to an external validation data set from Hospital Pablo Tobón Uribe, Medellín, Colombia. Both data sets were compiled into 1 validation data set to avoid institutional bias.
Validation using an age- and disease-matched cohort was conducted to assess the scoring system’s reproducibility and generalizability. We validated our score by running an external comparison with a unit similar to ours in terms of patients’ age group, type of disease, and transplant characteristics. Additionally, the physician who collected data from the GOSH center also collected data from her center in Colombia to ensure consistency and minimize personal bias. These data are presented in supplemental Table 3.
All statistical analyses were performed using Stata/MP v17.0 statistical software (StataCorp).
Results
Survival
The 2-year overall survival (OS) for the entire cohort was 73%. Among the 231 deceased patients, 2-year TRM was 16.6% (95% confidence interval, 14.2-19.2).
Failure to validate the HCT-CI
Seven out of 17 parameters were either not observed in our cohort (inflammatory bowel disease, depression, and moderate/severe renal disease) or not associated with 2-year TRM (arrhythmia, cardiovascular disease, rheumatological disease, and peptic ulcer). Of note, 70% of our cohort had HSCT before the age of 6 years, and thus no pulmonary function test (important parameter in HCT-CI) was performed.
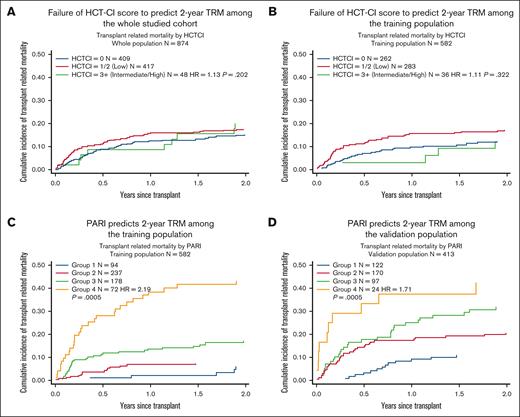
We ran the HCT-CI on our cohort of 874 pediatric HSCT recipients; 2-year TRM was 15.2%, 17.6%, and 20.1% in patients with HCT-CI scores 0, 1 to 2, and ≥3, respectively. HCT-CI was not a significant predictor of 2-year TRM neither as a continuous variable (P = .172; HR, 1.11) nor according to the categories above (P = .202; HR, 1.13).
Creation of PARI
Analysis of factors influencing the 2-year TRM: univariate analysis
The training data set of 582 patients was used to investigate RFs affecting the 2-year TRM, which was found to be 14.8% (Table 3).
PATIENT-RELATED FACTORS
Age at transplant (P = .471), weight (P = .73), sex (P = .34), and disease category (P = .53) had no influence on 2-year TRM.
TRANSPLANT-RELATED FACTORS
Donor/recipient sex mismatch, cytomegalovirus mismatch, and stem cell source did not influence 2-year TRM. Era of HSCT (2000-2005 vs 2006-2011 vs 2012-2016) did not influence 2-year TRM (P = .853). The use of mismatched donors was associated with a significantly higher 2-year TRM (24.1%) in comparison with 10/10 HLA-matched related or unrelated donors (P = .0005). Recipients of subsequent transplants had a slightly higher 2-year TRM than recipients of first transplant, although these results were not statistically significant (22% vs 14%; P = .32).
TREATMENT-RELATED FACTORS
The intensity of the conditioning regimen and use of serotherapy did not influence the 2-year TRM. The use of total body irradiation (TBI; TBI-based conditioning) was associated with a higher 2-year TRM of 18% vs 14% (P = .16). The type of graft-versus-host disease (GVHD) prophylaxis regimen used did not influence the 2-year TRM (P = .28).
CoM- AND ORGAN-SPECIFIC FACTORS
Pre-HSCT infections
Two-year TRM was significantly higher in the presence of respiratory viral infection as assessed by polymerase chain reaction on nasopharyngeal aspirate during conditioning, with TRM rates of 31% as opposed to 14% in the absence of respiratory viral infection (P = .039).
