Key Points
M+R induced durable responses (ORR, 52%; complete response, 30%; median DOR, 15.9 months; median OS, not reached) in R/R iNHL.
M+R remained well tolerated, with no new TEAEs in long-term follow-up.
Visual Abstract
Relapsed/refractory (R/R) indolent non-Hodgkin lymphoma (iNHL) is generally considered incurable with current treatment options. Previous phase 1b/2 results showed combining magrolimab (anti–cluster-of-differentiation [CD] 47 antibody) with the anti-CD20 antibody rituximab (M+R) has antitumor activity against R/R iNHL. We report 3-year follow-up data from this phase 1b/2 study assessing long-term safety and efficacy of M+R in R/R iNHL. After magrolimab priming, 4 patient groups in phase 1b M+R received 10 to 45-mg/kg magrolimab doses with 375 mg/m2 rituximab. Phase 2 explored 30 and 45 mg/kg magrolimab. Primary end points were treatment-emergent adverse events (TEAEs) and objective response rate (ORR). Secondary end points included duration of response (DOR), progression-free survival (PFS), and overall survival (OS). Exploratory analysis included circulating tumor DNA, biomarkers of magrolimab tumor penetration, and drug target expression assessments. Of 46 patients treated in phase 1b/2, 42 had follicular lymphoma and 4 had marginal zone lymphoma. All patients experienced ≥1 any-grade TEAE, and 44 reported ≥1 treatment-related TEAE. No additional toxicities were reported during long-term follow-up, and there were no treatment-related deaths. Median follow-up was 36.7 (range, 1.2-62.3) months. The ORR was 52.2%, with 30.4% achieving a complete response. The median DOR was 15.9 months, and median time-to-response was 1.8 months. Median PFS and OS were 7.4 (95% confidence interval, 4.8-13.0) months and not reached, respectively. These results demonstrate the long-term safety and efficacy of M+R in patients with iNHL and support further exploration of CD47-based treatment combinations. This trial was registered at www.ClinicalTrials.gov as #NCT02953509.
Introduction
Non-Hodgkin lymphomas (NHLs) are a heterogeneous group of malignancies, the majority of which arise from B-lymphocytes and proliferate in the lymph nodes.1 Indolent subtypes of NHL (iNHL) have a median survival of many decades; however, prognosis depends largely on histological nature, stage, and response to treatment.2,3 For example, ∼20% of patients develop rapidly evolving progressive disease (PD) within 2 years of first-line treatment (POD24) and have a 5-year overall survival (OS) of ∼50% vs ∼90% for those who do not experience early treatment failure.4-6
When “watch and wait” or radiation therapy is not an option, standard first-line therapy for iNHL includes the anti–cluster-of-differentiation (CD) 20 monoclonal antibody rituximab as a monotherapy or in combination with various chemotherapeutics.7,8 Patients whose iNHL relapses within 2 years after diagnosis or whose disease is refractory to rituximab monotherapy or combination therapies have limited options for effective treatment and, thus, shortened survival.4,9-11 Patients with iNHL that is relapsed/refractory (R/R) to second-line therapy (typically chemotherapy with rituximab that is sometimes combined with subsequent autologous stem cell transplant [ASCT]) are eligible for lenalidomide plus rituximab (if not used in second line),12,13 bispecific antibodies,14 enhancer of zeste homolog 2 (EZH2) inhibitor,15 or chimeric antigen receptor (CAR) T-cell therapy,16-19 among other treatments.20 Unfortunately, it is unclear whether any of the current options for R/R iNHL are curative, and some are not accessible to all patients for various reasons, such as advanced age, comorbidities, and inability to tolerate the therapy.20
Magrolimab is a first-in-class monoclonal antibody that blocks CD47, a widely expressed transmembrane protein that mediates the ability of cancer cells to evade phagocytosis by macrophages.21-23 CD47 is overexpressed on cancer cells, acting as a “don't eat me” signal.21,23 Magrolimab selectively eliminates malignant cells by blocking CD47, which exposes the prophagocytic “eat me” signal expressed on tumor cells.21,22,24 In a preclinical model of iNHL, magrolimab synergized with rituximab to enhance tumor cell phagocytosis; magrolimab blocks tumorigenic CD47 antiphagocytic signaling, whereas rituximab mediates activation of antibody-dependent cellular phagocytosis. Together, these monoclonal antibodies resulted in more effective tumor elimination than when either agent was used alone.21,22,24
In the clinical setting, we previously reported that magrolimab in combination with rituximab (M+R) appeared to be well tolerated, with promising activity in the phase 1b portion of a phase 1b/2 study of patients with R/R aggressive and iNHL (all follicular lymphoma [FL]).9 Overall, the objective response rate (ORR) was 50%; 36% of patients achieved complete response (CR; n = 22 total patients, including 15 with diffuse large B-cell lymphoma and 7 with iNHL). Among patients with R/R FL, the ORR was 71% and the CR rate was 43%.9 In the first 28 days of the phase 1b study, dose-limiting toxicities (DLTs) were assessed, and 1 patient with FL receiving 30 mg/kg magrolimab had a DLT (grade 4 neutropenia) that resolved, and the patient continued treatment.9 Here, we report the 3-year follow-up of the subgroup of patients with R/R iNHL treated with M+R from the phase 1b/2 study, assessing long-term safety and efficacy. Biomarker analysis of magrolimab tumor penetration, drug target expression, and circulating tumor DNA (ctDNA), to examine the feasibility of detecting molecular residual disease and monitoring response to treatment, were also assessed.
