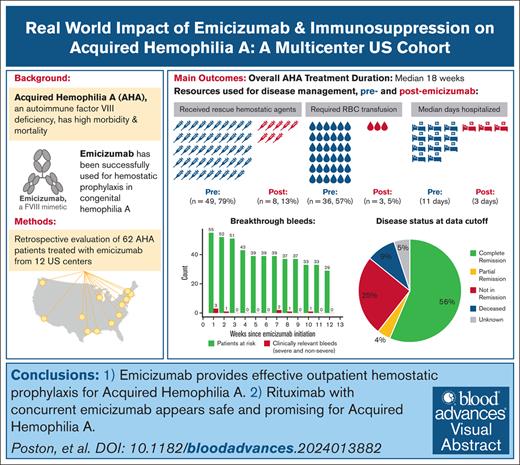
Key Points
Emicizumab provides effective outpatient hemostatic prophylaxis for AHA.
Rituximab with concurrent emicizumab seems to be safe and promising for AHA.
Visual Abstract
Acquired hemophilia A (AHA) is an autoimmune bleeding disorder that is caused by factor VIII (FVIII) autoantibodies with high morbidity and mortality due to bleeding and complications from immunosuppression (IST). To address the real-world implications of the FVIII mimetic antibody, emicizumab, and the role of IST, we retrospectively collected de-identified data on 62 patients with AHA who were treated off-label with emicizumab for a median of 10 weeks at 12 US-based hemophilia treatment centers. Most patients (95.2%) had acute bleeding at diagnosis, and 62.9% had partial or no control of bleeds despite the use of hemostatic agents at the time emicizumab was started. The main reason for initiating emicizumab was outpatient bleeding prophylaxis. After initiation of emicizumab, 87.1% had no additional bleeds. There were 6 breakthrough bleeds (2 spontaneous) in 5 patients and no fatal bleeding events during maintenance emicizumab treatment. The mean breakthrough bleed rate per patient-week was 0.02 (95% confidence interval, 0.0-0.03) during the first 12 weeks of emicizumab for the 55 patients with at least 12 weeks of follow-up. Of these patients, 92.7% received IST and 74.5% were prescribed rituximab-based regimens. Complete resolution of inhibitor and normalization of FVIII levels occurred in 56% overall and in 63% of the patients treated with rituximab. Overall, the median time to discontinuation of emicizumab and IST was 18 weeks. Two patients had thrombotic events while on emicizumab, but no adverse events were attributed to emicizumab and there were no infections attributed to IST. Emicizumab provides effective outpatient bleeding prophylaxis for AHA, and concurrent IST may further mitigate bleeding.
Introduction
Acquired hemophilia A (AHA) is a rare autoimmune bleeding disorder secondary to autoantibodies against factor VIII (FVIII). It can affect people of any age and sex, but it is more prevalent with advanced age, except when associated with pregnancy.1,2 Although approximately half of cases are idiopathic, AHA is sometimes linked to other autoimmune disorders or malignancies.1 Most patients present with severe, spontaneous bleeding that can be life- and limb-threatening.1 Treatment is often urgent and focused on restoring hemostasis while simultaneously eradicating the inhibitor using immunosuppression (IST). Around 30% of patients experience complications from IST, such as life-threatening infections, and overall, AHA is linked to a high mortality rate of 28% to 33%.1,3 The current standard of care hemostatic therapies (bypassing agents and recombinant porcine FVIII concentrate) require intravenous infusions, have short half-lives, and need close clinical and/or laboratory monitoring that often necessitate hospitalization. Bypassing agents, such as recombinant activated factor VII (rFVIIa) and activated prothrombin complex concentrate (aPCC), are effective for acute bleeding control but cannot be monitored with a laboratory assay and carry a prothrombotic risk. Recombinant porcine FVIII (rpFVIII) is another effective option for acute bleeding and can be monitored by evaluating the FVIII activity level, thereby conceivably avoiding supratherapeutic dosing and lessening the risk for thrombosis. The efficacy of rpFVIII is limited by cross-reacting antiporcine FVIII antibodies, which can occur de novo or develop after exposure to rpFVIII, thus necessitating frequent laboratory monitoring.4 Consequently, these hemostatic agents are generally not suitable for outpatient prophylactic bleeding prevention, which is an unmet need in AHA. Even after obtaining control of the initial bleed, the risk for rebleeding is high with 50% of patients experiencing breakthrough bleeding without hemostatic prophylaxis.5 Outcomes in AHA could be improved with outpatient-based hemostatic prophylaxis to mitigate the bleeding risks and reduce the urgency of eradicating the autoantibody with high intensity IST.
The FVIII mimetic bispecific antibody emicizumab is currently approved for patients with congenital hemophilia A with and without inhibitors (antibodies to FVIII) and is approved for AHA in Japan. Because of its long half-life of 28 days, emicizumab can be given subcutaneously and maintains steady state levels after an initial loading dose, thereby making it more feasible for outpatient administration. Emicizumab has a ceiling effect, and thrombin generation studies have estimated a FVIII equivalent activity of 15% to 30% at steady state.6,7 Although this does not normalize hemostasis, emicizumab improves the bleeding phenotype; patients with congenital hemophilia A on emicizumab therapy have a significant reduction in breakthrough bleeding, hospitalized days, and the need for bypassing agents in patients with inhibitors.8
Emicizumab is particularly attractive for AHA because of the ease of administration that enables outpatient hemostatic prophylaxis. Reducing the risk for recurrent bleeding may facilitate the use of lower intensity or delayed IST, thereby mitigating the risks for treatment-related adverse events. Therefore, emicizumab has been used off-label for hemostatic prophylaxis and in prospective studies, which, to date, have shown that emicizumab decreases but does not eliminate breakthrough bleeding in AHA.9,10 Eleven patients completed a prospective, multicenter, open-label trial of emicizumab for AHA that was conducted in Japan.11 Five patients (45%) had breakthrough bleeding after starting emicizumab, however, there were no major bleeds and the use of bypassing hemostatic agents decreased from use in 8 of 11 (73%) patients before starting emicizumab to 3 of 11 (27%) after emicizumab. The largest prospective trial to date included 47 patients with AHA who received emicizumab for 12 weeks without IST in Germany and Austria and showed a significant reduction in bleeding.12 Although patients on this trial were not started on IST initially, most patients ultimately required IST to eradicate the inhibitors; only 1 patient achieved spontaneous remission and 2 patients experienced partial remissions (PRs) without IST (endogenous FVIII levels improved close to 50% despite persistent inhibitors). Another prospective trial, Emicizumab in Patients with AHA (AHAEmi; NCT05345197), is currently enrolling participants in the United States to assess the efficacy of emicizumab with concurrent standard of care IST and may help to address the impact of emicizumab with concurrent IST. The purpose of this retrospective study was to understand the rationale behind starting emicizumab off-label and to determine its effectiveness in the real-world setting with and without concurrent IST.