The need to continue antimicrobials at day 0 (D0) and beyond, active cytomegalovirus, Epstein-Barr virus, or adenoviral viremia at the time of HSCT, previous fungal infection, or previous disseminated Bacillus Calmette-Guérin did not influence 2-year TRM (P = .26; P = .24; P = .65; P = .86; P = .591; and P = .944, respectively).
Cardiac CoMs
Cardiac functional or structural abnormalities were not significantly associated with 2-year TRM.
Pulmonary CoMs
The need for assisted ventilation at D0 and presence of any respiratory structural abnormalities (congenital or acquired) were associated with higher 2-year TRM rates of 25% and 23%, respectively, in comparison with 14% in the absence of any of these variables (P = .14; P = .19). Pulmonary hypertension and obstructive sleep apnea did not influence 2-year TRM.
Cerebrovascular CoMs
Prior central nervous system (CNS) infection, posterior reversible encephalopathy syndrome (PRES), epilepsy requiring medical intervention, or any CNS structural abnormality were associated with 2-year TRM rates of 50%, 67%, 50%, and 45%, as opposed to a rate of 14% in the absence of any of these variables (P = .0005; P = .007; P = .045; and P = .013, respectively). Encephalopathy, sinus thrombosis, and hydrocephalus were not associated with increased 2-year TRM.
Gastrointestinal CoMs
Patients with active infectious diarrhea (regardless of the pathogenic cause) at D0 and those who needed to remain on total parenteral nutrition at the time of HSCT had a 42% and 26.5% 2-year TRM, as opposed to almost 14% of patients who had none of these variables (P = .016; P = .08). Chronic diarrhea did not influence 2-year TRM.
Endocrine CoMs
The presence of any endocrine abnormalities was not associated with increased rates of 2-year TRM.
Hepatobiliary, renal, and biochemical CoMs
We used Common Terminology Criteria for Adverse Events version 5 for grading serum alanine transaminase (ALT), serum bilirubin, and serum creatinine. Regarding hepatobiliary disease, patients were divided into 3 groups: those with no hepatobiliary disease, those with mild hepatobiliary disease (ALT above the upper limit of normal [ULN] to 3 × ULN or serum bilirubin above ULN to 1.5 × ULN), and those with moderate hepatobiliary disease (ALT above 3× ULN or bilirubin above 1.5× ULN). Patients with moderate hepatobiliary disease and those with mild hepatobiliary disease had 2-year TRM of 18% and 16.6%, respectively (P = .014), as opposed to 10% among patients who had no evidence of hepatobiliary disease.
Renal factors including structural renal anomaly and serum creatinine above ULN to 1.5× ULN or >1.5× ULN for age were not associated with increased rates of 2-year TRM. Patients who had low serum albumin level <25 g/L or levels between ≥25 and <35 g/L had 2-year TRM of 50% and 20.7%, as opposed to 11.5% of patients who had albumin levels ≥35 g/L (P = .03).
Other CoMs
Prior malignancy (excluding the malignant indication for HSCT) was associated with higher 2-year TRM of 18.3% in comparison with 14.6% in the absence of this comorbidity (P = .18). History of autoimmunity or immune dysregulation (Omenn syndrome or hemophagocytic lymphohistiocytosis) did not influence 2-year TRM (P = .949). However, the need to continue prednisolone at a dose ≥0.3 mg/kg per day at D0 was associated with higher 2-year TRM rates of 27.5% vs 13.6% in the absence of this CoM (P = .026).
