TO THE EDITOR:
Chronic lymphocytic leukemia (CLL) is characterized by the accumulation of monoclonal B cells due to B-cell receptor (BCR) driven autonomous cell signaling, enhanced by the lymph node microenvironment.1,2 Thus, CLL is sensitive to the Bruton tyrosine kinase (BTK) inhibitor, ibrutinib, which blocks the BCR pathway and inhibits extracellular signal-regulated kinases (ERK) and NF-κB activation.3
Deletion 11q22-23 (del11q) effects 20% of patients with CLL and is associated with unique clinical and biological features.4 When >25% of CLL cells have del11q, patients experience a short time to first treatment and the deletion is associated with short telomeres and genomic instability.4-7 In addition, patients experience early relapse after chemotherapy but have an excellent response to ibrutinib.8 One copy of the ataxia-telangiectasia mutated (ATM) gene is lost in del11q, with 1 of 3 of patients having an ATM mutation on the second allele.4 Despite usually retaining a normal ATM allele, as the fraction of cells with a del11q increases, there is a decrease in ATM protein with a compensatory increase in activated/phosphorylated DNA–dependent protein kinase (pDNA-PKS2056).9 ATM is recruited to DNA double-strand breaks (DSBs), followed by high-fidelity DNA repair by homologous recombination.10,11 However, DNA-PK repairs DSBs by error-prone nonhomologous end-joining.9,12,13 This increase in activated DNA-PK in high-fraction del11q cases likely explains the rapid disease progression and resistance to chlorambucil.9,13 In this study, we evaluated the role of ATM for ibrutinib sensitivity in del11q CLL.
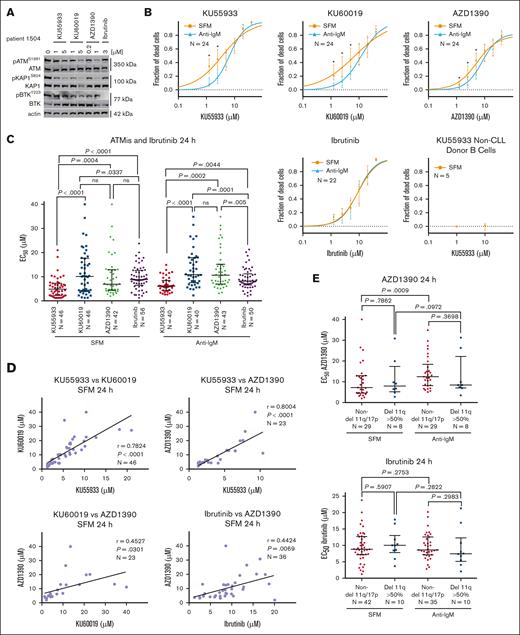
Three ATM inhibitors (ATMis; KU55933, KU60019, and AZD1390) were used to assess CLL sensitivity to chemotherapy and ibrutinib. AZD1390 is currently in clinical trials as a radiosensitizer10,14,15 and is >8 times more potent an ATMi than KU55933 and KU60019.10,16-18 Western analysis of CLL cells treated with the ATMis confirmed the increased potency of AZD1390 at inhibiting phosphorylation of ATM (pATMS1981) and KAP (pKAP1S824), whereas ibrutinib eliminated BTK phosphorylation (pBTKY223; Figure 1A).19
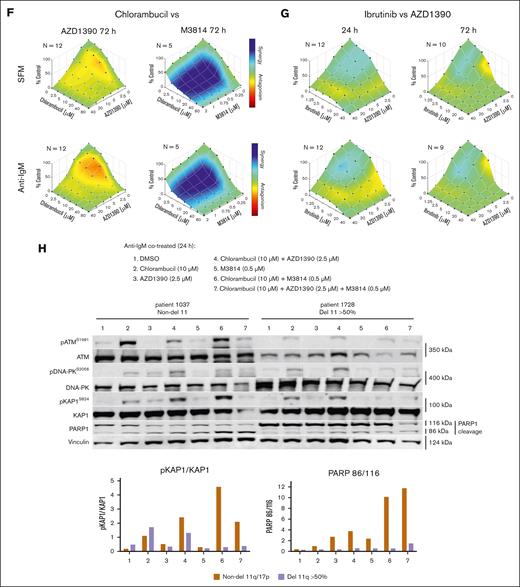
Influence of ATM inhibition and ibrutinib on cell death in CLL or normal B cells. (A) Western blot showing the effects of the ATMis on pATM and pKAP1 and inhibition of pBTK by ibrutinib. Cells were treated for 24 hours in serum-free media (SFM). (B) Survival curves for ibrutinib and the ATMis against CLL and normal B cells, incubated for 24 hours in SFM or with anti-IgM. Cytotoxicity was measured by annexin V/7-aminoactinomycin D staining and flow cytometry. (C) Effective concentration to reduce cell survival by 50% (EC50) for the ATMis and ibrutinib after