TO THE EDITOR:
Myeloma and its precursor, monoclonal gammopathy (MGUS), are more common in individuals with Black ancestry and are associated with immune dysfunction.1 T cells in patients with multiple myeloma (MM) express inhibitory immune checkpoints and were previously implicated to be exhausted, extrapolating from models of chronic viral infection.2 However, functional validation in single-cell transcriptomic/immunophenotypic studies is limited, and the clinical success of T-cell engagers in MM argues against global T-cell exhaustion.3,4
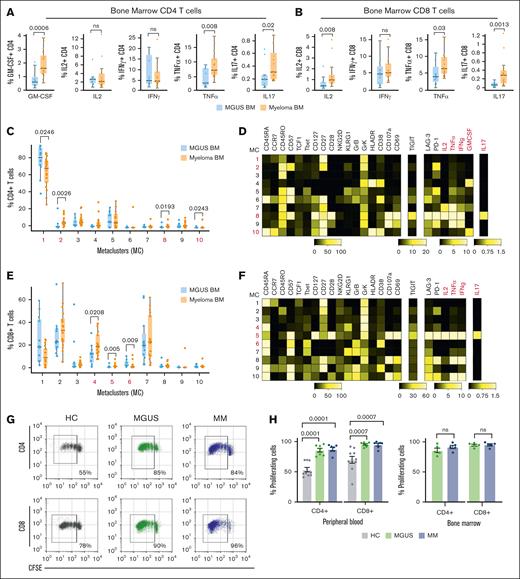
To understand the functional properties of immune cells in patients with plasma cell disorders, we combined functional assays with immune profiling in biospecimens from MGUS, newly diagnosed MM (NDMM), and age-matched healthy controls (patient characteristics in supplemental Table 1; see “Methods” in the supplemental Appendix). The capacity of T cells from blood/marrow to produce cytokines (interferon gamma [IFN-γ], tumor necrosis factor α [TNF-α], interleukin-2 [IL-2], granulocyte-macrophage colony-stimulating factor [GM-CSF], and IL-17) was analyzed by mass cytometry after stimulation by anti-CD3/CD2/CD28 antibodies. CD4 and CD8+ T cells from patients with NDMM/MGUS in both blood and bone marrow retained the capacity to produce all tested cytokines (Figure 1A-B; supplemental Figure 1). T cells in MM marrow exhibited higher production of several inflammatory cytokines (GM-CSF, IL-17, and TNF-α for CD4; and IL-2, TNF-α, and IL-17 for CD8) than MGUS counterparts (Figure 1A-B). Flow cytometry self-organizing map analysis of CD4 T cells identified 3 metaclusters (MCs) enriched in MM marrow: MC2 with CD57+ memory T cells; and 2 cytokine-producing MCs, MC10, which expressed GM-CSF, and MC8 with a polyfunctional phenotype, secreting IL-2, IFN-γ, IL-17, and TNF-α, which also expressed markers such as programmed cell death protein-1, lymphocyte activation gene-3, and T cell immunoreceptor with Ig and ITIM domains, previously linked to T-cell exhaustion (Figure 1C-D). Similar analysis of CD8 T cells identified 3 MCs as enriched in MM marrow, of which MC4 and MC6 had a terminal effector memory RA+ phenotype, whereas MC5 exhibited a polyfunctional phenotype, including IL-17 (Figure 1E-F). Most of the TNF-α– and IFN-γ–producing CD8 T cells in MM marrow had a terminal effector phenotype, and KLRG1+ differentiated T cells maintained the capacity for cytokine production (supplemental Figure 2). Both circulating and bone marrow T cells from patients with MGUS/MM also exhibited the capacity for T-cell proliferation in culture, again supporting the lack of global T-cell exhaustion (Figure 1G-H). Together, these data demonstrate that T cells in the MM marrow are enriched for phenotypes of advanced differentiation and exhibit distinct functional phenotypes, such as GM-CSF5 and IL-17 producers,6 previously implicated in tissue inflammation or bone disease.