Methods
At the time of this study, emicizumab was US Food and Drug Administration–approved for prophylactic use in congenital hemophilia A with and without inhibitors but was not US Food and Drug Administration–approved for use in AHA. In a survey of 87 hemophilia treatment centers (HTCs) in the United States, 12 adult HTCs with experience using off-label emicizumab for AHA were identified.13 Pediatric HTCs were excluded because of the negligible incidence of pediatric AHA. Adult hematologists at these 12 HTCs were prompted to provide de-identified retrospective data of all consecutive AHA cases treated with emicizumab at their HTC before 1 May 2023. Queries included demographics, bleeding history, treatment and procedures used for AHA management including IST course, emicizumab treatment and rationale, breakthrough bleeding, adverse events, and laboratory results as performed by the local institution’s standard of care. All institutions used chromogenic FVIII levels with bovine reagents once patients were started on emicizumab. Severe bleeding was defined according to the International Society on Thrombosis and Haemostasis classification as follows: drop in hemoglobin of >2 g/dL, required >2 packed red blood cell (pRBC) transfusions, and/or organ-, limb-, or life-threatening.14 Responses were compiled into a central database at the University of Washington under internal review board exemption. Study data were collected and managed using REDCap (Research Electronic Data Capture), an electronic data capture tool hosted at the University of Washington.15,16 Response to IST was assessed in cases with at least 12 weeks of follow-up. The bleeding rate for each patient was calculated by dividing the number of breakthrough bleeds by the time on emicizumab until week 12 or until end of treatment/dropout, whichever came first. The average of each patient’s bleed rate in the total cohort was then calculated and multiplied by 12 for the mean 12-week breakthrough bleed rate per patient.
Results
Demographics and comorbidities
A total of 62 patients with AHA were treated with off-label emicizumab at 12 US-based HTCs. The median age of the patients was 71 years (range, 34-93), 30 (48.4%) were female, and most (46 [74.2%]) were White (Table 1). Of those, 53 (85.5%) patients had preexisting comorbidities at the time of diagnosis. At the time of diagnosis, 10 were on therapeutic anticoagulants, which were discontinued in all cases. One patient who previously was on an anticoagulant for atrial fibrillation developed a deep venous thrombosis while off it (described hereafter). Eleven were on antiplatelet therapy, which was discontinued in 9 cases. One of these patients subsequently had a stroke while off antiplatelet therapy (described hereafter). Two patients continued antiplatelet therapy at the time of AHA diagnosis despite presenting with major bleeding (1 with congestive heart failure, the other with atrial fibrillation).
Baseline characteristics of the complete cohort
| Characteristic . | Total (N = 62) . |
|---|---|
| Sex | |
| Male | 32 (51.6%) |
| Female | 30 (48.4%) |
| Age, median (range) | 71 (34-93) |
| Race | |
| Asian/Pacific Islander | 2 (3.2%) |
| Black | 7 (11.3%) |
| Native American | 1 (1.6%) |
| Other | 2 (3.2%) |
| White | 46 (74.2%) |
| Unknown | 4 (6.5%) |
| Ethnicity | |
| Hispanic/Latino | 4 (6.5%) |
| Not Hispanic/Latino | 58 (93.5%) |
| Comorbidities∗ | |
| Vascular disease | 33 (53.2%) |
| Metabolic syndrome | 25 (40.3%) |
| Autoimmune disease | 14 (22.6%) |
| Cancer | 11 (17.7%) |
| Chronic kidney disease | 10 (16.1%) |
| Pulmonary disease | 9 (14.5%) |
| Venous thromboembolism | 7 (11.3%) |
| Dementia | 4 (6.5%) |
| Infection | 3 (4.8%) |
| Postpartum | 3 (4.8%) |
| Recurrent pancreatitis | 2 (3.2%) |
| None | 9 (14.5%) |
| Relevant medications at diagnosis∗ | |
| Antiplatelet† | 11 (17.7%) |
| Therapeutic anticoagulation‡ | 10 (16.1%) |
| Immunosuppression | 8 (12.9%) |
| Reason for AHA evaluation∗ | |
| Abnormal bleeding | 61 (98.4%) |
| Laboratory findings | 34 (54.8%) |
| Laboratory results at diagnosis | |
| Lowest FVIII activity level <1%§ | 38 (61.3%) |
| Lowest FVIII activity level 1%-5%§ | 19 (30.6%) |
| Lowest FVIII activity level >5%§ | 5 (8.1%) |
| Maximum FVIII inhibitor titer (BU/mL), median (range) | 74 (0.8-1100) |
| Maximum porcine inhibitor titer (BU/mL), median, (range)|| | 3 (0-24) |
| Lowest hemoglobin (g/dL)¶ | 6.8 (3.6-12.5) |
| Characteristic . | Total (N = 62) . |
|---|---|
| Sex | |
| Male | 32 (51.6%) |
| Female | 30 (48.4%) |
| Age, median (range) | 71 (34-93) |
| Race | |
| Asian/Pacific Islander | 2 (3.2%) |
| Black | 7 (11.3%) |
| Native American | 1 (1.6%) |
| Other | 2 (3.2%) |
| White | 46 (74.2%) |
| Unknown | 4 (6.5%) |
| Ethnicity | |
| Hispanic/Latino | 4 (6.5%) |
| Not Hispanic/Latino | 58 (93.5%) |
| Comorbidities∗ | |
| Vascular disease | 33 (53.2%) |
| Metabolic syndrome | 25 (40.3%) |
| Autoimmune disease | 14 (22.6%) |
| Cancer | 11 (17.7%) |
| Chronic kidney disease | 10 (16.1%) |
| Pulmonary disease | 9 (14.5%) |
| Venous thromboembolism | 7 (11.3%) |
| Dementia | 4 (6.5%) |
| Infection | 3 (4.8%) |
| Postpartum | 3 (4.8%) |
| Recurrent pancreatitis | 2 (3.2%) |
| None | 9 (14.5%) |
| Relevant medications at diagnosis∗ | |
| Antiplatelet† | 11 (17.7%) |
| Therapeutic anticoagulation‡ | 10 (16.1%) |
| Immunosuppression | 8 (12.9%) |
| Reason for AHA evaluation∗ | |
| Abnormal bleeding | 61 (98.4%) |
| Laboratory findings | 34 (54.8%) |
| Laboratory results at diagnosis | |
| Lowest FVIII activity level <1%§ | 38 (61.3%) |
| Lowest FVIII activity level 1%-5%§ | 19 (30.6%) |
| Lowest FVIII activity level >5%§ | 5 (8.1%) |
| Maximum FVIII inhibitor titer (BU/mL), median (range) | 74 (0.8-1100) |
| Maximum porcine inhibitor titer (BU/mL), median, (range)|| | 3 (0-24) |
| Lowest hemoglobin (g/dL)¶ | 6.8 (3.6-12.5) |
Percentages do not add up to 100% because respondents could select multiple answers for this variable.
The following indications for antiplatelet therapy (some had multiple) were provided: cardiovascular disease (n = 7), atrial fibrillation (n = 2), and stroke (n = 2).
The following indications for anticoagulation (some had multiple) were provided: atrial fibrillation (n = 7), venous thrombosis (n = 3), and prophylactic heparin with hemodialysis (n = 1).
FVIII activity level was measured with either a 1-stage or chromogenic assay before starting emicizumab.
Porcine inhibiter titer was only available for 42 patients.
Hemoglobin level was only available for 55 patients.
Most patients (61 [98.4%]) were evaluated for AHA because of abnormal bleeding. At diagnosis, the median FVIII level was <1% (range, <1%-23%). The median maximum inhibitor titer was 74 Bethesda unit (BU)/mL (range, 0.8-1100).