Methods
Trial oversight and conduct
This trial was conducted in accordance with the study protocol, the US Food and Drug Administration requirements, the International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use good clinical practice guidelines, the Declaration of Helsinki, and any applicable local health authority and institutional review board/independent ethics committee requirements. All participants gave written informed consent.
Study design
This trial was an open-label, multicenter, phase 1b/2 study (ClinicalTrials.gov identifier: NCT02953509) investigating combination M+R treatment in R/R B-cell NHL. Eligible patients (aged ≥18 years) had R/R B-cell NHL, including marginal zone lymphoma or FL (grades 1-3a), expressing CD20 and R/R to ≥2 prior lines of therapy with an Eastern Cooperative Oncology Group performance status (ECOG PS) score of 0 to 2. Patients whose disease relapsed after CAR T-cell therapy were ineligible. A hemoglobin of ≥9.5 g/dL before magrolimab administration was required. Patients needed an absolute neutrophil count of ≥1.0 × 106/μL and platelets of ≥50 × 106/mμL on enrollment. Adequate renal (serum creatinine) and hepatic (aspartate/alanine transaminase and bilirubin) function were also mandatory. Patients with known active or chronic infection with hepatitis B or C or HIV were excluded. The methods for the phase 1b portion of the study, which also included patients with diffuse large B-cell lymphoma, have been previously reported.9
In phase 1b, patients were primed with an initial dose of 1 mg/kg intravenous magrolimab on cycle 1 (each cycle is 28 days), day 1 to mitigate on-target anemia,9 followed by 10, 20, 30, or 45-mg/kg maintenance doses (supplemental Figure 1). In the 10 to 30-mg/kg magrolimab dose groups, maintenance doses were administered weekly starting on cycle 1, day 8. In the 45-mg/kg group, maintenance doses were administered weekly in cycles 1 and 2, and every 2 weeks for cycles 3 and later.
In phase 2, 30 and 45-mg/kg magrolimab maintenance doses were explored further because no additional DLTs or maximum tolerated doses occurred at these doses, and steady state serum dose levels were well above the targeted threshold.25 The phase 2 magrolimab dosing schedule was the same as that in the phase 1b, 45-mg/kg dose schedule: a priming dose on cycle 1, day 1, weekly maintenance dosing during cycles 1 and 2, followed by maintenance doses every other week thereafter. Intravenous rituximab was administered at 375-mg/m2 doses weekly starting on cycle 1, day 8, then monthly on day 1 of cycles 2 through 6, and on day 1 of every other cycle starting with cycle 8 according to prescribing recommendations. Patients enrolled in both phases received M+R treatment until loss of clinical benefit or occurrence of unacceptable toxicity. In case of severe hematologic toxicity, dose modifications or discontinuations were allowed per prescribing information (rituximab) or protocol (magrolimab) based on local institutional guidelines and the discretion of the investigator; administration of granulocyte colony-stimulating factor was performed according to local institutional standard of care.
End points
The primary end points of this study were incidence and severity of treatment-emergent adverse events (TEAEs) and DLTs (to establish a recommended phase 2 dose of magrolimab when used in combination with rituximab), and the ORR of combination treatment. TEAEs were investigator assessed using National Cancer Institute Common Terminology Criteria for Adverse Events version 4.03 criteria. DLTs were defined as any grade ≥3 TEAE determined to be related to the study drug (magrolimab and/or rituximab) that occurs during the first 4 weeks of treatment. Disease response was assessed by investigators according to the Lugano Classification for Lymphomas.26 Secondary end points included duration of response (DOR), progression-free survival (PFS), and OS.
The first response assessment scan occurred at cycle 3, day 1 (±7 days), with subsequent scans every 8 weeks (±4 weeks to coordinate with treatment cycle timing). Patients continuing on study medications for ≥18 months could have response assessments extended to every 3 to 4 cycles at the investigator’s discretion. Response assessments were obtained at treatment termination, unless a prior radiographic assessment was performed within the last 7 days or at a prior response assessment documenting PD. For patients with disease involvement in the bone marrow before treatment, bone marrow aspirate and biopsy were performed at the first response assessment (cycle 3, day 1). In addition, bone marrow assessments were conducted to confirm CR at any response assessment time point. For patients who achieved a CR, subsequent bone marrow aspirate and biopsies were not required but could be performed at the investigator’s discretion. All scans were investigator assessed.