Analysis of factors influencing 2-year TRM: multivariate analysis
Twelve out of 15 variables had HR >1.2 and were thus integrated into the PARI. CNS structural abnormality and albumin levels <25 g/L had received the highest weight of 3. The use of a mismatched donor, receiving a TBI-based conditioning, the presence of a respiratory viral infection at the time of transplantation, respiratory structural abnormality, and prior malignancy each was assigned a weight of 2. Having active infectious diarrhea at the time of transplantation, having mild or moderate/severe hepatobiliary disease, receiving prednisolone ≥0.3 mg/kg at the time of transplantation, having serum albumin levels between 25 and 35 g/dL each was assigned a weight of 1 (Table 3). Three factors were dropped because their HR was <1.2, including stem cell source, need for assisted ventilation before HSCT, and total parenteral nutrition dependence.
Development of the PARI
Patients were divided into 4 groups based on the weighted cumulative risk index. Groups 1 to 4 included 94, 238, 178, and 72 cases, respectively. Two-year TRM was 6.1% (95% CI, 2.3-12.7), 7.3% (95% CI, 4.4-11.1), 17.2% (95% CI, 12-23.2), and 43.2% (95% CI, 31.3-54.4) among patients in groups 1, 2, 3, and 4, respectively (Table 4, Figure 1).
Comparing HCT-CI with PARI in predicting 2-year TRM. (A and B) Demonstrates failure of HCT-CI to predict 2-year TRM among the whole population and training population. (C and D) Demonstrates that PARI can predict 2-year TRM among the whole population and the training population.
Comparing HCT-CI with PARI in predicting 2-year TRM. (A and B) Demonstrates failure of HCT-CI to predict 2-year TRM among the whole population and training population. (C and D) Demonstrates that PARI can predict 2-year TRM among the whole population and the training population.
Validation of PARI
PARI was applied to an internal validation data set from GOSH and to an external validation data set from Hospital Pablo Tobón Uribe, Medellín, Colombia. Both data sets were compiled into 1 validation data set to avoid institutional bias. This validation data set had 413 patients in total: 122 in group 1, 170 in group 2, 97 in group 3, and 24 patients in group 4. Two-year TRM was 10.9% (CI, 6.1-17.3), 18.9% (CI, 14.8-27.1), 31.8% (CI, 22.7-41.3), and 42.5% (CI, 22.6-61.2).
Comparing HCT-CI with the new index, PARI
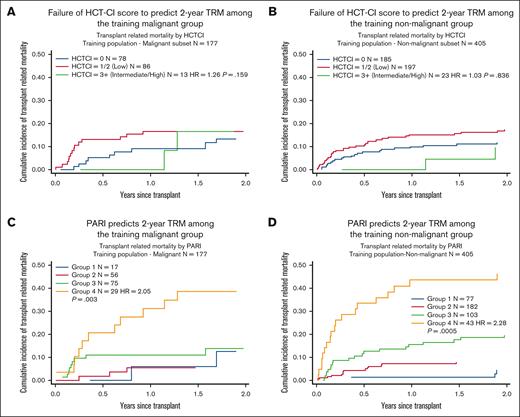
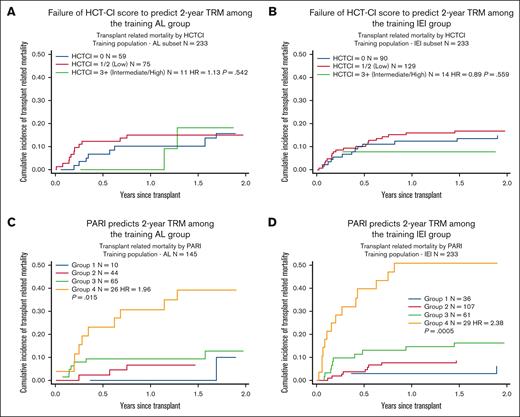
PARI was found to have better performance than HCT-CI in predicting 2-year TRM in children, with AIC and BIC values of 1069.245 and 1073.269, respectively, using PARI, as opposed to 1223.158 and 1227.051, respectively, using HCT-CI (Figure 1). We also compared PARI with HCT-CI in predicting 2-year TRM among individual groups within the training cohort: malignant (n = 177) and nonmalignant (n = 405), acute leukemia (n = 145), and IEI (n = 233). Again, PARI demonstrated superiority over HCT-CI in predicting 2-year TRM (Figures 2 and 3).