incubating CLL cells in SFM and with anti-IgM for 24 hours. (D) Correlation between the cytotoxicities of the ATMis and ibrutinib in CLL cells. Data derived from panel C. (E) Comparison of the EC50s in non-del11q and del11q (>50% cells with deletion) CLL samples. (F) Combenefit synergy plots of CLL cells incubated in SFM or with anti-IgM and exposed to chlorambucil (CLB) and AZD1390 or M3814 for 72 hours. (G) Combenefit synergy plots of CLL cells incubated in SFM or anti-IgM and exposed to ibrutinib and AZD1390 for 24 or 72 hours. (H) Western blot of del11q and non-del11q samples incubated for 24 hours with anti-IgM and treated with CLB and AZD1390 (ATMi), M3814 (DNA-PKi), or both. DMSO, dimethyl sulfoxide; ns, not significant.
Influence of ATM inhibition and ibrutinib on cell death in CLL or normal B cells. (A) Western blot showing the effects of the ATMis on pATM and pKAP1 and inhibition of pBTK by ibrutinib. Cells were treated for 24 hours in serum-free media (SFM). (B) Survival curves for ibrutinib and the ATMis against CLL and normal B cells, incubated for 24 hours in SFM or with anti-IgM. Cytotoxicity was measured by annexin V/7-aminoactinomycin D staining and flow cytometry. (C) Effective concentration to reduce cell survival by 50% (EC50) for the ATMis and ibrutinib after incubating CLL cells in SFM and with anti-IgM for 24 hours. (D) Correlation between the cytotoxicities of the ATMis and ibrutinib in CLL cells. Data derived from panel C. (E) Comparison of the EC50s in non-del11q and del11q (>50% cells with deletion) CLL samples. (F) Combenefit synergy plots of CLL cells incubated in SFM or with anti-IgM and exposed to chlorambucil (CLB) and AZD1390 or M3814 for 72 hours. (G) Combenefit synergy plots of CLL cells incubated in SFM or anti-IgM and exposed to ibrutinib and AZD1390 for 24 or 72 hours. (H) Western blot of del11q and non-del11q samples incubated for 24 hours with anti-IgM and treated with CLB and AZD1390 (ATMi), M3814 (DNA-PKi), or both. DMSO, dimethyl sulfoxide; ns, not significant.
We determined whether ATMi affected CLL cell viability ex vivo, measured by annexin V/7-aminoactinomycin D staining, after 24 hours in serum-free medium or with anti–immunoglobulin M (IgM), to simulate the microenvironment.20 Dose-dependent cytotoxicity was seen with all ATMis, with the median effective concentration to reduce cell survival by 50% (EC50) for KU55933 being 5.84 μM, 6.77 μM for AZD1390 (P = .01) and 10.33 μM for KU600019 (P = .001; Figure 1B-C; supplemental Table 3). Notably, normal B cells were not sensitive to ATMi. The 3 ATMis likely have different off-target effects, but as their cytotoxicities closely correlated in 20 CLL samples (P < .0001), this activity was likely mediated through ATM inhibition (Figure 1D).