Exploratory analyses
Exploratory objectives included assessing ctDNA using Signatera (Natera, Inc) molecular residual disease assay, biomarkers of magrolimab tumor penetration, and drug target expression. For ctDNA analysis, whole-exome sequencing was performed on formalin-fixed paraffin-embedded tumor (lymph node) biopsies (obtained at screening and cycle 2, day 8) and matched normal whole-blood samples. Whole-exome sequencing data were used to design and develop individual patient-specific ctDNA assays by targeting a set of up to 16 somatic, single-nucleotide variants identified in B-cell NHL and found in the associated patient’s tumor. These assays were used to track the presence of ctDNA in the associated patients’ blood plasma. Plasma samples for ctDNA testing were collected and banked from cycle 1, day 1 to cycle 4, day 1, but most patients had data only from cycle 1, day 1 to cycle 2, day 1. Plasma samples with at least 2 variants above the confidence threshold were considered ctDNA-positive.27,28 ctDNA concentration was reported in mean tumor molecules per milliliter of plasma. Patients were defined as having decreased ctDNA if the log10 fold-change at the patient’s furthest time point was less than −0.5 and increased/stable ctDNA if the log10 fold-change at the furthest time point was greater than or equal to −0.5.
Immunohistochemistry (immunoglobulin G4 [IgG4], CD20, and CD47) was performed on tumor biopsies to examine tumor penetration of magrolimab and whether baseline CD20 or CD47 staining was associated with clinical outcome. Tumor/lymph node biopsies were collected at screening and on C2D8. Biopsies were optional for phase 1b and mandatory for phase 2 unless the investigator determined that the biopsy was not feasible for investigating tumor penetration of magrolimab. An IgG4 assay (using clone EP4420) and scoring strategy was developed, and tumor cell membrane H-score at screening and on C2D8 was calculated. Tumor biopsies from screening were stained with an anti-CD20 (clone L26) antibody or an anti-CD47 antibody (clone SP279) to understand baseline CD20 and CD47 staining. A whole-tissue cell membrane–only H-score was calculated for CD20 staining, and a tumor cell H-score was calculated for CD47 staining.
Statistical analysis
All patients who received at least 1 dose of magrolimab were included in the efficacy analysis set; all patients who received at least 1 dose of either magrolimab or rituximab were included in the safety analysis set. For continuous variables, mean, standard deviation, median, and range were calculated. For categorical variables, the frequency and percentage in each category were calculated, along with 95% confidence intervals (CIs), as appropriate. For time-to-event variables, Kaplan–Meier estimates and corresponding 2-sided 95% CIs for medians and quartiles were calculated.
ORR was defined as the proportion of patients with objective response (CR or partial response [PR]) in the efficacy analysis set. DOR was measured from when the first objective response is met until the first date of objectively documented PD. PFS was measured from dose initiation until the first date of documented disease progression by, Lugano criteria, or death.29 OS was measured from dose initiation until death.
For immunohistochemical detection of IgG4, paired comparisons (screen vs C2D8) were run for data points with complete pairs. Incomplete pairs (eg, a patient with only screen) were excluded from statistical comparison. The H-score for CD47 and CD20 staining at screening was compared between responders (CR + PR) and nonresponders (stable disease + PD) using the Wilcoxon rank-sum test.
Results
Patient baseline characteristics and disposition
As of 28 February 2022 (data extraction date), 46 patients with R/R iNHL were enrolled and treated with M+R in phase 1b/2 (magrolimab maintenance doses: 10 mg/kg, n = 1; 30 mg/kg, n = 30; and 45 mg/kg, n = 15), including 42 patients with FL and 4 with marginal zone lymphoma. Baseline characteristics are summarized in Table 1. Median age was 61 (range, 28-87) years. A total of 28 (60.9%) patients had an ECOG PS score of 0, and 16 (34.8%) had an ECOG PS score of 1. Patients had received a median of 3 (range, 1-9) prior therapies, with 10 patients (21.7%) who had prior ASCT (the patient with only 1 prior line of therapy was a protocol deviation according to inclusion/exclusion criteria); 65.2% of patients had rituximab refractory disease, 63.0% of patients had disease that was refractory to their last regimen, 32.6% had primary refractory disease, and 56.5% had POD24 (progression of disease within 2 years of the start of their first prior anticancer therapy and first disease relapse/progression). The median duration of magrolimab treatment was 5.3 (range, 0.3-62.3) months. Patients received a median of 6.5 magrolimab cycles and 6 rituximab cycles. Forty patients (87%) discontinued magrolimab and/or rituximab because of either disease progression (magrolimab, 45.7%; rituximab, 43.5%), patient decision (magrolimab, 15.2%; rituximab, 13.0%), or TEAEs (magrolimab, 10.9%; rituximab, 13.0%). Additional details on extent of study drug exposure are summarized in supplemental Table 1. Six patients were still on treatment as of data cutoff.
