TO THE EDITOR:
Clinical trial requirements can be cumbersome and expensive. We previously established that bone marrow biopsies (BMB) can be eliminated from most clinical trials in follicular lymphoma (FL).1-3 These uncomfortable and costly procedures are required at baseline and to assess response to therapy in patients with positive BMB at baseline and complete response on imaging. We investigated nearly 1000 patients with FL enrolled on trials through Alliance for Clinical Trials in Oncology (Alliance/legacy Cancer and Leukemia Group B, CALGB), SWOG Research Network, and Eastern Cooperative Oncology Group and found that a minority of BMB affected response assessment.3 We recommended the procedures be eliminated from clinical trial requirements in FL.
We next investigated impact of imaging study frequency in FL clinical trials. Imaging is required at multiple time points for years while monitoring patients enrolled on trials. In 2013, the American Board of Internal Medicine and American Society of Hematology’s (ASH’s) Choosing Wisely campaign recommended against surveillance scanning in some patients who had been treated for lymphomas.4 This effort, which aims to eliminate unnecessary, potentially harmful, and expensive procedures, is applicable to imaging studies. These tests increase radiation exposure and secondary malignancy risk, anxiety, and cost.5-10 In clinical practice, surveillance scans have not been shown to improve overall survival in patients with lymphoma.11-13
A key end point in clinical trials enrolling patients with FL is progression-free survival (PFS). FL is largely not viewed as a curable disease but rather one in which patients typically achieve a remission with therapy, then later progress and require additional treatment. We sought to show that PFS would not be different when imaging is performed less frequently than required by trials. We aim to spare patients enrolled in trials from negative aspects of imaging studies in the prolonged period during which they are monitored.
We identified all trials through Alliance that enrolled untreated patients with FL from 2008 to 2016. We considered 2 imaging schedules: protocol specified (control schedule) vs relaxed (Schedule X) in which response assessments at every other required time point were omitted. For each trial, we estimated PFS using Kaplan-Meier methods for the 2 schedules then determined difference in PFS between schedules as percent change from control in 2-year and 4-year PFS rates. We further investigated impact of Schedule X on number of events with fixed study duration and impact of Schedule X on treatment effect estimation in the randomized trial CALGB(C)50904. We then performed simulation studies to approximate impact of less frequent imaging on PFS estimation using trials for which median PFS was observed: C50901 (ofatumumab for low-intermediate risk FL) and C50904 (ofatumumab/bendamustine for intermediate-high risk FL, control arm). We assumed exponential distribution for PFS, in which rate parameter was calculated based on observed median PFS with uniform censoring distribution. Study protocols mandated imaging every 4 months for 2 years then every 6 months for 10 years, which we called S0. We ran 10 000 iterations of 100 observations using 3 tumor assessment follow-up schedules: every 6 months for 2 years then annually (S1), every 8 months for 2 years then annually (S2), or annually (S3) for simulated PFS times. We calculated deviation of average PFS for each simulated schedule from truth (S0). Statistical analysis was conducted by the Alliance Statistics and Data Center.
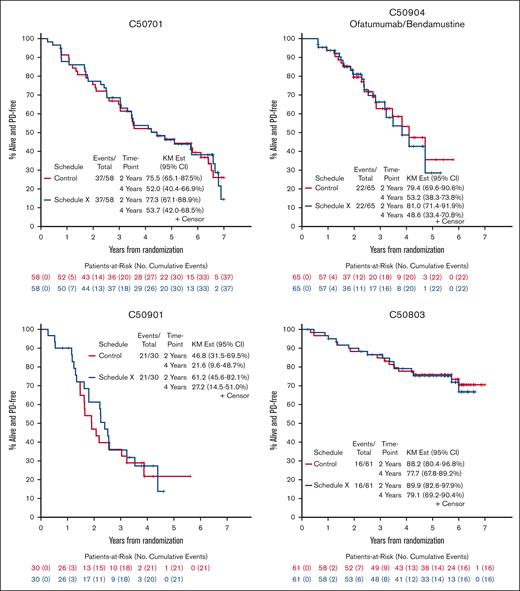
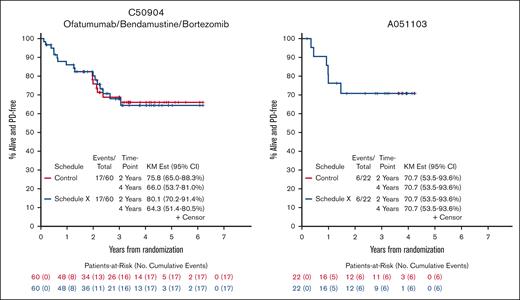
We identified 5 studies meeting inclusion criteria that completed enrollment of a total of 322 patients with untreated FL from 2008 to 2016 through Alliance; 296 were eligible for analysis.14-18 Reasons for exclusion were missing PFS data (3) and ineligibility/withdrawal from trial after enrollment (8). We did not include the ofatumumab 500 mg arm of C50901 because the study was amended to a single-arm design after slow accrual. Most of the patients had advanced-stage disease (97%) and intermediate/high risk Follicular Lymphoma International Prognostic Index scores (86%). Study and clinical characteristics are listed in the supplemental Table 2. Across 5 FL trials, median percent change of Schedule X from control schedule in estimated 2-year and 4-year PFS was 2.1% (range, 0.0%-30.8%) and 0.9% (range, −8.6% to 25.7%), respectively (Figure 1 and supplemental Table 3). In our analysis of impact of Schedule X on number of events, we found minimal difference, particularly with follow-up of 2 vs 4 years. We also found a trivial impact of Schedule X in our analysis of treatment effect estimation for C50904: hazard ratio was 0.81 vs 0.80 for Control vs Schedule X, respectively (supplemental Table 4). Simulation study results by trial were as follows: for C50901, true median PFS was 1.91 years and average median PFS for S1, S2, and S3 were 2.07, 2.11, and 2.22 years; for C50904 (control arm), true median PFS was 4.10 years and average median PFS for S1, S2, and S3 were 4.20, 4.16, and 4.09 years (Table 1). All studies were approved by the institutional review board at each participating site and informed consent forms were signed by all subjects enrolled on the trials.