A surprising finding was a striking correlation between the EC50s of AZD1390 and ibrutinib (P < .007) indicating overlapping mechanisms of cytotoxicity (Figure 1D; supplemental Figure 1). Moreover, both del11q (>50% cells deleted) and non-del11q cells had equal sensitivities to ATMis or ibrutinib (Figure 1E; supplemental Figure 2A-B). In contrast, del11q cases were more resistant to chlorambucil than non-del11q samples (supplemental Figure 2C).9
Next, we examined whether inhibition of ATM influenced the sensitivities of CLL cells to chlorambucil or ibrutinib (Figure 1F-G; supplemental Figure 3). CLL samples were treated with increasing doses of chemotherapy or ibrutinib in combination with AZD1390 for 24 hours or 72 hours and cytotoxicity assessed using Combenefit software and GraphPad Prism.9,21 Surprisingly, antagonism was seen between AZD1390 and chlorambucil, fludarabine, bendamustine, or etoposide (supplemental Figure 4). This confirms a previous observation in CLL cells that KU55933 did not sensitize HeLa or LoVo cells to alkylating agents.18 In contrast, as we previously observed, marked synergy was seen between chlorambucil and DNA-PK inhibition by M3814 (Figure 1F).9 Thus, antagonism between ATMi and chemotherapy is likely related to a compensatory increase in pDNA-PK, as seen in del11q cells.9 In contrast, ATM inactivation by ≤2.5 μM AZD1390–enhanced ibrutinib activity, indicating overlapping mechanisms of cytotoxicity (Figure 1G).
To determine whether there was a difference between non-del11q and del11q CLL cells in their usage of ATM or DNA-PK following chemotherapy, we measured pATMS1981 and pDNA-PKS2056 levels following chlorambucil ± AZD1390 and/or M3814 (Figure 1H). In non-del11q cells, chlorambucil preferentially activated ATM, whereas AZD1390 decreased the activation and produced a compensatory increase in pDNA-PKS2056 and pKAP1S824. This is similar to our previous study demonstrating an increase in pDNA-PKS2056 in del11q cells.9 In contrast, chlorambucil preferentially activated DNA-PK in del11q cells, and cells were unable to produce a compensatory increase in pATMS1981 and pKAP1S824 when DNA-PK was inhibited by M3814. Thus, chlorambucil preferentially activates ATM in non-del11q cells and DNA-PK in del11q cells, whereas both pATMS1981 and pDNA-PKS2056 can activate KAP1.
To investigate the cause for cytotoxic synergy between ATM inhibition and ibrutinib, we determined whether ATMi might prevent the phosphorylation of H2Axser139 (γH2Ax, a DNA DSBs marker) that we previously observed with ibrutinib in CLL (supplemental Figure 5A).21 CLL cells were incubated for 18 hours with ibrutinib with or without 1 μM or 10 μM KU55933. Induction of γH2Ax was observed with increasing doses of ibrutinib (0.6-5.0 μM), whereas KU55933 did not change γH2Ax levels (supplemental Figure 5A). To confirm that KU55933 could inhibit γH2Ax production, CLL cells were treated with 5 Gy irradiation and 1 μM KU55933 for 18 hours; γH2Ax measured at 0, 1, and 6 hours. KU55933 decreased γH2Ax induction but not DNA repair kinetics. Thus, the synergy observed between ATMi and ibrutinib is unrelated to alterations in DNA repair.