Baseline characteristics
| Characteristic . | All patients (N = 46) . |
|---|---|
| Age, median (range), y | 61.0 (28.0-87.0) |
| ≥65, n (%) | 20 (43.5) |
| Male, n (%) | 28 (60.9) |
| Race, n (%) | |
| White | 41 (89.1) |
| Black or African American | 2 (4.3) |
| Asian | 1 (2.2) |
| Not reported | 2 (4.3) |
| Ethnicity, n (%) | |
| Hispanic or Latino | 3 (6.5) |
| Not Hispanic or Latino | 43 (93.5) |
| Prior anticancer therapies, median (range) | 3 (1∗-9) |
| ECOG PS, n (%)† | |
| 0 | 28 (60.9) |
| 1 | 16 (34.8) |
| iNHL type, n (%) | |
| FL | 42 (91.3) |
| MZL | 4 (8.7) |
| Stage at initial diagnosis, n (%) | |
| I-II | 6 (13.0) |
| III-IV | 29 (63.0) |
| Unknown | 11 (23.9) |
| Rituximab refractory, n (%)‡ | |
| Any regimen | 30 (65.2) |
| Last regimen | 23 (50.0) |
| Refractory to last regimen, n (%) | 29 (63.0) |
| Primary refractory, n (%)§ | 15 (32.6) |
| Prior autologous transplant, n (%) | 10 (21.7) |
| Prior anticancer therapies, median (range) | 3 (1-9) |
| Anti-CD20 monoclonal antibodies, n (%) | 45 (97.8) |
| Chemotherapy, n (%) | 45 (97.8) |
| Steroids, n (%) | 38 (82.6) |
| Targeted therapy, n (%) | 19 (41.3) |
| Stem cell therapy, n (%) | 10 (21.7) |
| Antibody–drug conjugate, n (%) | 8 (17.4) |
| Immunotherapy, n (%) | 8 (17.4) |
| PD-1/PD-L1 inhibitors, n (%) | 4 (8.7) |
| Cytoprotectants, n (%) | 1 (2.2) |
| POD24, n (%) | 26 (56.5) |
| Time from diagnosis to study entry, median (range), mo | 75.2 (7.7-272.5)ǁ |
| Characteristic . | All patients (N = 46) . |
|---|---|
| Age, median (range), y | 61.0 (28.0-87.0) |
| ≥65, n (%) | 20 (43.5) |
| Male, n (%) | 28 (60.9) |
| Race, n (%) | |
| White | 41 (89.1) |
| Black or African American | 2 (4.3) |
| Asian | 1 (2.2) |
| Not reported | 2 (4.3) |
| Ethnicity, n (%) | |
| Hispanic or Latino | 3 (6.5) |
| Not Hispanic or Latino | 43 (93.5) |
| Prior anticancer therapies, median (range) | 3 (1∗-9) |
| ECOG PS, n (%)† | |
| 0 | 28 (60.9) |
| 1 | 16 (34.8) |
| iNHL type, n (%) | |
| FL | 42 (91.3) |
| MZL | 4 (8.7) |
| Stage at initial diagnosis, n (%) | |
| I-II | 6 (13.0) |
| III-IV | 29 (63.0) |
| Unknown | 11 (23.9) |
| Rituximab refractory, n (%)‡ | |
| Any regimen | 30 (65.2) |
| Last regimen | 23 (50.0) |
| Refractory to last regimen, n (%) | 29 (63.0) |
| Primary refractory, n (%)§ | 15 (32.6) |
| Prior autologous transplant, n (%) | 10 (21.7) |
| Prior anticancer therapies, median (range) | 3 (1-9) |
| Anti-CD20 monoclonal antibodies, n (%) | 45 (97.8) |
| Chemotherapy, n (%) | 45 (97.8) |
| Steroids, n (%) | 38 (82.6) |
| Targeted therapy, n (%) | 19 (41.3) |
| Stem cell therapy, n (%) | 10 (21.7) |
| Antibody–drug conjugate, n (%) | 8 (17.4) |
| Immunotherapy, n (%) | 8 (17.4) |
| PD-1/PD-L1 inhibitors, n (%) | 4 (8.7) |
| Cytoprotectants, n (%) | 1 (2.2) |
| POD24, n (%) | 26 (56.5) |
| Time from diagnosis to study entry, median (range), mo | 75.2 (7.7-272.5)ǁ |
MZL, marginal zone lymphoma; PD-1/PD-L1, programmed death receptor 1/programmed death-ligand 1.
The patient with only 1 prior line of therapy was a protocol deviation according to inclusion/exclusion criteria.
ECOG PS was missing for 2 patients.