Hemostatic efficacy of emicizumab
Bleeding before emicizumab initiation
Before starting emicizumab, nearly all patients presented with bleeding (59 [95.2%]), and 48 patients had severe bleeds (Table 2). Three patients did not present with active bleeding but had a history of abnormal bleeding before the AHA diagnosis. The majority (61.3%) of patients experienced spontaneous bleeds with 77.4% of those in soft tissues. Acute hemostatic treatment was administered to 49 patients (79%) before starting emicizumab, primarily with rFVIIa, aPCC, and rpFVIII. Thirty-six patients (58.1%) required transfusion with a median of 5 units (range, 1-30) of pRBC before starting emicizumab.
Acute bleeding events and hemostatic management before and after emicizumab initiation for the complete cohort (N = 62)
| . | Before emicizumab initiation . | After emicizumab initiation . |
|---|---|---|
| Bleeds | ||
| Patients with acute bleeding | 59 (95.2%) | 8† (12.9%) |
| Patients with severe acute bleeding∗ | 48 (77.4%) | 3 (4.8%) |
| Acute bleeding site‡ | ||
| Soft tissue | 48 (77.4%) | 3 (4.8%) |
| Hematuria | 8 (12.9%) | 2 (3.2%) |
| Gastrointestinal | 6 (9.7%) | 2 (3.2%) |
| Hemarthrosis | 5 (8.1%) | 3 (4.8%) |
| Retroperitoneal | 5 (8.1%) | 0 (0%) |
| Mucosal | 3 (4.8%) | 0 (0%) |
| Epistaxis | 2 (3.2%) | 0 (0%) |
| Central nervous system | 2 (3.2%) | 0 (0%) |
| Pulmonary | 1 (1.6%) | 0 (0%) |
| Retinal | 1 (1.6%) | 0 (0%) |
| None | 3§ (4.8%) | 54 (87.1%) |
| Bleeding trigger | ||
| Spontaneous | 38 (61.3%) | 4 (6.5%) |
| Procedural | 11 (17.7%) | 2 (3.2%) |
| Traumatic | 7 (11.3%) | 3 (4.8%) |
| Unknown | 3 (4.8%) | 1 (1.6%) |
| Rescue hemostatic agents used‡ | ||
| rFVIIa | 26 (41.9%) | 8 (12.9%) |
| Recombinant porcine FVIII | 21 (33.9%) | 1 (1.6%) |
| aPCC | 20 (32.3%) | 0 (0%) |
| FVIII replacement factor | 8 (12.9%) | 1 (1.6%) |
| Antifibrinolytic | 7 (11.3%) | 1 (1.6%) |
| Plasma | 4 (6.5%) | 0 (0%) |
| DDAVP | 2 (3.2%) | 0 (0%) |
| Cryoprecipitate | 2 (3.2%) | 0 (0%) |
| PCC | 1 (1.6%) | 0 (0%) |
| Platelet transfusion | 1 (1.6%) | 0 (0%) |
| None | 13 (21.0%) | 54 (87.1%) |
| RBC transfusions administered | ||
| No | 26 (41.9%) | 59 (95.2%) |
| Yes | 36 (58.1%) | 3 (4.8%) |
| pRBC units, median (range) | 5 (1-30) | 3 (1-7) |
| Procedures required for hemostasis | ||
| Yes|| | 19 (30.6%) | 3 (4.8%) |
| No | 43 (69.4%) | 59 (95.2%) |
| Hospitalization (d), median (range) | 11 (0-60) | 3 (0-30) |
| Control of initial bleeding | ||
| Complete resolution or NA | 23 (37.1%) | 62¶ (100.0%) |
| Partial resolution | 22 (35.5%) | 0 (0%) |
| No control | 17 (27.4%) | 0 (0%) |
| Mean breakthrough bleed rate per patient-week, (95% CI)# | — | 0.02 (0.00-0.03) |
| Mean 12-wk breakthrough bleed rate per patient# | — | 0.18 (0.03-0.34) |
| . | Before emicizumab initiation . | After emicizumab initiation . |
|---|---|---|
| Bleeds | ||
| Patients with acute bleeding | 59 (95.2%) | 8† (12.9%) |
| Patients with severe acute bleeding∗ | 48 (77.4%) | 3 (4.8%) |
| Acute bleeding site‡ | ||
| Soft tissue | 48 (77.4%) | 3 (4.8%) |
| Hematuria | 8 (12.9%) | 2 (3.2%) |
| Gastrointestinal | 6 (9.7%) | 2 (3.2%) |
| Hemarthrosis | 5 (8.1%) | 3 (4.8%) |
| Retroperitoneal | 5 (8.1%) | 0 (0%) |
| Mucosal | 3 (4.8%) | 0 (0%) |
| Epistaxis | 2 (3.2%) | 0 (0%) |
| Central nervous system | 2 (3.2%) | 0 (0%) |
| Pulmonary | 1 (1.6%) | 0 (0%) |
| Retinal | 1 (1.6%) | 0 (0%) |
| None | 3§ (4.8%) | 54 (87.1%) |
| Bleeding trigger | ||
| Spontaneous | 38 (61.3%) | 4 (6.5%) |
| Procedural | 11 (17.7%) | 2 (3.2%) |
| Traumatic | 7 (11.3%) | 3 (4.8%) |
| Unknown | 3 (4.8%) | 1 (1.6%) |
| Rescue hemostatic agents used‡ | ||
| rFVIIa | 26 (41.9%) | 8 (12.9%) |
| Recombinant porcine FVIII | 21 (33.9%) | 1 (1.6%) |
| aPCC | 20 (32.3%) | 0 (0%) |
| FVIII replacement factor | 8 (12.9%) | 1 (1.6%) |
| Antifibrinolytic | 7 (11.3%) | 1 (1.6%) |
| Plasma | 4 (6.5%) | 0 (0%) |
| DDAVP | 2 (3.2%) | 0 (0%) |
| Cryoprecipitate | 2 (3.2%) | 0 (0%) |
| PCC | 1 (1.6%) | 0 (0%) |
| Platelet transfusion | 1 (1.6%) | 0 (0%) |
| None | 13 (21.0%) | 54 (87.1%) |
| RBC transfusions administered | ||
| No | 26 (41.9%) | 59 (95.2%) |
| Yes | 36 (58.1%) | 3 (4.8%) |
| pRBC units, median (range) | 5 (1-30) | 3 (1-7) |
| Procedures required for hemostasis | ||
| Yes|| | 19 (30.6%) | 3 (4.8%) |
| No | 43 (69.4%) | 59 (95.2%) |
| Hospitalization (d), median (range) | 11 (0-60) | 3 (0-30) |
| Control of initial bleeding | ||
| Complete resolution or NA | 23 (37.1%) | 62¶ (100.0%) |
| Partial resolution | 22 (35.5%) | 0 (0%) |
| No control | 17 (27.4%) | 0 (0%) |
| Mean breakthrough bleed rate per patient-week, (95% CI)# | — | 0.02 (0.00-0.03) |
| Mean 12-wk breakthrough bleed rate per patient# | — | 0.18 (0.03-0.34) |
CI, confidence interval; DDAVP, desmopressin; NA, not applicable.
Severe acute bleeding defined as a drop in hemoglobin of >2 g/dL, requiring >2 RBC transfusions, and/or organ-, limb-, or life-threatening.
Three of the 8 patients had breakthrough bleeding only during the emicizumab loading phase, including 1 who had a severe bleed that required pRBCs. Supplemental Table 1 further describes breakthrough bleeds during the emicizumab maintenance phase.