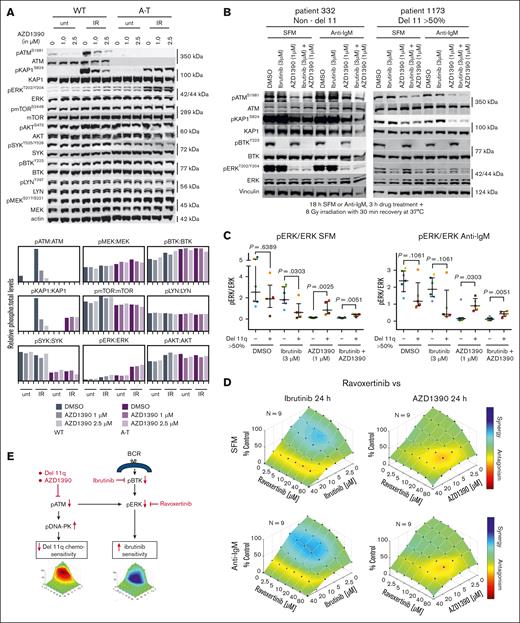
AZD1390 is a highly specific inhibitor of ATM,15 but in this study, we evaluated the possible effects of AZD1390 on other key kinases in the BCR pathway. We examined AZD1390-treated B cells derived from a normal/wild-type individual and from a patient with ataxia-telangiectasia, with inactivating mutations in ATM.22 AZD1390 only specifically affected ATM and downstream KAP1, whereas the B-cell relevant mTOR, PI3K (AKT), MEK, LYN, SYK, BTK, and ERK pathways were unaffected (Figure 2A). Interestingly, a decrease in ERK activation has been reported with ATMi in glioma cell lines, and ibrutinib in CLL cells.3,16,17 We, thus, determined whether the cytotoxicity of ATMi and its synergy with ibrutinib in CLL cells were related to changes in ERK levels or activation. Twelve CLL samples (7 non-del11q and 5 del11q) were treated with 3 μM ibrutinib, 1 μM AZD1390, or ibrutinib/AZD1390 for 3 hours followed by activation of ATM by irradiation (8 Gy; Figure 2B; supplemental Figures 6 and 7). With irradiation alone, pERKT202/Y204 levels trended lower in del11q than non-del11q cells (Figure 2C). Ibrutinib reduced pERKT202/Y204 to a greater extent in del11q cells than in non-del11q cells, whereas AZD1390 and ibrutinib together produced a marked reduction in pERKT202/Y204 in both non-del11q and del11q cells. These results suggest that the sensitivity to ibrutinib in del11q CLL and in AZD1390-treated non-del11q CLL is related to decreased ERK activity through loss of ATM (Figure 2C; supplemental Figures 6 and 7). To confirm that inhibition of ERK activation by AZD1390 was responsible for its activity in CLL cells, we combined the ERK inhibitor, ravoxertinib, with ibrutinib or AZD1390. Ravoxertinib was synergistic with ibrutinib but antagonistic with AZD1390 confirming that the cytotoxicity of AZD1390 and its synergy with ibrutinib were related to a reduction in ERK activity (Figure 2D). Responses were similar in samples with or without del11q (data not shown).
Protein expressions in del11q and non-del11q cells, with and without irradiation, and the interaction of ravoxertinib with AZD1390 and ibrutinib. (A) Western analysis of human B cells derived from individuals without (control) and with ataxia-telangiectasia (A-T; compound inactivating mutations in ATM exon 4) cotreated with AZD1390 and 8 Gy irradiation. AZD1390 reduced pATMS1981 and pKAP1S824 formation in control cells, whereas AZD1390 did not affect other B-cell–specific signaling pathways (mTOR, BTK, ERK, AKT, SYK, and LYN) in control/wild-type (WT) and A-T cells. Interestingly, genetic loss of ATM resulted in alterations to mTOR, SYK, and ERK activity, independent of AZD1390 treatment. (B) Western analysis of CLL cells from a representative non-del11 case and a del11q case (>50% cells effected) following incubation ex vivo for 18 hours in SFM or with anti-IgM, 3-hour drug treatment + 8 Gy irradiation with 30 minute recovery at 37°C. (C) Expression of pERK in 7 non-del11q/del17p cases and 5 del11q cases obtained by densitometry from western analysis in panel B, showing a trend toward decreased pERK in del11q cases coinciding with decreased pATM and pKAP1 (supplemental Figure 6). (D) Ibrutinib (synergism) or AZD1390 (antagonism) cotreatment with ravoxertinib on CLL cell survival. Combenefit plots represent data combined from 9 independent cells from patients with CLL. (E) Postulated mechanisms to explain the chemotherapy resistance, more rapid disease progression, and sensitivity to ibrutinib in del11q CLL. DMSO, dimethyl sulfoxide; IR, irradiation; unt, untreated.