Defined as failure to achieve an objective response or progression during any previous rituximab-containing regimen (monotherapy or in combination) or progression within 6 months of last rituximab dose.
Defined as either no response to treatment, progression during the first treatment regimen, or progression within 6 months of the last dose of the first treatment regimen. Patients discontinuing the first treatment regimen because of toxicity are not considered to have primary refractory disease.
Seven patients were missing their original diagnosis date.
Safety
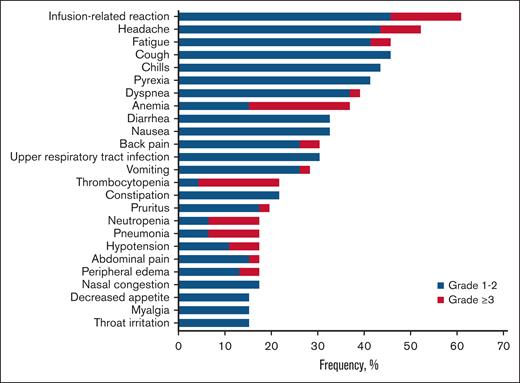
All 46 patients (100%) reported at least 1 TEAE of any grade, and 44 patients (95.7%) reported at least 1 treatment-related TEAE of any grade. The majority of TEAEs were grade 1 to 2, with few exceptions (Figure 1). The most common any-grade TEAEs were infusion-related reaction (60.9%), headache (52.2%), and fatigue (45.7%). The most common treatment-related TEAEs of any grade were infusion-related reaction (60.9%), headache (39.1%), and chills (37.0%). Thirty-three patients (71.7%) reported at least 1 grade ≥3 TEAE. The most common grade ≥3 TEAEs were anemia (21.7%; an on-target effect of magrolimab), thrombocytopenia (17.4%), and neutropenia (10.9%). Of those who discontinued treatment because of any TEAEs, only 2 patients (4.3% of the safety analysis set) discontinued treatment because of treatment-related TEAEs: grade 4 thrombocytopenia (n = 1) and grade 2 pneumonitis/dyspnea/cough (n = 1). There were no deaths due to TEAEs. There was no apparent association between the incidence of TEAEs and magrolimab dose across the 4 doses examined.
Efficacy
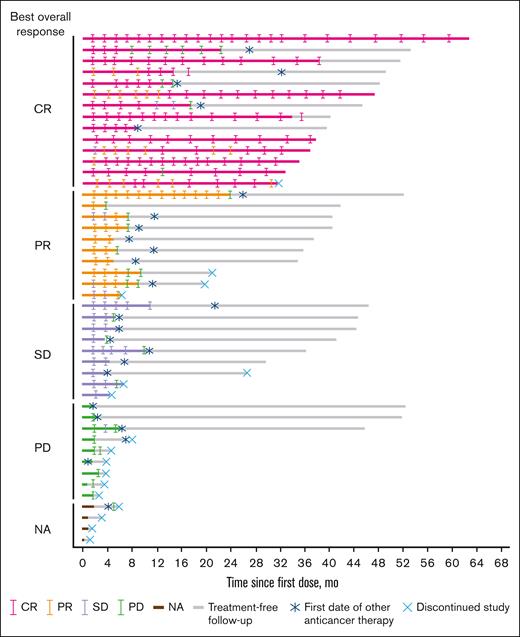
The ORR was 52.2%, with 14 patients (30.4%) achieving a CR (Table 2; Figure 2). The ORR was 43.3% in patients with rituximab refractory disease (n = 30), 44.8% in patients with disease that was refractory to their last regimen (n = 29), and 33.3% in patients with primary refractory disease (n = 15). In patients who had prior ASCT (n = 10), the ORR was 90.0%. The ORR for the POD24 subgroup was 57.7%. Responses were comparable across patient subgroups regardless of number of prior lines of therapy or POD24.
