TO THE EDITOR:
While hematopoietic cell transplantation (HCT) is often curative for patients with hematological malignancies, significant challenges remain due to long-term side effects from the conditioning regimen and their impact on quality of life. New regimens, including treosulfan (Treo), a busulfan analog, have demonstrated effectiveness while having a favorable acute toxicity profile.1,2
A study using Treo/fludarabine in combination with total body irradiation (TBI) for patients with matched related (MRD), matched or mismatched unrelated (URD) donors, and those undergoing cord blood transplantation (CBT) began enrolling in 2005.3-5 Short-term clinical outcomes have been reported.3-5 Here we present quality of life data and long-term outcomes from those studies.
This is a retrospective study of all patients who underwent HCT following Treo-based conditioning at the Fred Hutchinson Cancer Center (FHCC) between 2005 and 2019. The study included patients who underwent unrelated 4/6 to 6/6 HLA-matched single or double CBT, MRD, or matched or mismatched URD HCT for hematologic malignancies. Patients gave written consent allowing the use of medical records for research in accordance with the Declaration of Helsinki, and the institutional review board of FHCC approved the study. Patients received Treo IV on days −6 to −4 at doses of 10 or 14 g/m2 per day (total 30 or 42 g/m2) and fludarabine IV on days −6 to −2 at doses of 30 or 40 mg/m2 per day (total 150-200 mg/m2). In all patients but CBT, if HCT-comorbidity index was ≥5, the Treo total dose was 30 g/m2. All CBT patients received a total dose of 42 g/m2. In accordance with findings from a randomized study conducted at our institution,4 a single dose of 2 Gy TBI was added to the conditioning regimen in most patients (n = 255). The preferred source of hematopoietic cells was granulocyte colony-stimulating factor–mobilized peripheral blood stem cells for MRD and URD, but bone marrow was allowed when peripheral blood stem cells were unavailable. Graft-versus-host disease (GVHD) prophylaxis consisted of cyclosporine and mycophenolate mofetil for CBT recipients and tacrolimus and methotrexate for others. Survivors' work status was determined using data from the FHCC database, which was populated with information from annual long-term follow-up unit surveys. The specific question regarding work status on the post-HCT surveys was: “What is the recipient’s current or most recent work status during the reporting period?” The response options were full-time work, part-time work, unemployed, medical disability, or unknown. A question specific to minors was also included for school-age children with the following response options: under school age, part-time or full-time student, or home-schooled. The questions changed slightly during the period studied. Therefore, responses were categorized according to school, work, unemployed, homemaker, medical disability, and none of the above. Percentages of work status categories (full-time work, part-time work, unemployed, retired, and medical disability) are reported at different time points. This study reports 1-year, 3-year, and 5-year data after HCT. In addition, we investigated whether patients developed subsequent malignant neoplasms, whether they or their partners became pregnant, and whether systemic immunosuppressive therapy (IST) was discontinued. Discontinuation of systemic IST was defined as cessation of treatment for at least 6 months after resolution of GVHD. In the absence of GVHD, IST was tapered either per protocol or per treating physicians. Chronic GVHD (cGVHD) was evaluated according to the National Institutes of Health cGVHD consensus criteria.6 Chronic GVHD-free relapse-free survival (CRFS) was defined as the absence of cGVHD requiring IST, relapse, and death. GVHD-free, relapse-free survival (GRFS) was defined as the absence of grades 3 to 4 acute GVHD, cGVHD requiring IST, relapse, and death. Kaplan-Meier or cumulative incidence estimates were used to summarize point estimates of CRFS, GRFS, relapse, RFS, overall survival, and nonrelapse mortality, considering appropriate competing risk.
Overall, 345 patients with a median age at HCT of 50.2 (range, 0.7-70.5) years were included. Nearly half (n = 171) were male and 45 (13%) were pediatric patients with a median age of 8 (range, 0.7-18) years. Most patients had acute myeloid leukemia (n = 186, 54%) or myelodysplastic neoplasm (n = 106, 31%). Most received peripheral blood grafts (n = 199, 58%), followed by cord blood (n = 120, 35%), and bone marrow (n = 26. 8%). About 60% (n = 133) of MRD and URD were cytomegalovirus seronegative. Most patients were White (n = 272, 81.2%)/non-Hispanic or Latino (n = 318, 92.2%). The median follow-up among survivors was about 65 (range, 12-165.7) months (Table 1).