Functional profiles of immune cells in MGUS/MM. (A) Cytokine secretion (GM-CSF, IL-2, IFN-γ, TNF-α, and IL-17) by CD4 T cells in bone marrow (BM) mononuclear cells from MGUS (n = 12) and MM (n = 17), after stimulation with anti-CD2/3/28. (B) Cytokine secretion (IL-2, IFN-γ, TNF-α, and IL-17) by CD4 T cells in BM mononuclear cells from MGUS (n = 12) and MM (n = 17), after stimulation with anti-CD2/3/28. Flow cytometry self-organizing map clustering of mass cytometry data on BM CD4+ T cells (C-D) and CD8+ T cells (E-F) from MGUS (n = 12) and MM (n = 17). Panels C,E show proportions of MC in patients with MGUS/MM. Panels D,F are heat maps showing phenotypes of MC. (G-H) T-cell proliferation after stimulation with anti-CD2/3/28. In panel G, representative plot shows proliferation of carboxyfluorescein succinimidyl ester-labeled peripheral blood T cells. In panel H, bar graph shows proliferating CD4 and CD8 T cells in blood (right image; healthy controls, n = 10; MGUS, n = 7; MM, n = 7) and BM (left image; MGUS, n = 5; MM, n = 5). In panels A-C and E, box plots represent median with Q1 to Q3 and error bars as minimum-maximum. P values were calculated using a 2-tailed Mann-Whitney. In panel H, bar graph shows proliferating CD4 and CD8 T cells in blood (left image; HC, n = 10; MGUS, n = 7; MM, n = 7) and BM (right image; MGUS, n = 5; MM, n = 5). P values were calculated using a 2-tailed Mann-Whitney. ns, not significant.
Functional profiles of immune cells in MGUS/MM. (A) Cytokine secretion (GM-CSF, IL-2, IFN-γ, TNF-α, and IL-17) by CD4 T cells in bone marrow (BM) mononuclear cells from MGUS (n = 12) and MM (n = 17), after stimulation with anti-CD2/3/28. (B) Cytokine secretion (IL-2, IFN-γ, TNF-α, and IL-17) by CD4 T cells in BM mononuclear cells from MGUS (n = 12) and MM (n = 17), after stimulation with anti-CD2/3/28. Flow cytometry self-organizing map clustering of mass cytometry data on BM CD4+ T cells (C-D) and CD8+ T cells (E-F) from MGUS (n = 12) and MM (n = 17). Panels C,E show proportions of MC in patients with MGUS/MM. Panels D,F are heat maps showing phenotypes of MC. (G-H) T-cell proliferation after stimulation with anti-CD2/3/28. In panel G, representative plot shows proliferation of carboxyfluorescein succinimidyl ester-labeled peripheral blood T cells. In panel H, bar graph shows proliferating CD4 and CD8 T cells in blood (right image; healthy controls, n = 10; MGUS, n = 7; MM, n = 7) and BM (left image; MGUS, n = 5; MM, n = 5). In panels A-C and E, box plots represent median with Q1 to Q3 and error bars as minimum-maximum. P values were calculated using a 2-tailed Mann-Whitney. In panel H, bar graph shows proliferating CD4 and CD8 T cells in blood (left image; HC, n = 10; MGUS, n = 7; MM, n = 7) and BM (right image; MGUS, n = 5; MM, n = 5). P values were calculated using a 2-tailed Mann-Whitney. ns, not significant.