Comparing HCT-CI with PARI in predicting 2-year TRM among malignant and nonmalignant training groups. (A and B) Demonstrates failure of HCT-CI to predict 2-year TRM among the training malignant and non-malignant groups. (C and D) Demonstrates that PARI can predict 2-year TRM among both the training malignant and non-malignant groups.
Comparing HCT-CI with PARI in predicting 2-year TRM among malignant and nonmalignant training groups. (A and B) Demonstrates failure of HCT-CI to predict 2-year TRM among the training malignant and non-malignant groups. (C and D) Demonstrates that PARI can predict 2-year TRM among both the training malignant and non-malignant groups.
Comparing HCT-CI with PARI in predicting 2-year TRM among AL and IEI training groups. (A and B) Demonstrates failure of HCT-CI to predict 2-year TRM among the training AL and IEI groups. (C and D) Demonstrates that PARI can predict 2-year TRM among both the training AL and IEI groups. AL, acute leukemia.
Comparing HCT-CI with PARI in predicting 2-year TRM among AL and IEI training groups. (A and B) Demonstrates failure of HCT-CI to predict 2-year TRM among the training AL and IEI groups. (C and D) Demonstrates that PARI can predict 2-year TRM among both the training AL and IEI groups. AL, acute leukemia.
Discussion
To our knowledge, this is the first hematopoietic cell transplantation index specifically tailored for pediatric HSCT, combining RF with CoM in 1 scoring model, through refining some of the HCT-CI criteria and adding RF and CoM unique to the pediatric group.
Since the development of the Charlson Comorbidity Index in 1987,17 several indices have been used to predict NRM and OS after HSCT, but most were relevant in adults undergoing HSCT for malignant diseases.4 HCT-CI was the first index to include both children and adults, incorporating patient-specific variables for the first time.5 However, this scoring system is less relevant in children because of some RFs and comorbidities being seldom encountered in this age group. Another limitation of the HCT-CI is the lack of sensitivity. Upon validating the HCT-CI, we and other investigators noted that at least one-third to half of the patients received a score of 0, indicating the failure of HCT-CI to capture some relevant CoMs.5,7,10,11
This has led researchers to look for other parameters that influence the 2-year mortality in children13 or consider the use of a refined HCT-CI.9,18 Realizing the weaknesses of HCT-CI, Sorror et al6 performed a prospective Center for International Blood and Marrow Transplant Research multicenter study on 8115 recipients of allogeneic HSCT including 944 children. They found that children with HCT-CI scores of 1 to 2 had NRM equivalent to those with HCT-CI of 0, whereas adult HSCT recipients had a statistically significant increase in NRM with increasing risk scores from 0 to 3. Only children with HCT-CI scores of ≥3 had significantly higher risk of NRM compared with children with scores of 0. We obtained similar results in our study cohort. Given the challenges in applying HCT-CI in pediatrics, Matthes-Martin et al19 (2008) created a risk-adjusted score to predict 1-year TRM among pediatric and adult HSCT recipients with malignant and nonmalignant conditions. Five RFs were selected with reference to published data, including patient age (<10 vs ≥10 years), underlying disease (malignant vs nonmalignant), donor type (alternative donor vs matched sibling donor), T-cell depletion (T cell–depleted graft vs T cell–replete graft), number of transplants (>1 vs 1 transplant), and disease remission status (in the malignant setting). In univariate analysis, all factors except the prior transplant correlated significantly with TRM. In multivariate analysis, disease status, donor other than matched sibling donors, and age remained significantly correlated with TRM. This study created a score for both malignant and nonmalignant disease. However, it only focused on 5 RFs that were selected by the authors. Moreover, the age group of HSCT recipients was wide ranging (0.3-26.2 years). To create a comprehensive and inclusive risk-assessment index, patient-, transplant-, treatment-, and organ-related RFs and CoMs were evaluated. Among the transplant-related factors, the use of mismatched grafts was associated with significantly higher rates of 2-year TRM in comparison with the use of matched related or unrelated grafts (weight of 2). This is possibly related to the increased risk of complications including GVHD and infections related to delayed immune reconstitution when a T-cell depletion approach is used. Advances in graft manipulation technology and use of cellular therapies after HSCT may improve the outcome with mismatched donors.20-25 Among the treatment-related factors, TBI-based conditioning was associated with increased 2-year TRM (weight of 2). Although TBI is a known RF with a significant impact on long-term survival after allogeneic HSCT in childhood,26,27 it was not previously included in any scoring systems. It is important that we carefully assess its use when the patient has other RFs that could negatively impact their survival.