Percentages do not add up to 100% because respondents could select multiple answers for this variable.
All 3 patients had remote bleeding before the AHA diagnosis.
Procedures included arterial embolization, endoscopy, intubation, fasciotomy, drainage, cauterization, and cystoscopy.
All patients had resolution of initial bleeding after starting emicizumab. Four patients experienced new breakthrough bleeding during loading, and there were 6 bleeds in 5 patients during maintenance. All ultimately had resolution of bleeding while on emicizumab.
Calculations based on patients with at least 12 weeks of follow-up (n = 55).
Emicizumab initiation
The main reason to initiate emicizumab was for bleeding prophylaxis and/or to facilitate outpatient management (Table 3). Dosing varied but most received the standard loading regimen used for congenital hemophilia A. One patient (who was not actively bleeding at emicizumab initiation) received an accelerated loading dose of emicizumab (6 mg/kg on day 1 and 3 mg/kg on day 2).11,12 At the time emicizumab was started, 39 (62.9%) patients had partial or no control of acute bleeding, and 32 received concurrent alternative hemostatic agents, which were discontinued in 20 patients (Table 2). No patients continued to receive aPCC after starting emicizumab. After initiation of emicizumab in patients who had ongoing bleeding, all bleeding resolved within a median of 7 days (1-32 days) (Table 3). Eight patients experienced new breakthrough bleeds during both the loading and maintenance phases of emicizumab (Table 2; Figure 1; supplemental Figures 1 and 2). Four patients experienced bleeds during the loading phase of emicizumab (<4 weeks of therapy); of those, 3 were nonsevere and 1 was a severe spontaneous bleed. The 3 nonsevere bleeds all occurred within the first week of starting emicizumab; the severe bleed was ongoing hematuria that eventually required embolization, rFVIIa, and transfusion with 3 units of pRBCs. One patient with nonsevere bleeding during loading subsequently had a nonsevere traumatic breakthrough bleed during maintenance emicizumab. Breakthrough bleeding did not recur on maintenance emicizumab in the other 3 patients with breakthrough bleeds during loading.
Emicizumab treatment for the complete cohort
| . | Total cohort (N = 62) . |
|---|---|
| Reason for emicizumab initiation∗ | |
| Bleeding prophylaxis | 50 (80.6%) |
| Facilitate outpatient management | 37 (59.7%) |
| Decrease immunosuppression | 21 (33.9%) |
| Bleeding despite use of other hemostatic agents | 20 (32.3%) |
| Failure to respond to immunosuppression | 10 (16.1%) |
| Presumed or confirmed antibody to porcine FVIII | 5 (8.1%) |
| Time from diagnosis to emicizumab initiation (d), median (range) | 19 (0-1461) |
| Time to bleeding resolution after emicizumab initiation (d), median (range)† | 7 (1-32) |
| Emicizumab impact on immunosuppression plan | |
| Allowed reduced or no concurrent steroid regimen | 21 (33.9%) |
| Allowed no concurrent immunosuppression therapy | 4 (6.5%) |
| Other impact‡ | 4 (6.5%) |
| No impact | 33 (53.2%) |
| Emicizumab loading dosage | 52 (83.9%) |
| 2.5-3 mg/kg weekly§ | 2 (3.2%) |
| 1.5 mg/kg weekly for 4 wk | 5 (8.1%) |
| 6 mg/kg once, then 3 mg/kg weekly | 1 (1.6%) |
| 3 mg/kg once | 1 (1.6%) |
| No loading regimen | 1 (1.6%) |
| Other | |
| Emicizumab maintenance dosage | |
| 1.5 mg/kg weekly | 26 (41.9%) |
| 3 mg/kg every 2 wk | 15 (24.2%) |
| 1.5 mg/kg every 2 wk | 2 (3.2%) |
| 6 mg/kg every 4 wk | 2 (3.2%) |
| 3 mg/kg every 4 wk | 0 (0%) |
| None | 14 (22.6%) |
| Other | 3 (4.8%) |
| Emicizumab dose adjustments | |
| Reduced|| | 2 (3.2%) |
| Increased (for breakthrough bleed) | 1 (1.6%) |
| No change | 59 (95.2%) |
| Emicizumab duration (wk), median (range)¶ | 10 (1-52) |
| Emicizumab discontinued at time of survey | 46 (74.2%) |
| Reason(s) for emicizumab discontinuation,∗n = 46 | |
| Resolution of inhibitor | 27 (58.7%) |
| Resolution of bleeding and/or rising FVIII | 20 (43.4%) |
| Death or loss to follow-up | 4 (8.7%) |
| Adverse event (arthralgias) | 1 (2.2%) |
| Cost/insurance coverage | 1 (2.2%) |
| . | Total cohort (N = 62) . |
|---|---|
| Reason for emicizumab initiation∗ | |
| Bleeding prophylaxis | 50 (80.6%) |
| Facilitate outpatient management | 37 (59.7%) |
| Decrease immunosuppression | 21 (33.9%) |
| Bleeding despite use of other hemostatic agents | 20 (32.3%) |
| Failure to respond to immunosuppression | 10 (16.1%) |
| Presumed or confirmed antibody to porcine FVIII | 5 (8.1%) |
| Time from diagnosis to emicizumab initiation (d), median (range) | 19 (0-1461) |
| Time to bleeding resolution after emicizumab initiation (d), median (range)† | 7 (1-32) |
| Emicizumab impact on immunosuppression plan | |
| Allowed reduced or no concurrent steroid regimen | 21 (33.9%) |
| Allowed no concurrent immunosuppression therapy | 4 (6.5%) |
| Other impact‡ | 4 (6.5%) |
| No impact | 33 (53.2%) |
| Emicizumab loading dosage | 52 (83.9%) |
| 2.5-3 mg/kg weekly§ | 2 (3.2%) |
| 1.5 mg/kg weekly for 4 wk | 5 (8.1%) |
| 6 mg/kg once, then 3 mg/kg weekly | 1 (1.6%) |
| 3 mg/kg once | 1 (1.6%) |
| No loading regimen | 1 (1.6%) |
| Other | |
| Emicizumab maintenance dosage | |
| 1.5 mg/kg weekly | 26 (41.9%) |
| 3 mg/kg every 2 wk | 15 (24.2%) |
| 1.5 mg/kg every 2 wk | 2 (3.2%) |
| 6 mg/kg every 4 wk | 2 (3.2%) |
| 3 mg/kg every 4 wk | 0 (0%) |
| None | 14 (22.6%) |
| Other | 3 (4.8%) |
| Emicizumab dose adjustments | |
| Reduced|| | 2 (3.2%) |
| Increased (for breakthrough bleed) | 1 (1.6%) |
| No change | 59 (95.2%) |
| Emicizumab duration (wk), median (range)¶ | 10 (1-52) |
| Emicizumab discontinued at time of survey | 46 (74.2%) |
| Reason(s) for emicizumab discontinuation,∗n = 46 | |
| Resolution of inhibitor | 27 (58.7%) |
| Resolution of bleeding and/or rising FVIII | 20 (43.4%) |
| Death or loss to follow-up | 4 (8.7%) |
| Adverse event (arthralgias) | 1 (2.2%) |
| Cost/insurance coverage | 1 (2.2%) |
Percentages do not add up to 100% because respondents could select multiple answers for this variable.
Time to bleeding resolution only calculated for the 30 patients who had ongoing bleeding at the time of initiation.
