TO THE EDITOR:
In a recent issue of Blood Advances, Valtis et al1 found that patients with lymphoma who underwent chimeric antigen receptor T-cell therapy (CAR-T) and those with cancer cachexia, defined as a decrease in body mass index (BMI, kg/m2) >5% during a 3-month period before lymphodepleting chemotherapy, had worse overall survival even when controlling for important confounders, including disease burden, performance status, age, and disease type. Poorer survival in their cohort was driven by a combination of earlier relapse and an increase in nonrelapse mortality (NRM) among those with cancer cachexia when compared with those without. The authors appropriately noted the limitations of their work, including a lack of data on comorbidities and a retrospective design. CAR-T continues to change the landscape of treatment for patients with lymphoma. There are now 4 US Food and Drug Administration–approved products for aggressive and indolent subtypes with increasingly earlier integration of these therapies into the management plan. Despite improvements in prognostic modeling and management of CAR-T toxicities, including immune effector cell associated neurotoxicity, cytokine release syndrome, and immune effector cell associated hematotoxicity with consequent B-cell aplasia, NRM remains a concern. In a recent analysis by Rejeski et al, the NRM rates for those with lymphoma who undergo CAR-T ranged from 3.8% to 7.4%, depending on the product, and the majority (50.8%) of these deaths were a consequence of infection.2 Thus, CAR-T is not without its risks and patient selection is of significant importance. Given the relative newness of CAR-T in the armamentarium of lymphoma therapies, little is known about defining and optimizing patient fitness for CAR-T. Comorbidities, physician assigned performance status, frailty, sarcopenia, malnutrition, and a comprehensive geriatric assessment have all been used to assess patient fitness for CAR-T. The work of Valtis et al1 adds cancer cachexia to that list.
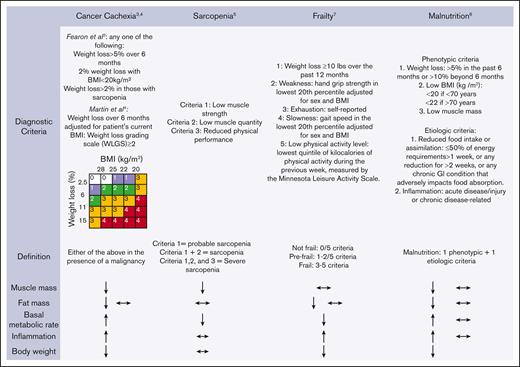
Cancer cachexia, malnutrition, sarcopenia, and frailty (Figure 1) have all been indicted as contributors to poor outcomes for those who are undergoing CAR-T. These 4 entities are intertwined yet distinct, both diagnostically and in their management, causing significant confusion for hematologists. Cancer cachexia is a catabolic inflammatory syndrome with complex metabolic changes to the host, which leads to weight loss and loss of skeletal muscle mass, driven by the underlying malignancy. The diagnostic criteria for cancer cachexia reflect manifestations of the syndrome rather than a deep understanding of the biologic processes that drive cancer cachexia, which are poorly described to date. The diagnostic criteria3,4 currently use a combination of weight loss, BMI, symptoms (eg, fatigue, anorexia), inflammatory markers (eg, C-reactive protein, interleukin-6, albumin), and phenotypic criteria (low muscle strength). Sarcopenia, defined as loss of muscle mass and function, is part of the normal aging process but is also frequently a complication of cancer through cancer cachexia and myotoxic chemotherapeutic or supportive care agents (eg, steroid-induced myopathy).5 Malnutrition is an imbalance between the growth and breakdown of body tissues and nutrient stores and leads to a loss of muscle and organ mass, either because of nutrient deficiencies or inflammation from an underlying disease.6 Finally, frailty, commonly diagnosed using the Fried Frailty phenotype,7 is a syndrome characterized by decreased physiological reserve and a heightened vulnerability to stressors. When considering how to intervene in a patient who meets the diagnostic criteria for these entities, clearly defining which limitation a patient has may have significant impact on their clinical improvement. For example, for a patient with cancer cachexia who are experiencing sarcopenia and malnutrition secondarily, management without targeting the underlying biologic mechanisms of cancer cachexia is unlikely to yield clinical benefit. In contrast, a patient with malnutrition and secondary sarcopenia owing to low nutrition intake only as a consequence of resource scarcity would likely rapidly improve with dietary intervention alone. Not surprisingly, a previous pilot report indicated the heterogeneous ability of body metric and laboratory-based criteria to predict cachexia-related clinical and functional outcomes after CAR-T, suggesting a possible avenue for the identification of subphenotypes of muscle wasting.8
Diagnostic criteria and clinical features of cancer cachexia, sarcopenia, frailty, and malnutrition. ↑ indicates increase; ↓, decrease; and ↔, unchanged. The presence of 2 arrows indicates that either of the changes in the variable may occur in the syndrome (eg, cancer cachexia may be accompanied by a decrease or no change in fat mass).