We counted each patient only once, but we deemed subsequent HSCT as a RF that might increase 2-year TRM. We consider it important to include subsequent HSCTs because not an insignificant number of children undergo second HSCTs for malignant and nonmalignant disorders. It would be important for the counseling physician to consider this in the context of the patient’s other comorbidities. In a patient with normal organ function and no major comorbidities, a subsequent HSCT may not significantly increase TRM. However, in the presence of other RFs, a subsequent HSCT adds further, sometimes unacceptable risk. In our study, we found that a subsequent HSCT using a mismatched donor and with TBI conditioning increased the 2-year TRM.
Infections are inarguably one of the leading causes of TRM after HSCT. In PARI, respiratory viral infections had the highest weight of 2, corroborating our previous observation of high TRM in the presence of a positive nasopharyngeal aspirate for viral infections before HSCT.28 The presence of a positive nasopharyngeal aspirate for viral infections was a RF for TRM irrespective of the underlying diagnosis.
Within organ-related CoMs, respiratory structural abnormality had a significant association with TRM (weight of 2). Bronchiectasis is a particularly common complication in patients with IEI and recurrent respiratory infections.29 Pulmonary complications after allogeneic HSCT remain a leading cause of mortality, and having a pre-existing structural lung disease with decreased respiratory reserve or propensity to infections further increases this risk.30
Previous CNS infection, PRES, epilepsy requiring medical treatment, and known CNS structural anomaly were associated with increased 2-year TRM and had a weight of 3. Pre-HSCT PRES may predispose to post-HSCT PRES or to severe GVHD due to the inability to use calcineurin inhibitors.31-36
Besides the organ-specific RF/CoM variables, PARI included biochemical parameters already present in HCT-CI but modified in their definitions (modified definition for raised serum ALT, bilirubin, and creatinine) and a new parameter that was not previously evaluated (albumin levels). Although serum albumin is commonly used as a clinical nutritional parameter, it is regarded as a nonspecific predictor of nutritional status because of its relatively long half-life and susceptibility to stress and disease. Nevertheless, hypoalbuminemia has been shown to be an independent predictor of higher mortality.37 Sivgin et al38 conducted retrospective research on 102 patients with HSCT and demonstrated that serum albumin levels were linked to transplant outcome: patients with low albumin (3.2 g/dL) had lower median OS (230 days) than those with greater albumin levels (570 days).
Although each of the previous publications have added valuable information about factors that can affect 2-year TRM in a transplant setting, our work has several notable strengths. Firstly, it was conducted with a large number of patients at a single tertiary center with accurate and detailed data on CoMs. Furthermore, we focused solely on the pediatric age group and included patients with both malignant (30%), IEI (40%), metabolic (8%) and nonmalignant hematologic conditions (8%). Including a broad range of diagnoses enabled us to capture comorbidities unique to specific disease types. In contrast to recent attempts to modify the HCT-CI, where the authors used expert opinions to define comorbidities,18,19 we systematically tested each comorbidity in univariate analysis before finalizing PARI. We have attempted to devise an age-specific and not a disease-specific scoring system that will have universal applicability (except in the case of hemoglobinopathies) in the field of pediatric HSCT. Many of the organ-specific criteria are not disease-specific, and we have included all transplant characteristics with no selection bias.