PFS for Control Schedule and Schedule X for 5 Alliance trials enrolling patients with untreated FL from 2008-2016. Kaplan-Meier curves for C50701 (phase 2, epratuzumab/rituximab), C50803 (phase 2, lenalidomide/rituximab), C50904 (phase 2, ofatumumab/bendamustine vs ofatumumab/bendamustine/bortezomib), C50901 (phase 2, ofatumumab), and A051103 (phase 1, rituximab/lenalidomide/ibrutinib) demonstrate minimal difference in 2- and 4-year PFS when scans are performed according to protocol (Control Schedule) vs omitting every other scan (Schedule X).
PFS for Control Schedule and Schedule X for 5 Alliance trials enrolling patients with untreated FL from 2008-2016. Kaplan-Meier curves for C50701 (phase 2, epratuzumab/rituximab), C50803 (phase 2, lenalidomide/rituximab), C50904 (phase 2, ofatumumab/bendamustine vs ofatumumab/bendamustine/bortezomib), C50901 (phase 2, ofatumumab), and A051103 (phase 1, rituximab/lenalidomide/ibrutinib) demonstrate minimal difference in 2- and 4-year PFS when scans are performed according to protocol (Control Schedule) vs omitting every other scan (Schedule X).
Simulation study results for C50901 and C50904 (Control Arm) assessing different imaging schedules
| Follow-up schedule . | C50901 . | ||
|---|---|---|---|
| Average median PFS (% difference from true median PFS) . | Absolute difference from true median PFS (mo) . | Bias (MSE) . | |
| S1: every 6 mo for 2 y then annually | 2.07 (8.3%) | 1.9 | 0.16 (0.13) |
| S2: every 8 mo for 2 y then annually | 2.11 (10.7%) | 2.4 | 0.20 (0.14) |
| S3: annually | 2.22 (16.2%) | 3.7 | 0.31 (0.18) |
| Follow-up schedule . | C50901 . | ||
|---|---|---|---|
| Average median PFS (% difference from true median PFS) . | Absolute difference from true median PFS (mo) . | Bias (MSE) . | |
| S1: every 6 mo for 2 y then annually | 2.07 (8.3%) | 1.9 | 0.16 (0.13) |
| S2: every 8 mo for 2 y then annually | 2.11 (10.7%) | 2.4 | 0.20 (0.14) |
| S3: annually | 2.22 (16.2%) | 3.7 | 0.31 (0.18) |
| Follow-up schedule . | C50904, Control Arm . | ||
|---|---|---|---|
| Average median PFS (% difference from true median PFS) . | Absolute difference from true median PFS (mo) . | Bias (MSE) . | |
| S1: every 6 mo for 2 y then annually | 4.20 (2.5%) | 1.2 | 0.10 (0.63) |
| S2: every 8 mo for 2 y then annually | 4.16 (1.5%) | 0.7 | 0.06 (0.60) |
| S3: annually | 4.09 (−0.3%) | 0.1 | −0.01 (0.56) |
| Follow-up schedule . | C50904, Control Arm . | ||
|---|---|---|---|
| Average median PFS (% difference from true median PFS) . | Absolute difference from true median PFS (mo) . | Bias (MSE) . | |
| S1: every 6 mo for 2 y then annually | 4.20 (2.5%) | 1.2 | 0.10 (0.63) |
| S2: every 8 mo for 2 y then annually | 4.16 (1.5%) | 0.7 | 0.06 (0.60) |
| S3: annually | 4.09 (−0.3%) | 0.1 | −0.01 (0.56) |
True median PFS for the C50901 arm was 1.91 years.
True median PFS for the C50904 (Control Arm) was 4.10 years.
m, months; MSE, mean squared error; PFS, progression-free survival; y, years.