Patient characteristics
| . | N = 345 . | % . |
|---|---|---|
| Age median (range), y | 50.2 (0.7-70.5) | |
| Sex | ||
| Female | 174 | 50.4 |
| Male | 171 | 49.6 |
| Disease | ||
| AML | 186 | 53.9 |
| MDS | 106 | 30.7 |
| ALL | 36 | 10.4 |
| Others∗ | 17 | 4.9 |
| Donor type | ||
| Matched URD | 128 | 37.1 |
| MRD | 85 | 24.6 |
| CB | 120 | 34.8 |
| Mismatched URD | 12 | 3.5 |
| Graft source | ||
| PBSC | 199 | 57.7 |
| CB | 120 | 34.8 |
| BM | 26 | 7.5 |
| GVHD prophylaxis | ||
| CNI + MMF ± other | 221 | 64.8 |
| CNI + MTX ± other | 120 | 35.2 |
| Recipient CMV | ||
| Seropositive | 225 | 65.2 |
| Seronegative | 113 | 32.8 |
| Missing | 7 | 2.0 |
| ABO | ||
| Matched | 127 | 54.0 |
| Major mismatch | 43 | 18.3 |
| Minor mismatch | 51 | 21.7 |
| Bidirectional mismatch | 14 | 6.0 |
| Race | ||
| White | 272 | 78.8 |
| Asian | 37 | 10.7 |
| Other† | 36 | 10.4 |
| Ethnicity | ||
| Not Hispanic or Latino | 318 | 92.2 |
| Hispanic or Latino | 22 | 6.4 |
| Unknown | 5 | 1.4 |
| Follow-up among survivors, median (range), mo | 64.6 (12-165.7) |
| . | N = 345 . | % . |
|---|---|---|
| Age median (range), y | 50.2 (0.7-70.5) | |
| Sex | ||
| Female | 174 | 50.4 |
| Male | 171 | 49.6 |
| Disease | ||
| AML | 186 | 53.9 |
| MDS | 106 | 30.7 |
| ALL | 36 | 10.4 |
| Others∗ | 17 | 4.9 |
| Donor type | ||
| Matched URD | 128 | 37.1 |
| MRD | 85 | 24.6 |
| CB | 120 | 34.8 |
| Mismatched URD | 12 | 3.5 |
| Graft source | ||
| PBSC | 199 | 57.7 |
| CB | 120 | 34.8 |
| BM | 26 | 7.5 |
| GVHD prophylaxis | ||
| CNI + MMF ± other | 221 | 64.8 |
| CNI + MTX ± other | 120 | 35.2 |
| Recipient CMV | ||
| Seropositive | 225 | 65.2 |
| Seronegative | 113 | 32.8 |
| Missing | 7 | 2.0 |
| ABO | ||
| Matched | 127 | 54.0 |
| Major mismatch | 43 | 18.3 |
| Minor mismatch | 51 | 21.7 |
| Bidirectional mismatch | 14 | 6.0 |
| Race | ||
| White | 272 | 78.8 |
| Asian | 37 | 10.7 |
| Other† | 36 | 10.4 |
| Ethnicity | ||
| Not Hispanic or Latino | 318 | 92.2 |
| Hispanic or Latino | 22 | 6.4 |
| Unknown | 5 | 1.4 |
| Follow-up among survivors, median (range), mo | 64.6 (12-165.7) |
ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; BM, bone marrow; CB, cord blood; CMV, cytomegalovirus; CNI, calcineurin inhibitor; MDS, myelodysplastic syndrome; MMF, mycophenolate mofetil; MTX, methotrexate; PBSC, peripheral blood stem cell.
Other: biphenotypic acute leukemia (n = 2), chronic lymphocytic leukemia (n = 1), chronic myeloid leukemia (n = 2), chronic myelomonocytic leukemia (n = 7), leukemia, not otherwise specific (n = 1), myeloproliferative disorder (n = 3), and non-Hodgkin lymphoma (n = 1).
Other: Black or African American (n = 7), American Indian or Alaska Native (n = 7), Native Hawaiian or other Pacific Islander (n = 9), multiple (n = 3), and unknown (n = 10).
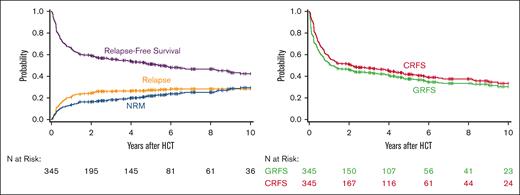
The estimated overall survival at 5 years was 56% (95% confidence interval [CI], 51-61) and RFS was 51% (95% CI, 46-56). The cumulative incidence of relapse at 5 years was 27% (95% CI, 23-32) and nonrelapse mortality was 21% (95% CI, 17-26). The 5-year CRFS was 42% (95% CI, 36-47) and GRFS was 38% (95% CI, 32-43) (Figure 1).