Because the functional studies discussed above did not support the global T-cell exhaustion often implicated in cancer, we hypothesized that immunophenotypic changes, such as an increase in terminal effectors and inhibitory checkpoints previously described in MM,2,3 may instead be related to altered trajectories of immune aging. Aging of the immune system is associated with altered immune composition, which has been used to quantify immune aging.7 Because prior studies quantifying immune aging did not include racially diverse cohorts, we first analyzed a cohort of healthy individuals (n = 107) across 7 decades of life using a custom mass cytometry panel to identify immune phenotypes that correlated with age (supplemental Figure 3). Specimens from patients with MGUS/MM were analyzed with the same panel, and data from aging-associated variables were interpolated to compute aging-associated immune score (AAIS) in patients with MM/MGUS, similar to methods described earlier.7 In a cohort of patients with MGUS (n = 36) and NDMM (MM_1; n = 41) matched for chronologic age, patients with NDMM had significantly higher AAIS than those with MGUS (Figure 2A). Together, these data suggest that malignant transformation in MM may be associated with progressive immune aging. Both MGUS and MM are more common in individuals with Black ancestry. Black patients with MGUS also had higher AAIS than age-matched White counterparts (Figure 2B); however, no race-dependent differences were observed for the NDMM cohort. Together, these data suggest that an impact of racial ancestry on immune-aging trajectories may begin early in MGUS and provide insights into prior studies showing MM development at an earlier age in Black patients.1
Correlates of immune aging. (A) Chronologic age (ChrAge) and AAIS in patients with MGUS (n = 36) and NDMM (MM_1; n = 41). (B) ChrAge and AAIS in Black (n = 16) and White patients with MGUS (n = 18). (C) Transcriptional profiles related to immunologic aging in MM and MGUS. Data shown are top enriched pathways based on pathway analysis (hallmark, _H; reactome, _R) of differentially expressed genes from immune-old (n = 5) vs immune-young patients with MM (n = 4) and immune-old (n = 7) and immune-young patients with MGUS (n = 7) (split by median AAIS) in CD4 T cells, CD8 T cells, myeloid cells, and tumor cells. Pathways in pink are enriched in immune-aged and those in blue are enriched in immune-young. Detection of baseline levels of phosphoproteins (pSTAT1, pSTAT3, pSTAT5, p38, and pERK) by mass cytometry in circulating CD3+ T cells (D), CD14+16– monocytes (E), and CD14+16+ monocytes (F) in healthy controls (n = 10) and MM (n = 6). (G) ChrAge and AAIS in patients with MM who developed receptor-binding domain (RBD–) binding antibodies after severe acute respiratory syndrome coronavirus 2 vaccination (RBD+; n = 69) vs those who did not (RBD–; n = 14). Each dot is a unique patient. P values were calculated using a 2-tailed Mann-Whitney. Bar graphs represent mean ± SEM. P values were calculated using a 2-tailed Mann-Whitney. EMT, epithelial-mesenchymal transition; G2M, G2/mitosis; H, hallmark; MMI, median metal intensity; ns, not significant; R, reactome; TCR sig, T cell receptor signature.
Correlates of immune aging. (A) Chronologic age (ChrAge) and AAIS in patients with MGUS (n = 36) and NDMM (MM_1; n = 41). (B) ChrAge and AAIS in Black (n = 16) and White patients with MGUS (n = 18). (C) Transcriptional profiles related to immunologic aging in MM and MGUS. Data shown are top enriched pathways based on pathway analysis (hallmark, _H; reactome, _R) of differentially expressed genes from immune-old (n = 5) vs immune-young patients with MM (n = 4) and immune-old (n = 7) and immune-young patients with MGUS (n = 7) (split by median AAIS) in CD4 T cells, CD8 T cells, myeloid cells, and tumor cells. Pathways in pink are enriched in immune-aged and those in blue are enriched in immune-young. Detection of baseline levels of phosphoproteins (pSTAT1, pSTAT3, pSTAT5, p38, and pERK) by mass cytometry in circulating CD3+ T cells (D), CD14+16– monocytes (E), and CD14+16+ monocytes (F) in healthy controls (n = 10) and MM (n = 6). (G) ChrAge and AAIS in patients with MM who developed receptor-binding domain (RBD–) binding antibodies after severe acute respiratory syndrome coronavirus 2 vaccination (RBD+; n = 69) vs those who did not (RBD–; n = 14). Each dot is a unique patient. P values were calculated using a 2-tailed Mann-Whitney. Bar graphs represent mean ± SEM. P values were calculated using a 2-tailed Mann-Whitney. EMT, epithelial-mesenchymal transition; G2M, G2/mitosis; H, hallmark; MMI, median metal intensity; ns, not significant; R, reactome; TCR sig, T cell receptor signature.
To further understand transcriptional changes associated with immune-aging phenotypes, we analyzed bone marrow cells from patients with MM/MGUS with cellular indexing of transcriptomes and epitope sequencing (supplemental Figure 4). Patients were divided into immunologically younger and older cohorts based on median AAIS. These groups were comparable in terms of chronologic age (supplemental Figure 4D). We compared transcriptomes of immunologically younger and older cohorts to identify differentially expressed pathways in specific cell types. Pathway analysis of differentially expressed genes in CD4/CD8 T cells between immunologically younger and older patients with MM revealed greater PD1 signaling in older patients with MM, whereas interferon-response signatures were enriched in older patients with MGUS and MM (Figure 2C). Enriched pathways in myeloid and tumors cells from immunologically older patients with MM were also consistent with greater immune activation (Figure 2C).