Other IST modifications included allowed steroid monotherapy (n = 2) and altered timing and intensity of IST regimens (besides steroids; n = 2).
Loading dosage was given weekly for 2 to 5 weeks.
Emicizumab was reduced in 2 patients; 1 developed a deep venous thrombosis after which emicizumab was stopped before the dose was reduced, the other had the dose reduced after the patient’s bleeding resolved.
Data available for only 46 patients who had completed their emicizumab course at the time of survey submission.
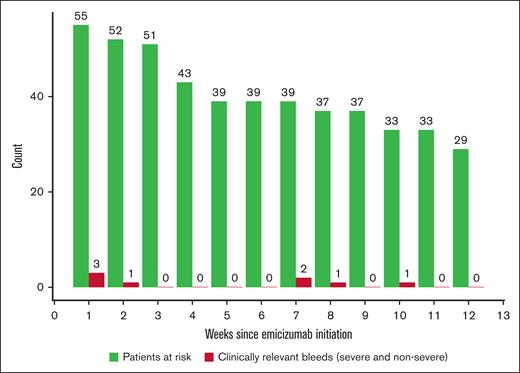
Breakthrough bleeds during the first 12 weeks after emicizumab initiation (n = 55). Patients at risk were defined as the number of patients on emicizumab until week 12 or until the end of treatment/dropout, whichever came first. No patients died during this time period.
Breakthrough bleeds during the first 12 weeks after emicizumab initiation (n = 55). Patients at risk were defined as the number of patients on emicizumab until week 12 or until the end of treatment/dropout, whichever came first. No patients died during this time period.
Bleeding on maintenance emicizumab
Of the 62 patients, 5 (8%) had 6 breakthrough bleeds on maintenance emicizumab; 2 of those (3.2%) were severe spontaneous gastrointestinal bleeds and 4 were nonsevere traumatic/procedural bleeds (supplemental Table 1). One patient had 2 separate nonsevere traumatic bleeding events. rFVIIa was used to treat all breakthrough bleeds. Three breakthrough bleeds required additional hemostatic agents, namely FVIII concentrate (1), rpFVIII (1), and an antifibrinolytic (1). Both spontaneous gastrointestinal bleeds required pRBC transfusions (1 and 7 units of pRBCs, respectively) and an endoscopy to manage the bleeds, in addition to hemostatic agents. There were no fatal bleeding events.
Two patients continued antiplatelet therapy after the AHA diagnosis and 1 patient started anticoagulation therapy for a venous thromboembolism while on emicizumab (described below). No patients on either antiplatelet therapy or anticoagulants experienced breakthrough bleeding with emicizumab.
Breakthrough bleeding after starting emicizumab was uncommon in the overall study population and the median duration of hospitalized days decreased from 11 days (range, 0-60) before emicizumab to 3 days (range, 0-30) after starting emicizumab (Table 2). The mean breakthrough bleed rate per patient-week was 0.02 (95% confidence interval, 0.0-0.03) during the first 12 weeks of emicizumab treatment for the 55 patients with at least 12 weeks of follow-up (Table 2; Figure 1). The mean 12-week breakthrough bleed rate per patient was 0.18 (95% confidence interval, 0.03-0.34). Most of these patients received IST. Breakthrough bleeding occurred in 50% (2/4) of patients who did not receive IST (supplemental Table 2).
Baseline factor activity and inhibitor titer
Most patients presented with baseline FVIII levels of <1% at diagnosis (n = 38 [61.3%]), which previously has been shown to be a poor prognostic risk factor for AHA remission (Table 1).17 There was a wide range in the maximum inhibitor titers, but the median maximum inhibitor titers were lowest in patients with a nadir FVIII of >5% (median, 19 BU/mL [range, 11-47]). Of the 19 patients with a baseline FVIII of between 1% and 5%, the median maximum inhibitor titer was 32 BU/mL (range, 0.8-243). Of the 38 patients whose FVIII was <1%, the median maximum inhibitor titer was 105 BU/mL (range, 3.8-1100).
Immunosuppression
IST in overall cohort
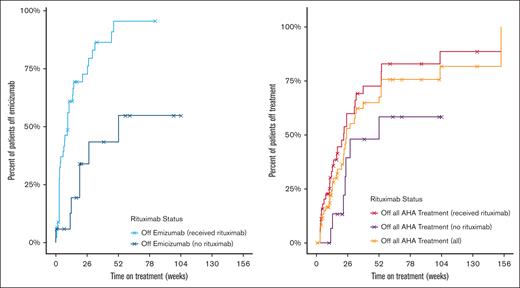
Most patients (56 [90%]) were on a single agent or combinations of IST. Overall, patients received rituximab (45 [72.6%]), glucocorticoids (39 [62.9%]), cyclophosphamide (19 [30.6%]), mycophenolate mofetil (6 [9.7%]), and daratumumab (1 [1.6%]). For the 37 patients in the cohort who completed emicizumab and IST at the time of data cutoff, the median time on treatment was 18 weeks (Figure 2).
Duration of emicizumab course and duration of all AHA treatment by rituximab status (N = 62). Duration of emicizumab course was calculated for entire cohort (N = 62). Duration of all AHA treatment was defined as the amount of time patients were on emicizumab and/or immunosuppression therapy (if applicable) for the indication of AHA. The median time for the 37 patients in the cohort who had completed emicizumab and IST (if applicable) at the time of data collection cutoff was 18 weeks. Patients were censored at death or if follow-up time ended before discontinuing therapy (indicated with an X on graph).
Duration of emicizumab course and duration of all AHA treatment by rituximab status (N = 62). Duration of emicizumab course was calculated for entire cohort (N = 62). Duration of all AHA treatment was defined as the amount of time patients were on emicizumab and/or immunosuppression therapy (if applicable) for the indication of AHA. The median time for the 37 patients in the cohort who had completed emicizumab and IST (if applicable) at the time of data collection cutoff was 18 weeks. Patients were censored at death or if follow-up time ended before discontinuing therapy (indicated with an X on graph).
Response to IST on concurrent emicizumab
Disease status and response to IST, if applicable, were assessed in 55 patients who were followed for a minimum of 12 weeks (Table 4; supplemental Figure 3). Half of these patients (28/55 [50.9%]) began IST at least 1 week before starting emicizumab (supplemental Table 2). Complete remission (CR) was defined as a normalized FVIII level without an inhibitor while off IST. A normalized FVIII was defined as levels >50% measured using a chromogenic FVIII assay with bovine reagents; however, 2 patients were considered to be in CR with FVIII levels at 48% without detectable inhibitors while off IST and emicizumab. Patients were considered to be in PR when the level of FVIII was at least 50% while on IST and/or with a persistent inhibitor. Overall, 56% (31/55) of patients were in CR, 4% (2/55) were in PR, and 25% (14/55) continued to have active AHA at the time of data collection cutoff (Table 4).