Protein expressions in del11q and non-del11q cells, with and without irradiation, and the interaction of ravoxertinib with AZD1390 and ibrutinib. (A) Western analysis of human B cells derived from individuals without (control) and with ataxia-telangiectasia (A-T; compound inactivating mutations in ATM exon 4) cotreated with AZD1390 and 8 Gy irradiation. AZD1390 reduced pATMS1981 and pKAP1S824 formation in control cells, whereas AZD1390 did not affect other B-cell–specific signaling pathways (mTOR, BTK, ERK, AKT, SYK, and LYN) in control/wild-type (WT) and A-T cells. Interestingly, genetic loss of ATM resulted in alterations to mTOR, SYK, and ERK activity, independent of AZD1390 treatment. (B) Western analysis of CLL cells from a representative non-del11 case and a del11q case (>50% cells effected) following incubation ex vivo for 18 hours in SFM or with anti-IgM, 3-hour drug treatment + 8 Gy irradiation with 30 minute recovery at 37°C. (C) Expression of pERK in 7 non-del11q/del17p cases and 5 del11q cases obtained by densitometry from western analysis in panel B, showing a trend toward decreased pERK in del11q cases coinciding with decreased pATM and pKAP1 (supplemental Figure 6). (D) Ibrutinib (synergism) or AZD1390 (antagonism) cotreatment with ravoxertinib on CLL cell survival. Combenefit plots represent data combined from 9 independent cells from patients with CLL. (E) Postulated mechanisms to explain the chemotherapy resistance, more rapid disease progression, and sensitivity to ibrutinib in del11q CLL. DMSO, dimethyl sulfoxide; IR, irradiation; unt, untreated.
AZD1390 has clinical potential as a radiosensitizer, particularly in p53 mutant cancers, reversing drug resistance and enhancing immunotherapy in a variety of cancers.23 Here, we show that AZD1390 is synergistic with ibrutinib, through downregulation of pERK, but antagonistic to chemotherapy. AZD1390 is given orally with tolerable toxicity raising its potential for combination studies with ibrutinib.14 Furthermore, the decrease in pERK with ATM loss likely contributes to the sensitivity of del11q CLL cells to ibrutinib, whereas increased pDNA-PKS2056 causes chemotherapy resistance and short time to first treatment (Figure 2E).
Acknowledgments: This study was supported by the Manitoba Tumour Bank, Winnipeg, Manitoba, a member of the Canadian Tissue Repository Network.
Funding for this study was provided by Research Manitoba, the Canadian Institutes of Health Research, the Leukemia Lymphoma Society, and the CancerCare Manitoba Foundation.
Contribution: S.E.F.K., A.S., J.B., and S.H.Y. carried out the experiments with technical assistance from B.K.; S.E.F.K., A.S., J.B.J., and S.K. designed the research, collected, analyzed, and interpreted data; L.Y., V.B., and J.B.J. cared for patients contributing samples; S.E.F.K., J.B.J., and S.K. wrote the manuscript; S.B.G. and V.B. revised the manuscript; and all authors approved the manuscript in its final format.
Conflict-of-interest disclosure: V.B. and L.Y. report membership on advisory boards for, and grant funding from, AbbVie, AstraZeneca, BeiGene, Janssen, Merck, and Lily Oncology. The remaining authors declare no competing financial interests.
Correspondence: James B. Johnston, CancerCare Manitoba, ON5044-675 McDermot Ave, Winnipeg, MB R3E 0V9, Canada; email: jjohnsto@cancercare.mb.ca; and Sachin Katyal, CancerCare Manitoba, ON5044-675 McDermot Ave, Winnipeg, MB R3E 0V9, Canada; email: Sachin.Katyal@umanitoba.ca.
References
Author notes
J.B.J. and S.K. contributed equally to this study.
Materials and methods, details of antibodies, and patient characteristics are provided in supplemental files (supplemental Tables 1 and 2).
The full-text version of this article contains a data supplement.