Response among all patients and in patient subsets
| . | All patients (N = 46) . | Rituximab refractory (n = 30) . | Refractory to last regimen (n = 29) . | Primary refractory (n = 15) . | POD24 (n = 26) . | Prior ASCT (n = 10) . | Prior LOT . | |
|---|---|---|---|---|---|---|---|---|
| ≤2 (n = 10) . | >2 (n = 36) . | |||||||
| ORR, n (%)∗ | 24 (52.2) | 13 (43.3) | 13 (44.8) | 5 (33.3) | 15 (57.7) | 9 (90.0) | 5 (50.0) | 19 (52.8) |
| CR | 14 (30.4) | 8 (26.7) | 7 (24.1) | 3 (20.0) | 9 (34.6) | 6 (60.0) | 2 (20.0) | 12 (33.3) |
| PR | 10 (21.7) | 5 (16.7) | 6 (20.7) | 2 (13.3) | 6 (23.1) | 3 (30.0) | 3 (30.0) | 7 (19.4) |
| SD, n (%) | 9 (19.6) | 7 (23.3) | 8 (27.6) | 3 (20.0) | 5 (19.2) | 0 | 2 (20.0) | 7 (19.4) |
| PD, n (%) | 9 (19.6) | 7 (23.3) | 7 (24.1) | 4 (26.7) | 3 (11.5) | 0 | 1 (10.0) | 8 (22.2) |
| NA, n (%)† | 4 (8.7) | 3 (10.0) | 1 (3.4) | 3 (20.0) | 3 (11.5) | 1 (10.0) | 2 (20.0) | 2 (5.6) |
| . | All patients (N = 46) . | Rituximab refractory (n = 30) . | Refractory to last regimen (n = 29) . | Primary refractory (n = 15) . | POD24 (n = 26) . | Prior ASCT (n = 10) . | Prior LOT . | |
|---|---|---|---|---|---|---|---|---|
| ≤2 (n = 10) . | >2 (n = 36) . | |||||||
| ORR, n (%)∗ | 24 (52.2) | 13 (43.3) | 13 (44.8) | 5 (33.3) | 15 (57.7) | 9 (90.0) | 5 (50.0) | 19 (52.8) |
| CR | 14 (30.4) | 8 (26.7) | 7 (24.1) | 3 (20.0) | 9 (34.6) | 6 (60.0) | 2 (20.0) | 12 (33.3) |
| PR | 10 (21.7) | 5 (16.7) | 6 (20.7) | 2 (13.3) | 6 (23.1) | 3 (30.0) | 3 (30.0) | 7 (19.4) |
| SD, n (%) | 9 (19.6) | 7 (23.3) | 8 (27.6) | 3 (20.0) | 5 (19.2) | 0 | 2 (20.0) | 7 (19.4) |
| PD, n (%) | 9 (19.6) | 7 (23.3) | 7 (24.1) | 4 (26.7) | 3 (11.5) | 0 | 1 (10.0) | 8 (22.2) |
| NA, n (%)† | 4 (8.7) | 3 (10.0) | 1 (3.4) | 3 (20.0) | 3 (11.5) | 1 (10.0) | 2 (20.0) | 2 (5.6) |
LOT, line of therapy; NA, not available; SD, stable disease.
Per Lugano criteria.
Patients with no postbaseline overall assessment.
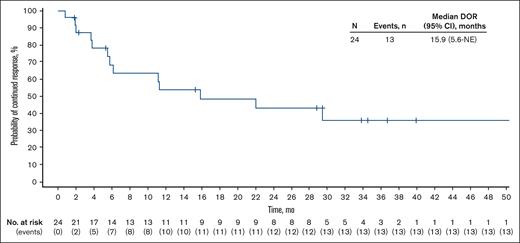
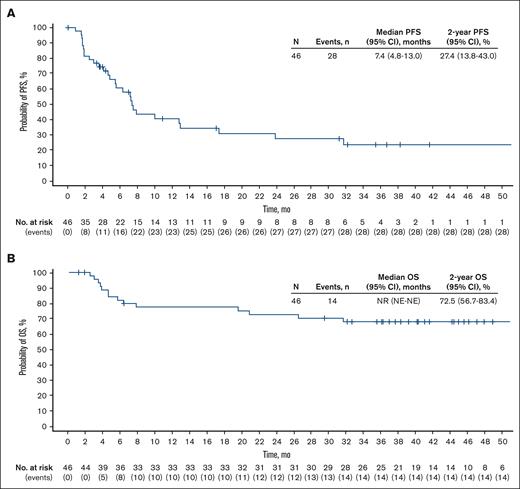
With a median follow-up of 36.7 (range, 1.2-62.3) months, the median DOR in the 24 patients who achieved a response was 15.9 (95% CI, 5.6 to not estimable) months (Figure 3); the median duration of CR was not reached (NR; 95% CI, 11.3 to not estimable). The median time to response was 1.8 (range, 1.6-5.5) months. Among all patients, median PFS was 7.4 (95% CI, 4.8-13.0) months, and median OS was NR (Figure 4). The 2-year PFS and OS rates were 27.4% (95% CI, 13.8-43.0) and 72.5% (95% CI, 56.7-83.4), respectively.
Exploratory analyses
ctDNA data were collected from 7 patients across multiple time points cycle 1, day 1; cycle 1, day 8; cycle 1, day 15; cycle 2, day 1; cycle 3, day 1; and cycle 4 day 1 (supplemental Figure 2A). Evaluation of ctDNA dynamics revealed a change in ctDNA levels relative to cycle 1 day 1 (baseline). The 3 patients who responded to treatment (CR, n = 1; PR, n = 2) demonstrated decreases or clearance of ctDNA (supplemental Figure 2B). Patients with stable disease (n = 1) or PD (n = 3) showed relatively stable or increased ctDNA.