Laboratory presentation and remission status of patients with AHA with at least 12 weeks of follow-up on IST regimen (n = 55)
| . | None, n = 4 . | Glucocorticoids monotherapy, n = 6 . | Glucocorticoids & cyclophosphamide, n = 3 . | Rituximab & glucocorticoids, n = 10 . | Rituximab monotherapy, n = 14 . | Rituximab and other IST, n = 17 . | MMF monotherapy n = 1 . | Total, n = 55 . |
|---|---|---|---|---|---|---|---|---|
| Lowest FVIII activity level,∗ median (range) | 2% (<1%-3%) | <1% (<1%-23%) | <1% (<1%-<1%) | <1% (<1%-9%) | <1% (<1%-7%) | <1% (<0.3%-5%) | <1% | <1% (<0.3%-23%) |
| Maximum FVIII inhibitor titer,† median (range) | 19 (0.8-243) | 16 (4-749) | 100 (69-368) | 30 (3.8-660.5) | 44 (9.2-169) | 140 (10-1100) | 33 | 69 (0.8-1100) |
| Remission status‡ | ||||||||
| CR | 2 (50%) | 3 (50%) | 0 (0%) | 6 (60%) | 9 (64%) | 11 (65%) | 0 (0%) | 31 (56%) |
| PR | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (7%) | 1 (6%) | 0 (0%) | 2 (4%) |
| Not in remission/relapse | 1 (25%) | 3 (50%) | 2 (67%) | 3 (30%) | 2 (14%) | 2 (12%) | 1 (100%) | 14 (25%) |
| Deceased | 1 (25%) | 0 (0%) | 0 (0%) | 1 (10%) | 0 (0%) | 3 (18%) | 0 (0%) | 5 (9%) |
| Unknown | 0 (0%) | 0 (0%) | 1 (33%) | 0 (0%) | 2 (14%) | 0 (0%) | 0 (0%) | 3 (5%) |
| . | None, n = 4 . | Glucocorticoids monotherapy, n = 6 . | Glucocorticoids & cyclophosphamide, n = 3 . | Rituximab & glucocorticoids, n = 10 . | Rituximab monotherapy, n = 14 . | Rituximab and other IST, n = 17 . | MMF monotherapy n = 1 . | Total, n = 55 . |
|---|---|---|---|---|---|---|---|---|
| Lowest FVIII activity level,∗ median (range) | 2% (<1%-3%) | <1% (<1%-23%) | <1% (<1%-<1%) | <1% (<1%-9%) | <1% (<1%-7%) | <1% (<0.3%-5%) | <1% | <1% (<0.3%-23%) |
| Maximum FVIII inhibitor titer,† median (range) | 19 (0.8-243) | 16 (4-749) | 100 (69-368) | 30 (3.8-660.5) | 44 (9.2-169) | 140 (10-1100) | 33 | 69 (0.8-1100) |
| Remission status‡ | ||||||||
| CR | 2 (50%) | 3 (50%) | 0 (0%) | 6 (60%) | 9 (64%) | 11 (65%) | 0 (0%) | 31 (56%) |
| PR | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (7%) | 1 (6%) | 0 (0%) | 2 (4%) |
| Not in remission/relapse | 1 (25%) | 3 (50%) | 2 (67%) | 3 (30%) | 2 (14%) | 2 (12%) | 1 (100%) | 14 (25%) |
| Deceased | 1 (25%) | 0 (0%) | 0 (0%) | 1 (10%) | 0 (0%) | 3 (18%) | 0 (0%) | 5 (9%) |
| Unknown | 0 (0%) | 0 (0%) | 1 (33%) | 0 (0%) | 2 (14%) | 0 (0%) | 0 (0%) | 3 (5%) |
FVIII activity level was measured with either a 1-stage or chromogenic assay.
FVIII inhibitor titer units are BU/mL.
CR was defined as a normalized FVIII without an inhibitor while off IST. Patients were considered in a PR with a FVIII of at least 50% while on IST and/or with a persistent inhibitor.
IST regimens without rituximab
A total of 10 patients with at least 12 weeks of follow-up were treated with IST regimens without rituximab, specifically glucocorticoid monotherapy (n = 6), cyclophosphamide and glucocorticoid (n = 3), and mycophenolate mofetil monotherapy (n = 1; Table 4). Of those, 7 had active inhibitors at the time of data collection cutoff. Three patients who were treated with glucocorticoid monotherapy were in CR, 3 of whom had a maximum inhibitor titer of <20 BU/mL. Among the 3 patients who achieved CR, the median time to discontinuation of emicizumab was 13 weeks and the median time to discontinuation of all AHA treatment was 24 weeks.
Rituximab-based IST regimens
A total of 41 (74.5%) patients received rituximab of which 14 were treated with rituximab monotherapy, 10 with rituximab and a glucocorticoid, and 17 with other IST regimens (Table 4). Most (26/41 [63%]) who received rituximab achieved CR with resolution of their bleeding and inhibitor with no further need for IST or emicizumab. Among the patients who reached CR, the median time to discontinue emicizumab was 9 weeks and the median time to discontinue all AHA treatment was 19 weeks. The median maximum inhibitor titer was 30 BU/mL (range, 3.8-660.5) for the 10 patients who were treated with rituximab and glucocorticoid; 60% (6/10) were in a CR at the time of data collection cutoff. Of the 14 patients who received rituximab monotherapy, the median maximum inhibitor titer was 44 BU/mL (range, 9.2-169), and 64% (9/14) were in CR at the time of data collection cutoff.
Patients not treated with IST
Four patients were not treated with IST and had at least 12 weeks of follow-up (Table 4). The median maximum inhibitor titer was 19 BU/mL (range, 0.8-243). Two patients experienced spontaneous remission after a median duration of 32 weeks of emicizumab.
Adverse events before starting emicizumab
Before starting emicizumab, there were 13 adverse events in 12 patients (19.4%), 5 of which were related to IST (Table 5). Four thrombotic events occurred before starting emicizumab, namely arterial thrombosis (2 [3.2%]) and demand cardiac ischemia (2 [3.2%]). The arterial thrombotic events included 1 case of mesenteric ischemia that correlated with the use of aPCC and 1 stroke attributed to arterial embolization for an acute neck bleed. The patient with mesenteric ischemia did not have known cardiovascular risk factors. The patient who developed a stroke had been using aspirin for a previous stroke. Aspirin use was discontinued after the diagnosis of AHA and was subsequently restarted after the new stroke 2 weeks before starting emicizumab. Both patients with demand ischemia had a history of cardiovascular disease but were not on antiplatelet or anticoagulation therapy at the time of AHA diagnosis.
AEs before and after emicizumab initiation for the complete cohort (N = 62)
| AE outcomes . | Before emicizumab initiation . | After emicizumab initiation . |
|---|---|---|
| Patients with AEs | 12 (19.4%) | 4 (6.5%) |
| AEs of special interest | ||
| AE related to IST∗ | 5 (8.1%) | 0 (0%) |
| Arterial thrombosis | 2 (3.2%) | 0 (0%) |
| NSTEMI/demand ischemia | 2 (3.2%) | 0 (0%) |
| TIA | 0 (%) | 1 (1.6%) |
| Venous thromboembolism | 0 (0%) | 1 (1.6%) |
| Infections owing to IST | 0 (0%) | 0 (0%) |
| Disseminated intravascular coagulation | 0 (0%) | 0 (0%) |
| Thrombotic microangiopathy | 0 (0%) | 0 (0%) |
| Other AEs | ||
| Infection (not related to IST) | 2 (3.2%) | 0 (0%) |
| Syncope | 1 (1.6%) | 0 (0%) |
| Weight loss | 1 (1.6%) | 0 (0%) |
| Arthralgia | 0 (0%) | 1 (1.6%) |
| Shortness of breath | 0 (0%) | 1 (1.6%) |
| AE outcomes . | Before emicizumab initiation . | After emicizumab initiation . |
|---|---|---|
| Patients with AEs | 12 (19.4%) | 4 (6.5%) |
| AEs of special interest | ||
| AE related to IST∗ | 5 (8.1%) | 0 (0%) |
| Arterial thrombosis | 2 (3.2%) | 0 (0%) |
| NSTEMI/demand ischemia | 2 (3.2%) | 0 (0%) |
| TIA | 0 (%) | 1 (1.6%) |
| Venous thromboembolism | 0 (0%) | 1 (1.6%) |
| Infections owing to IST | 0 (0%) | 0 (0%) |
| Disseminated intravascular coagulation | 0 (0%) | 0 (0%) |
| Thrombotic microangiopathy | 0 (0%) | 0 (0%) |
| Other AEs | ||
| Infection (not related to IST) | 2 (3.2%) | 0 (0%) |
| Syncope | 1 (1.6%) | 0 (0%) |
| Weight loss | 1 (1.6%) | 0 (0%) |
| Arthralgia | 0 (0%) | 1 (1.6%) |
| Shortness of breath | 0 (0%) | 1 (1.6%) |
AE, adverse event; NSTEMI, non–ST-elevation myocardial infarction.