To further validate transcriptional evidence of enhanced inflammation in MM at a proteomic level, we performed single-cell phosphoproteomic analysis at baseline and after in vitro stimulation in a cohort of MM and age-matched healthy donors. T cells and CD16+/CD16– monocytes from MM expressed higher baseline levels of several phosphoproteins (pSTAT1, pSTAT3, pSTAT5, pERK, and p38; Figure 2D-F). After stimulation with lipopolysaccharide and IFN-α, MM T cells exhibited reduced fold increase in phosphoproteins compared with healthy controls (supplemental Figure 5). In contrast, myeloid cells (particularly CD16+ monocytes) expressed higher levels of phosphoproteins relative to age-matched healthy donors (supplemental Figure 5). These data further support enhanced basal inflammatory signaling in MM immune cells and are remarkably similar to phosphoproteomic profiles described in studies of immune aging.8
Reduced immune response to vaccines has been recognized as a major consequence of immune aging in humans.9 To evaluate the impact of immune-aging phenotypes in MM on immune function in vivo, we analyzed a cohort of patients with MM (MM_2; n = 83) who had received severe acute respiratory syndrome coronavirus 2 vaccination.10 Biospecimens from this cohort were analyzed to assess immune aging. Evaluation of immune aging in this cohort was based on 15 aging-associated subsets analyzed in these patients (supplemental Figure 3). Similar to the initial MM cohort, patients in this cohort had significantly higher AAIS than patients with MGUS of similar chronologic age (supplemental Figure 6). Patients who developed vaccine-induced immunity, defined as positive titers for receptor-binding domain antibodies, had lower AAIS than patients lacking these responses (Figure 2G).
These data demonstrate that in spite of transcriptional similarities with exhausted T cells described in prior studies,2 T cells in MM marrow lack global T-cell exhaustion, underscoring the need for functional validation to interpret transcriptome-based studies. For example, PD1 signaling in MM T cells may reflect aging,11 as opposed to canonical T-cell exhaustion. T cells in MM marrow are instead enriched for inflammatory phenotypes that may contribute to disease pathogenesis. The finding that patients with MM exhibit greater immune aging than age-matched MGUS cohorts suggests a role for aging-associated changes in immune dysfunction linked to malignant transition. Racial ancestry may affect the early trajectories of immune aging in MGUS pathogenesis.12 Immune aging correlated with vaccine response, illustrating a link with immune function in vivo. Transcriptomes from patients with MM in the ongoing immune atlas effort also suggest enhanced aging-associated inflammatory signaling, providing external validation to these findings.13 Further studies are needed to better understand the mechanisms underlying immune aging and the clinical impact of this biology on emerging immune therapies in patients with MM.
Acknowledgments: M.V.D. is supported, in part, by funds from the National Institutes of Health (NIH; grant R35CA197603), the Paula and Rodger Riney Foundation, and a Specialized Center of Research Program award from the Leukemia and Lymphoma Society. K.M.D. is supported, in part, by funds from the NIH (grants CA238471 and AR077926). The authors acknowledge Winship immune monitoring resource, supported by NIH grant P30CA138292.
Contribution: S.V.P. performed experiments, analyzed data, and wrote the manuscript; S.K. performed bioinformatic analyses; M.I.A. and R.R. performed the experiments and analyzed the data; K.L. assisted with specimen collection; N.S.J., J.L.K., C.C.H., A.K.N., V.A.G., and S.L. performed clinical research, analyzed the data, and reviewed the manuscript; K.M.D. and M.V.D. designed and supervised the project, analyzed the data, and wrote the first draft of the manuscript; and all authors reviewed and approved the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Madhav V. Dhodapkar, Emory University, 1365 Clifton Rd, Atlanta, GA 30322; email: madhav.v.dhodapkar@emory.edu; and Kavita M. Dhodapkar, Emory University, 1365 Clifton Rd, Atlanta, GA 30322; email: kavita.dhodapkar@emory.edu.
References
Author notes
K.M.D. and M.V.D. contributed equally to this study.
Deidentified primary data linked to this study are available on reasonable request from the corresponding authors, Madhav V. Dhodapkar (madhav.v.dhodapkar@emory.edu) and Kavita M. Dhodapkar (kavita.dhodapkar@emory.edu).
The full-text version of this article contains a data supplement.