Twenty-six patients had samples with CD20, CD47, or IgG4 staining completed; 24 of them had all 3 stains completed. Paired baseline and cycle 2, day 8 samples were available for 19 (CD20), 13 (CD47), and 12 (IgG4) patients. Magrolimab (via IgG4 staining) was detected in tumor biopsy after treatment (supplemental Figure 3A), supporting magrolimab penetration into tumor tissue. Baseline CD47 and CD20 staining was comparable for responders and nonresponders (supplemental Figure 3B).
Discussion
The results of this 3-year follow-up study support and extend initial findings of the phase 1b study.9,20 At 3-year follow-up, combination M+R treatment continued to provide durable responses in heavily pretreated patients with iNHL. The ORR was 52.2%, independent of line of therapy and POD24 status, and responses were observed in multiple patient subgroups, including patients whose disease was refractory to rituximab, primary refractory, and refractory to last regimen. The median DOR was 15.9 months, and the median OS was NR. M R continued to be well tolerated at 3 years, with no new or concerning safety signals observed during long-term follow-up, demonstrating the long-term safety of M R treatment.
Our biomarker analyses demonstrated the feasibility of a novel, customized approach to detecting ctDNA using a tumor-informed assay approach (Signatera). Although limited by small patient numbers and sample collection beyond 3 cycles, we found ctDNA levels decreased in responders and increased or remained stable in patients with PD. One patient with a CR achieved ctDNA clearance, suggesting that M+R may have the potential to induce long-term CRs. Immunohistochemistry analyses of tumor biopsy samples showed that neither baseline CD47 nor CD20 levels appeared to correlate with response to treatment. Thus, factors affecting response to M+R therapy remain unclear and warrant further study.
Since the design of this study, lenalidomide plus rituximab,12,13 T-cell immunotherapies (eg, CAR T-cells16-19 and bispecific T-cell engagers14), and EZH2 inhibitors,15 among others, have emerged as effective options for patients with R/R iNHL.20,30 Although all have demonstrated efficacy in patients with R/R iNHL, it is unclear whether any are curative; some have toxicities that are challenging for many practices, and some (eg, CAR T-cell therapy) are primarily limited to academic centers.20,31 Interestingly, preclinical data indicate that CD47 blockade facilitates cross-priming of T cells by dendritic cells, thus enhancing T-cell–mediated tumor elimination.32 Considering the current long-term efficacy and safety results, it is possible that combining M+R with other T-cell immunotherapies may enhance efficacy and/or help maintain a deep CR without adding substantial toxicity.
The limitations of this phase 1b/2 study include the small sample size and large proportion of patients with FL, as well as the exclusion of other types of iNHL. Nonetheless, these results provide further evidence for the tolerability and efficacy of the M+R combination in a heavily pretreated population with iNHL and support further exploration of CD47-based treatment combinations with standard-of-care and novel agents.
Acknowledgments
The authors thank Himanshu Sethi (Natera, Inc) for his contributions to the biomarker analyses. Medical writing support for the development of this manuscript, under the direction of the authors, was provided by Miranda Bader-Goodman of Ashfield MedComms, an Inizio company, and funded by Gilead Sciences, Inc.
This study was funded by Gilead Sciences, Inc.
Authorship
Contribution: G.H.-K. and Y.H. performed statistical analysis; L.S.-F. participated in biomarker exploratory analysis; A.M., L.P., G.P.C., S.M.S., I.W.F., N.L.B., N.G., C.R., R.A., and M.R. contributed to data collection; R.A. and G.P.C. led the study. All authors had full access to all the data in the study and have read, provided critical revisions, and approved the manuscript; C.R. is currently affiliated with the Department of Clinical Sciences, Obsidian Therapeutics, Redwood City, CA. All authors confirm that they meet International Committee of Medical Journal Editors authorship criteria.