AEs related to steroids included encephalopathy and hyperglycemia. AEs related to rituximab were infusion reactions; AEs related to daratumumab were hypersensitivity reactions. AEs related to bortezomib were infusion reactions.
Adverse events during emicizumab treatment
The majority (58, 93.5%) tolerated emicizumab without complications. Four patients experienced adverse events while on emicizumab, and these included venous thrombosis (n = 1), transient ischemic attack (TIA; n = 1), arthralgia (n = 1), and dyspnea (n = 1; Table 5). No patients experienced disseminated intravascular coagulation or thrombotic microangiopathy while on emicizumab, however, none were treated with aPCC while on emicizumab.
The patient who developed venous thrombosis had no history of thrombosis but had been on apixaban for atrial fibrillation before the AHA diagnosis (discontinued at AHA diagnosis). This patient subsequently developed a proximal lower extremity deep vein thrombosis (DVT) while on maintenance emicizumab at a dose of 3 mg/kg every other week and had a chromogenic FVIII activity level of <1% with no recent use of bypassing agents and no recent bleeding.18 The DVT occurred 60 days after the diagnosis of AHA and 35 days after starting emicizumab. Emicizumab administration was stopped for 4 weeks during which time the FVIII remained at <1% with no recurrent bleeding. Four weeks after the DVT diagnosis, emicizumab was restarted at 1.5 mg/kg every other week along with reinitiation of anticoagulation therapy with no additional adverse events.
One patient with no history of stroke or use of antiplatelet or anticoagulant therapy experienced a TIA when the patient was on emicizumab monotherapy (with no recent use of bypassing agents). The TIA was not attributed to emicizumab by the treating physician and emicizumab was continued without any dose modifications.
Another patient developed fever and dyspnea the day after receiving the last dose of rituximab. The symptoms spontaneously resolved without a clear underlying etiology. Emicizumab was discontinued 6 months after starting emicizumab in a patient with underlying rheumatoid arthritis after preexisting arthralgias worsened.
Adverse events related to IST
Before starting emicizumab, 5 patients had adverse events attributed to IST, namely hyperglycemia (1 on glucocorticoid monotherapy), encephalopathy (1 on glucocorticoid monotherapy), and hypersensitivity or infusion reactions (3 separate reactions to rituximab, bortezomib, and daratumumab) (Table 5). After starting emicizumab, no patients experienced adverse events attributed to IST. Notably, there were no infections related to IST before or after starting emicizumab.
Status at final data cutoff
At the time of the final data collection cutoff, 5 of 62 (8%) patients had died, and 2 of those were still on emicizumab treatment (1 died of cancer progression and had been on emicizumab since diagnosis for 10 months, and the other died of unknown causes after being on emicizumab for 4 months since AHA diagnosis; Table 4; supplemental Figure 3). The other deaths that occurred while off emicizumab were attributed to COVID-19 (n = 1; exact date of death and timing from last dose of emicizumab unknown; died at least 6 months after AHA diagnosis), heart failure (n = 1; died 19 months after AHA diagnosis and 5 months after discontinuation of emicizumab), cancer progression (n = 1; died 21 months after AHA diagnosis and 18 months after discontinuation of emicizumab). According to the treating physicians, no deaths were attributed to emicizumab, bleeding, or IST (even in the cases of death from otherwise unknown causes). None of the patients who died experienced breakthrough bleeding or adverse events while on emicizumab.
Of the 55 patients with at least 12 weeks of follow-up, 13 (24%) remained on emicizumab (supplemental Table 2). Thirty-one patients (56% [31/55]) were in CR at the time of the data collection cutoff (Table 4). Of the 7 patients with less than 12 weeks of follow-up, 2 were in a CR, 3 were in a PR, and 2 were not in remission at the time of data collection cutoff.
Discussion
To our knowledge, this study offers the largest cohort of real-world outcomes of patients with AHA who were treated with emicizumab in conjunction with various IST regimens. Like other historic series and clinical trials of AHA, this retrospective cohort captured older individuals with multiple comorbidities. Previous retrospective data showed that most patients present with serious bleeding and prospective data established that the bleeding or rebleeding risk remained high when FVIII activity was < 50% in the first 12 weeks after diagnosis.5 In our cohort, emicizumab seemed to provide successful hemostatic prophylaxis in most with no breakthrough bleeding in 87.1% of patients in the real-world setting. Our study also showed that emicizumab was given to facilitate the transition to outpatient management, and most patients experienced fewer hospitalization days after emicizumab. Adverse events were uncommon in this cohort despite high rates of comorbidities, thereby supporting the safety of emicizumab for patients with AHA. Although vascular risk factors are a consideration when starting emicizumab and were present in 63% of this cohort, just 2 patients with vascular comorbidities experienced thrombotic events while on emicizumab, neither of which were attributed to emicizumab.
The need for IST in AHA
Before the availability of emicizumab, IST was associated with a 4% to 16% rate of fatal infections,1,17 thus prompting some to investigate whether emicizumab provides sufficient hemostatic prophylaxis to defer IST use. This strategy assumes that delaying IST until recovery from the initial bleeding event will lead to improved performance status when IST is initiated and thus reduce adverse events. In our retrospective cohort, we found minimal adverse events related to IST with no infections reported. This discrepancy with some historic data could be because of the retrospective nature of our query but may also be because of the relatively higher treatment rates with rituximab in our cohort. Notably, other retrospective series have also found lower rates of complications with use of IST.19,20 Most patients in this cohort (74.5% [41/55]) received a rituximab-based regimen with complete response rates similar to historic registries of higher intensity IST regimens.17 CR occurred in 64% of patients who received rituximab monotherapy despite a low baseline median nadir FVIII level of <1% (<1%-7%) and a median maximum inhibitor titer of 44 BU/mL (range, 9.2-169; Table 4). Per the international AHA guidelines,21 patients with these high-risk features should be treated with a combination of glucocorticoid and cyclophosphamide or rituximab. Of note, these guidelines were released before off-label use of emicizumab. The maximal inhibitor titer and minimal FVIII levels did not seem to correlate with response to IST in this cohort (supplemental Figure 4). This, along with the excellent response to rituximab, including rituximab monotherapy, suggests that the need for higher intensity IST may not be necessary with concurrent emicizumab. The role of IST will be further elucidated by comparing the GTH-AHA-EMI trial (emicizumab without upfront IST)12 and the ongoing parallel trial, AHAEmi (NCT05345197, emicizumab with upfront IST).