Diagnostic criteria and clinical features of cancer cachexia, sarcopenia, frailty, and malnutrition. ↑ indicates increase; ↓, decrease; and ↔, unchanged. The presence of 2 arrows indicates that either of the changes in the variable may occur in the syndrome (eg, cancer cachexia may be accompanied by a decrease or no change in fat mass).
What does the current and future of optimization of cancer cachexia look like in clinical practice? To begin, timing is key. Many events generally occur between the initial consultation and receipt of CAR-T, including leukapheresis, receipt of bridging therapy, and conditioning chemotherapy. As the authors point out, the median time from consultation with a cell therapy physician to infusion of CAR-T is 52 days. This provides a lengthy runway to optimize patient fitness, including cancer cachexia, but requires detection at the earliest time point possible. At the time of the initial discussions around CAR-T candidacy, at a minimum, weight loss in the past 3 or 6 months and BMI at the current time point, data which are readily available in the electronic medical record, should be assessed. Furthermore, a patient’s comorbidities, frailty, functional status, malnutrition, cognition, and sarcopenia ought to be assessed because limitations in these domains may impair cancer cachexia optimization. Those who have limitations in activities of daily living and significant cognitive impairment are often poor candidates for optimization and may benefit from alternative therapies. Comorbidities, especially those that impair oral intake, malabsorption, and the patient’s ability to participate in exercise (eg, cardiopulmonary), ought to be evaluated and managed by referral to appropriate subspecialist if not well controlled. This comprehensive fitness assessment may best be accomplished by referral to so-called optimization programs, which are increasingly available at academic centers worldwide and have shown to improve outcomes for those who undergo CAR-T.9 From there, cancer cachexia optimization involves a combination of dietary, physical activity, and pharmacologic intervention. A high-protein diet (1.5-2.0 g/kg per day), function-focused exercise training (customized by rehabilitation clinicians), nutrition intake–related symptom management, and anti-inflammatory therapy are proposed as the basis of multimodal approaches to cachexia.10,11 Low-dose (2.5 mg daily) olanzapine, mirtazapine, megestrol acetate, cannabinoids, and the Ghrelin mimetic anamorelin are all pharmacologic approaches used across the globe, although there is no standard of care and significant variability in clinical practice. A recent phase 2 trial of ponsegromab, a monoclonal antibody that targets growth differentiation factor-15, an inflammatory cytokine, in patients with an advanced stage solid tumor provided promising results in improving both appetite and physical activity with trials in patients with hematologic malignancies eagerly awaited.12 These recent findings also raise the possibility that specific biologic mechanisms can lead to targeted improvement in multiple clinical domains, including appetite and function, and that further study of these nuanced immune factors is needed.13
In summary, the work of Valtis et al1 is an important contribution to the field of fitness assessment and optimization in those undergoing CAR-T. This work adds to the body of literature that supports the urgent need for interventional trials to mitigate the negative effects of cancer cachexia and poor patient fitness more broadly.
Contribution: S.J.Y. conceptualized, wrote, and edited the manuscript; and I.R. conceptualized and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Samuel J. Yates, Section of Hematology and Oncology, The University of Chicago, 5841 S. Maryland Ave, MC7082, Chicago, IL 60637; email: samuel.yates@uchicagomedicine.org.