Our findings support decreased frequency of imaging studies in FL clinical trials. We found minimal differences in PFS rates at 2 and 4 years in the study schedule (Control Schedule) vs Schedule X in which imaging results at every other time point were omitted. The disparate result in C50901 is related to small shift in events with low number of patients at risk for progression. Our simulation provides further evidence of low impact on median PFS when imaging is performed less frequently. We recognize that most trials in this analysis were immunotherapy-based. We believe the results would also apply to chemoimmunotherapy-based trials, particularly because the latter are more likely to have higher PFS rates at 2 and 4 years, and therefore imaging studies at earlier time points are less likely to show relapse. A similar analysis in primarily chemoimmunotherapy-based trials may even find smaller differences in PFS than we report.
Though our study comprises a relatively small group of patients, the simulation allowed us to estimate the impact of scanning on a larger scale. Patients enrolled on C50901 and C50904 could have received up to 22 scans during follow-up (S0), whereas in our simulation, patients would have only received up to 12 (S1), 11 (S2), or 10 scans (S3). With estimated radiation exposure of 40 mSV/scan, the difference in radiation between S0 vs S3 is 480 mSV.5 Based on a cost effectiveness analysis of surveillance imaging published in 2015, when these studies were enrolling, additional scanning would have been ∼$40 000 more per patient in S0 vs S3.8 Performing scans annually rather than multiple times per year for 10 years would have saved ∼$5 000 000 in C50904, a phase 2 study enrolling 130 patients.
We continue to recommend frequent study visits with history, exams, and labs. Close communication between patients and physicians is critical to our strategy of decreased imaging frequency. In our analysis, we were unable to differentiate whether relapses were detected based on scheduled imaging vs scans ordered because of concerns raised at or between visits. We matched imaging studies done at closest time points to those mandated by trials. If some relapses were detected by imaging ordered because of clinical suspicion rather than study protocol, that would further support our conclusion. We recommend that imaging studies be performed annually in trials for FL, and additional scans tailored to each patient’s clinical situation if an investigator is concerned about relapse.
Modernizing clinical trial requirements is a key initiative of ASH and the American Society of Clinical Oncology. Decreasing scan frequency is a straightforward way in which clinical trials can be simplified without significantly impacting study end points. In addition to being patient-centered by decreasing radiation exposure and anxiety, this strategy, if combined with other ways of decreasing costs of clinical trials, would enable more studies to open for patients to gain access to novel therapeutic approaches. To be widely implemented, this approach will require collaboration by investigators, the National Clinical Trials Network, and the pharmaceutical industry. We plan a larger analysis of industry-sponsored chemoimmunotherapy-based trials in patients with lymphoma to confirm our results.
Acknowledgments: Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under award numbers U10CA180821 and U10CA180882 (to the Alliance for Clinical Trials in Oncology), UG1CA233339, UG1CA233247. It was also supported in part by Celgene (C50803) and GSK (C50901, C50904).
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Contribution: S.C.R., J.Y., L.D.P., S.J.M., and J.P.L. designed the research; S.C.R., J.Y., and L.D.P performed the research and analyzed data; all authors interpreted data; J.Y., L.D.P., and S.J.M. performed statistical analysis; and all authors wrote and reviewed the manuscript.
Conflict-of-interest disclosure: S.C.R. received research funding from Constellation, Genentech/Roche, and Karyopharm Therapeutics and has consulted for ADC Therapeutics, Bristol Myers Squibb(BMS), Genmab, Karyopharm, Kite, and Seagen. P.M. has consulted for AbbVie, AstraZeneca, BeiGene, BMS, Daiichi Sankyo, Epizyme, Genentech, Gilead, Janssen, Merck, and Takeda. N.L.B. has received research funding from ADC Therapeutics, Autolus, BMS/Celgene, Forty Seven, Inc, Gilead/Kite Pharma, Janssen, Merck, Millennium, Pharmacyclics, Roche/Genentech, and Seagen and has participated in an advisory board with ADC Therapeutics, Foresight Diagnostics, Kite, Roche/Genentech, and Seagen. J.P.L. has received research funding from the National Cancer Institute, Leukemia and Lymphoma Society, Genentech, Epizyme, Janssen; and has consulted for AbbVie, Astellas, AstraZeneca, Bayer, BeiGene, BMS, Calithera, Constellation, Eisai, Lilly, Epizyme, Genmab, Grail, Incyte, Janssen, Karyopharm, Lilly, Merck, Mustang Bio, Pfizer, Roche/Genentech, Second Genome, and Sutro.
Correspondence: Sarah C. Rutherford, Weill Cornell Medicine, 1305 York Avenue, 7th Floor, Y-764, New York, NY 10021; email: sar2014@med.cornell.edu.
References
Author notes
Presented in abstract form at the 2023 annual meeting of the American Society of Clinical Oncology, Chicago, IL, 5 June 2023.
ClinicalTrials.gov identifier NCT00553501 (C50701), NCT01145495 (C50803), NCT01190449 (C50901), NCT01286272 (C50904), and NCT01829568 (A051103).
Data are available upon reasonable request from the corresponding author, Sarah C. Rutherford (sar2014@med.cornell.edu).
The full-text version of this article contains a data supplement.