Another strength was the application of 2-staged assessments for factors that can influence 2-year TRM. We analyzed our factors using a univariate model, excluding those with a P value ≥ .2 or those not associated with 2-year TRM. We believe that this is a statistically stronger model than other models14 that were created through running factors only in a multivariate model.5,18
Our use of an internal and external control cohort to validate our data, thereby eliminating selection bias, is another strength of our approach.
PARI clearly demonstrated a rise in TRM across groups 1 to 4, with patients having 2-year TRM rates of 10% or less in the absence of any RF/CoM, whereas 2-year TRM reached 40% to 50% in patients from group 4. Furthermore, we developed a simple index with 12 parameters including RF/CoM relevant to the pediatric cohort. Moreover, we used c-statistics to assess model fitness. Comparing models, PARI was found to have better performance than HCT-CI in predicting 2-year TRM in children across both malignant and nonmalignant cohorts.
This study has some limitations. The retrospective design of the study could have affected the quality of the data given that some variables may not have been captured in all patients despite accurate records, or definitions may have changed over the years. We believe that the external validation would have circumvented some of these issues. Another limitation is that patients with hemoglobinopathies, an increasingly common indication for HSCT, are under-represented in our cohort (only 10 patients). These patients have unique CoMs, such as iron overload, which are not included in PARI; this is a noted drawback of the HCT-CI.39 We thus cannot recommend the use of PARI for children undergoing HSCT for hemoglobinopathies. A prospective study across different centers is needed to evaluate the validity of PARI in predicting 2-year TRM across a range of diseases.
The heterogeneity of the disease population could be viewed as a limitation, but we consider this as one of the strengths of our scoring system relative to other scoring systems that have mainly focused on malignant disorders. Many of the comorbidities that we identified as significant are not disease-specific (respiratory viral infection, structural lung abnormality, or prior malignancy). The transplant factors (use of mismatched graft) are also independent of the disease being treated. Hence, a single scoring system, irrespective of the disease being treated, is in our opinion an improvement on existing systems.
Increasingly, second transplants are being offered/needed for malignant conditions because with medical and cellular advances in this field, children are surviving longer with life-prolonging treatments.40 Similarly, as we learn more about newer diagnoses and indications for transplantation in the field of IEI, a second transplant is needed in an increasing number of patients who have had partial correction or graft failure after their first transplant.41,42 The risk of a second transplant is undoubtedly higher, but knowledge of other comorbidities and RFs could put the risk into perspective for families and treating physicians, especially when there may be alternatives to a second transplant.
In conclusion, we have identified important variables that were not previously incorporated into risk models, and we have developed a highly specific scoring system in pediatric HSCT. We hope that after further prospective revalidation, PARI will facilitate patient/guardian counseling and perhaps prompt transplant physicians to consider treatment of some pretransplant RFs, if feasible, or nontransplant medical approaches, if available.
Acknowledgments
The authors thank the patients and their families, and the physicians, advanced practice providers, nurses, and other providers and staff members who participated in the care of these patients at Great Ormond Street Hospital.
Authorship
Contribution: N.B. and R.E. contributed to material preparation and data collection, and prepared the first draft of the manuscript; S.K. and R.E. analyzed the data; R.P., R.E., N.B., S.K., and K.R. summarized and interpreted the findings; and all authors contributed to the study’s conception and design, commented on previous versions of the manuscript, read and approved the final manuscript version to be published, and agreed to be accountable for all aspects of the work and ensure that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Reem Elfeky, Great Ormond Street Hospital, Great Ormond St, London WC1N 3J, United Kingdom; email: reem.elfeky@gosh.nhs.uk.
References
Author notes
R.E. and N.B. contributed equally to this study.
Derived data supporting the findings of this study are available on request from the corresponding author, Reem Elfeky (reem.elfeky@gosh.nhs.uk).
The full-text version of this article contains a data supplement.