Conflict-of-interest disclosure: A.M. reports consultancy for AstraZeneca, BeiGene, Bristol Myers Squibb, Gilead Sciences, Inc, Incyte Corporation, Kyowa Kirin, MorphoSys/Incyte Corporation, Novartis, Pharmacyclics, Seattle Genetics, and TG Therapeutics Inc; reports research funding from Affimed, Celgene/Bristol Myers Squibb, Forty Seven, Inc/Gilead Sciences, Inc, I-MAB Biopharma, Incyte Corporation, Innate Pharmaceuticals, Juno Pharmaceuticals/Bristol Myers Squibb, Kite Pharma/Gilead Sciences, Merck, Roche/Genentech, Seattle Genetics, Takeda, and TG Therapeutics; and reports speakers bureau participation for AstraZeneca, BeiGene, Bristol Myers Squibb, Gilead Sciences, Inc, Kyowa Kirin, Incyte Corporation, MorphoSys/Incyte, Pharmacyclics, and Seattle Genetics. L.P. reports honoraria from Pfizer and Roche; and reports travel support from Novartis. G.P.C. has received honoraria for speaker or consultancy work from Roche, Takeda, Kite Pharma/Gilead Sciences, Incyte, AstraZeneca, BeiGene, Novartis, SecuraBio, and AbbVie; and received research funding from Pfizer, BeiGene, AstraZeneca, Amgen, Inc, and Bristol Myers Squibb. S.M.S. reports consultancy for MorphoSys/Incyte, Janssen, Bristol Myers Squibb, Karyopharm, TG Therapeutics, and Celgene; and received research funding from FortySeven, TG Therapeutics, Pharmacyclics, Acerta, Karyopharm, Portola, Celgene, Novartis, Roche/Genentech, and Epizyme. I.W.F. reports consultancy for AbbVie, AstraZeneca, BeiGene, Century Therapeutics, Genentech, Inc, Genmab, Hutchison MediPharma, Iksuda Therapeutics, InnoCare Pharma, Janssen, Kite Pharma, MorphoSys, Myeloid Therapeutics, Novartis, Nurix Therapeutics, Pharmacyclics, F. Hoffmann-La Roche Ltd, Secura Bio, Servier Pharmaceuticals, Takeda, TG Therapeutics Inc, Verastem, Vincerx Pharma, and Xencor; and reports institutional research grants from AbbVie, Acerta Pharma, Agios, ArQule, AstraZeneca, BeiGene, Biopath, Bristol Myers Squibb, Calibr, Cancer and Leukemia Group B, Celgene, City of Hope National Medical Center, Constellation Pharmaceuticals, Curis, CTI Biopharma, Epizyme, Fate Therapeutics, Forma Therapeutics, Forty Seven, Genentech, Inc, Gilead Sciences, Inc, InnoCare Pharma, IGM Biosciences, Incyte, Infinity Pharmaceuticals, Janssen, Kite Pharma, Loxo, Merck, Millennium Pharmaceuticals, MorphoSys, Myeloid Therapeutics, Novartis, Nurix, Pfizer, Pharmacyclics, Portola Pharmaceuticals, Rhizen Pharmaceuticals, F. Hoffmann-La Roche Ltd, Seattle Genetics, Tessa Therapeutics, TCR2 Therapeutics, TG Therapeutics, Trillium Therapeutics, Triphase Research and Development Corporation, Unum Therapeutics, and Verastem. N.L.B. reports research funding from ADC Therapeutics, Autolus, Bristol Myers Squibb, Celgene, Forty Seven, Janssen, Kite Pharma, Merck, Millennium, Pharmacyclics, F. Hoffmann-La Roche/Genentech, and Seattle Genetics; and reports board of directors membership or advisory committee membership for ADC Therapeutics, F. Hoffmann-La Roche/Genentech, and Seattle Genetics. N.G. has received consulting fees from Seagen, TG Therapeutics, AstraZeneca, Pharmacyclics, Janssen, Bristol Myers Squibb, Gilead Sciences, Inc, Kite Pharma, Syncopation, Lava Therapeutics, BeiGene, Incyte, Karyopharm, Roche/Genentech, Novartis, Loxo Oncology, Genmab, Adaptive Biotech, and ADC Therapeutics; previously served on speakers bureau for Gilead Sciences, Inc, AstraZeneca, Bristol Myers Squibb, Pharmacyclics, Janssen, Epizyme; and has received research funding from TG Therapeutics, Roche/Genentech, Bristol Myers Squibb, Gilead Sciences, Inc, MorphoSys, and AbbVie. G.H.-K. and Y.H. are employed by Gilead Sciences, Inc, and hold stock in Gilead Sciences, Inc. L.S.-F. is employed by, and holds stock in, Gilead Sciences, Inc. C.R., at the time of the study, was employed by Gilead Sciences, Inc, and held stock in Gilead Sciences, Inc and Alphabet Inc, Class A. R.A. has received research funding from BeiGene, Merck, Roche/Genentech, Seattle Genetics, ADC Therapeutics, Pharmacyclics, Cyteir, and Daiichi Sankyo; and has served on advisory boards for Roche/Genentech, MorphoSys, and Epizyme. M.R. declares no competing financial interests.
Correspondence: Amitkumar Mehta, Department of Medicine, University of Alabama at Birmingham, 1720 2nd Ave S, NP2556, Birmingham, AL 35294; Phone: +1 (205) 996-8400; email: amitkumarmehta@uabmc.edu.
References
Author notes
Gilead Sciences shares anonymized individual patient data upon request or as required by law or regulation with qualified external researchers on the basis of submitted curriculum vitae and reflecting nonconflict of interest. The proposal must also include a statistician. Approval of such requests is at Gilead Science’s discretion and is dependent on the nature of the request, the merit of the research proposed, the availability of the data, and the intended use of the data. Data requests should be sent to datarequest@gilead.com.
The full-text version of this article contains a data supplement.