In addition, delaying IST may increase the bleeding risk, even with the use of emicizumab, by prolonging the duration that patients are at risk for breakthrough bleeding. The prospective GTH-AHA-EMI trial of 47 patients with AHA showed a reduced bleeding rate when compared with historic controls among patients on emicizumab without IST for the first 12 weeks after AHA diagnosis.12 Breakthrough bleeding occurred in 14 patients (29.8%) with 2 fatal bleeding events (gastrointestinal bleed in week 11 and intracranial bleed in week 13), and nearly all ultimately required IST to control their inhibitors. It is unclear if concurrent IST with emicizumab further reduced bleeding in this retrospective cohort; breakthrough bleeds occurred in only 4 patients (7%) on concurrent IST with emicizumab with a minimum of 12 weeks of follow-up and with no fatal bleeding events in the overall cohort. Notably, there were only 2 spontaneous gastrointestinal breakthrough bleeds on maintenance emicizumab, a common site of bleeding in the general older population. To our knowledge, this is the largest cohort of patients with AHA without a fatal bleeding event; fatal bleeds occurred in 2.9% to 9.1% of patients with AHA in historic registries.1,3,17,22 Given that the historic time to response to IST is 6 to 11 weeks, delaying IST poses a prolonged period of bleeding risk. Prospective data support the efficacy of emicizumab in reducing bleeding risk, and this retrospective data suggests concurrent use of IST and emicizumab may further improve bleeding rates. However, in our cohort, there were insufficient patients who did not receive IST to definitively determine if withholding IST impacts the bleeding risk. Considering the minimal adverse and bleeding events in this older cohort with concurrent comorbidities, this suggests that emicizumab and upfront IST, especially rituximab, may provide the most effective strategy for managing bleeding risk for patients with AHA.
Reducing bleeding risk with accelerated loading
Most patients in this cohort received the standard emicizumab loading doses used in congenital hemophilia A and not the accelerated loading dose (6 mg/kg on day 1 and 3 mg/kg on day 2) that was used in prospective trials of emicizumab in AHA.11,12 There were 4 breakthrough bleeds (1 severe and 3 nonsevere) during emicizumab loading in our study. Although bleeds, especially nonsevere bleeding, were likely underreported because of the retrospective nature of this study, it is possible that bleeding would have improved with accelerated loading of emicizumab that enables achieving steady state emicizumab levels within 7 days.11,12 The ongoing prospective AHAEmi trial will help to clarify if bleeding is further mitigated by upfront IST with concurrent accelerated loading doses of emicizumab. However, there is a scarcity of data on whether an accelerated loading dose reduces the use of bypassing agents or improves the cost of care and quality of life when compared with conventional emicizumab loading doses.
Although this retrospective cohort offers the largest real-world data of patients with AHA treated with emicizumab, limitations include the varied duration of follow-up, which could have led to some missed side effects. Particularly, rituximab can have delayed adverse events that may not have been captured, including delayed neutropenia, reduced response to vaccinations, and prolonged infectious risk. Patients were followed by their HTCs for bleeding, but it is possible that some breakthrough bleeds could have been missed, especially mild bleeding, owing to a lack of prospective monitoring. There was considerable variability in the completeness of data collection and no standardized approach to emicizumab or IST regimens.
Emicizumab could improve AHA outcomes by providing outpatient hemostatic prophylaxis with lower intensity IST. Additional safety and dosing data are required to clarify the role of emicizumab in AHA.
Acknowledgments
The authors thank the patients and staff at our hemophilia treatment centers. The authors are grateful to Angela Dove for her assistance in managing the Research Electronic Data Capture (REDCap) database. The REDCap instance used was supported by the Institute of Translational Health Sciences, which was funded by the National Center for Advancing Translational Sciences of the National Institutes of Health (award number UL1TR002319).
Authorship
Contribution: J.N.P. and R.K.-J. designed the study, contributed cases, analyzed the data, and wrote the manuscript; J.N.P. wrote the first draft of the manuscript; C.B. analyzed the data; A.v.D. and K.A.B. assisted in designing the data query and contributed cases; J.Y.Z., A.P., E.C.C., O.K., P.E., L.C., C.W., M.A.E., J.F.W., L.M.M., C.M.K., and M.J. contributed cases to the database; and all authors assisted in preparing the manuscript.
Conflict-of-interest disclosure: A.v.D. reports receiving honoraria for participating in scientific advisory board panels, consultations, and speaking engagements for BioMarin, Regeneron, Pfizer, Bioverativ/Sanofi, CSL Behring, Novo Nordisk, Precision Medicine, SparX Therapeutics, Takeda, Genentech, and uniQure, and is a cofounder and member of the board of directors of Hematherix LLC, a biotech company that is developing superFVa therapy for bleeding complications. A.P. reports receiving research support from Shire/Takeda, Genentech/Hoffman LaRoche; serving on advisory boards for Genentech, Shire/Takeda, Sigilon, and uniQure; and serving as a consultant for Aspa, I-Mab, and Sunovion. C.M.K. reports receiving research support from Bayer, Genentech, Novo Nordisk, Octapharma, and Regeneron; serving on advisory boards for Bayer, Genentech, Novo Nordisk, Octapharma, Pfizer, and Takeda; and serving on data and safety monitoring boards for Bayer and Octapharma. C.W. reports serving as a consultant for Genentech, Novo Nordisk, CSL Behring, BioMarin, and Sanofi. E.C.C. reports serving as a consultant for Guidepoint, Sentiment, Enos Health, Lumanity, and AbbVie. J.N.P. reports serving as a consultant for TeraImmune. J.Y.Z. reports serving as a consultant for Diagonal Therapeutics. L.M.M. reports serving as a consultant for Sanofi, Sobi, CSL Behring, Spark, BioMarin, Novo Nordisk, Takeda, and Pfizer; receiving research funding from Sanofi; and serving on speakers' bureaus for Sanofi and CSL Behring. M.A.E. reports serving as a consultant for Genentech/Roche, Novo Nordisk, CSL Behring, Takeda, Sanofi, Bayer, HEMA Biologics, LFB, Regeneron, BioMarin, and the National Bleeding Disorders Foundation. M.J. reports serving as a consultant for and receiving research funding from Genentech and serving as a consultant for Takeda, Sanofi, Bioamarin, CSL Behring, and Octapharma. O.K. reports serving as a consultant for BioMarin, Sanofi, Pfizer, Genentech, Kedrion, Bayer, and Novo Nordisk. P.E. reports serving as a consultant for Genentech. R.K.-J. reports receiving research support from Genentech, Sanofi, and Pfizer, and serving on advisory boards and as a consultant for Roche/Genentech and Regeneron. The remaining authors declare no competing financial interests.
Correspondence: Jacqueline N. Poston, Hematology/Oncology Division, Department of Medicine, Larner College of Medicine at the University of Vermont, 89 Beaumont Ave, Given E214, Burlington, VT 05405; email: jacqueline.poston@med.uvm.edu.
References
Author notes
Data are available on request from the corresponding author, Jacqueline N. Poston (jacqueline.poston@med.uvm.edu).
The full-text version of this article contains a data supplement.