Long-term clinical outcomes in patients receiving a Treo-based conditioning regimen.
Long-term clinical outcomes in patients receiving a Treo-based conditioning regimen.
Regarding work status and other long-term outcomes, we surveyed patients who survived at least 1-year after HCT. Patients were sent an long-term follow-up unit questionnaire annually and results are from 1, 3, and 5 years after HCT. Response rates were 196 out of 259 (76%), 91 out of 185 (49%), and 62 out of 124 (50%), respectively. The distribution of their activities across these time points are as follows: at year 1, 43% reported working, while 34% indicated disability. Others were in school (11%), unemployed (3%), staying at home (7%), or fell under the "other" category (3%). A similar trend was noted at 3 years, but with a slightly higher proportion working (53%). The percentage reporting limitations due to health remained similar at 30%; 7% were in school, 3% unemployed, and 7% at home. These proportions changed little by year 5, when 52% reported working, 32% reported limitations due to health, 6% were in school, 6% were unemployed, and only 2% were staying at home (Figure 1).
At 1, 3, and 5 years after HCT, 85 out of 124 (68%), 36 out of 68 (53%), and 19 out of 54 (35%) patients reported being on immunosuppression. Eighteen patients/partners reported successful pregnancies without assisted methods. Of these 18 patients, the median age at the time of transplant was 40 years (range, 26-52); 9 (50%) patients were female, 10 (55%) had acute myeloid leukemia, 9 (50%) received a matched unrelated donor, 5 (27%) received a CBT, and 15 (83%) received TBI. At the 1-year follow-up, 9 patients (50%) did not have cGVHD, and 5 reported being off IST at the time of completing the questionnaire. Eleven patients reported a new malignancy after HCT.
This large single center study in patients with hematological diseases treated with Treo-based conditioning showed an acceptable long-term survival with favorable CRFS and potentially improved preservation of gonadal function. Our data indicate that Treo is an effective conditioning regimen with long-term outcomes being comparable to previous studies with TBI-based or busulfan-based conditioning regimens.7
However, it is noteworthy that more than one-third of patients reported receiving immunosuppression at 1-year after HCT, a proportion that declined slightly over 3 and 5 years after HCT. Thirty-five percent of patients remained on IST at the 5-year mark which compare relatively favorable to other studies where ∼42% of patients were able to discontinue immunosuppression. Moreover, roughly 37% of those who successfully ceased immunosuppression subsequently required its reinstatement due to the development of GVHD.8 Whether the inclusion of novel GVHD prophylaxis regimens with Treo-based conditioning can improve these outcomes warrants further investigation. In addition, while many patients have returned to work, a significant number continues to face health problems. Approximately one-third of responding patients reported work limitations due medical disability at both 1 and 5 years after HCT. The specific nature of these limitations, whether related to GVHD, organ damage, other physical disabilities, psychological well-being, or additional factors, could not be determined. These outcomes align with other reported findings.9,10
Sexual dysfunction and fertility-related issues are major post-HCT late effects in young patients, particularly women, significantly impacting their quality of life.11-13 The 16 pregnancies observed in our cohort are encouraging, contrasting with the 4 reported pregnancies in a very large registry study following nonmyeloablative HCT.14 While our data are promising, it is important to acknowledge the limitations inherent in relying on self-reported questionnaires, as we lack a reliable means to correlate this information with critical clinical data, such as gonadal function and GVHD status at the time of the pregnancies.
Our results suggest that Treo may serve as a viable alternative to TBI- or busulfan-based conditioning regimens, particularly for patients aiming to preserve fertility; however, this will require confirmation in prospective studies.
Acknowledgments: The authors are grateful to the patients and families who consented to the use of clinical research results.
This work was supported by a research grant from Medexus Pharmaceuticals Inc. F.M. and L.T. were supported by the George and Fay Young Foundation.
Contribution: F.M. and R.S.M. participated in the study design, data analysis, and interpretation of data for the manuscript; T.G. performed the statistical analyses; and H.J.D., C.D., S.J.L., P.V., L.T., A.D., and B.G. provided revisions and critical review of the final manuscript.
Conflict-of-interest disclosure: F.M. received a research grant from and has been part of the advisory board for Medexus Pharmaceuticals Inc. The remaining authors declare no competing financial interests.
Correspondence: Filippo Milano, Translational Science and Therapeutics, Fred Hutchinson Cancer Center, 1100 Fairview Ave N, Seattle, WA 98109; email: fmilano@fredhutch.org.
References
Author notes
A deidentified data set is available on request from the corresponding author, Filippo Milano (fmilano@fredhutch.org).