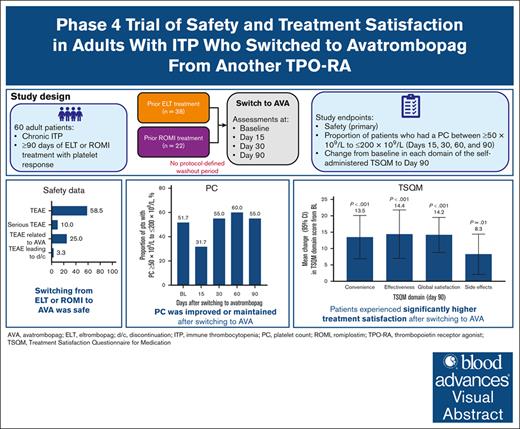
Key Points
Switching to avatrombopag from another TPO-RA was safe in adult patients with ITP.
Patients experienced sustained effectiveness and enhanced overall treatment satisfaction after the switch.
Visual Abstract
This phase 4, multicenter, open-label study was conducted to evaluate the safety, efficacy, and treatment satisfaction of switching to avatrombopag from another thrombopoietin receptor agonist (TPO-RA) in patients with immune thrombocytopenia (ITP). Adults who had received ≥90 days of treatment with eltrombopag or romiplostim and had a response (2 platelet counts [PCs] ≥50 × 109/L) switched to avatrombopag with no protocol-defined washout period. The primary end point was the incidence of treatment-emergent adverse events (TEAEs) and serious TEAEs. Secondary end points were the proportion of patients who had a PC between ≥50 × 109/L and ≤200 × 109/L (days 15, 30, 60, and 90) and change from baseline in each domain of the self-administered Treatment Satisfaction Questionnaire for Medication (TSQM) to day 90. Among 60 enrolled patients, 58.3% experienced TEAEs and 10.0% experienced serious TEAEs (1 related to avatrombopag [thrombocytopenia that resolved]; 5 unrelated [1 unrelated death]). A PC ≥50 × 109/L to ≤200 × 109/L was reported for 51.7%, 31.7% (mean PC, 256.2 × 109/L [standard deviation, 176.7 × 109/L]), 55.0%, 60.0%, and 55.0% at baseline and on days 15, 30, 60, and 90, respectively. TSQM scores increased from baseline to day 90 across all domains (mean change: convenience, +13.5; effectiveness, +14.4; global satisfaction, +14.2; side effects, +8.3). There was no correlation between stable avatrombopag dose (day 90) and previous TPO-RA dose (high or low). Patients with ITP may safely switch from another TPO-RA to avatrombopag and maintain adequate PCs while experiencing improved treatment satisfaction. This trial was registered at www.ClinicalTrials.gov as #NCT04638829
Introduction
Immune thrombocytopenia (ITP) is an autoimmune disease in which patients have a platelet count (PC) <100 × 109/L and an increased risk of bleeding.1 First-line treatments for ITP include corticosteroids, IV immune globulin, and anti-D immune globulin.2 Thrombopoietin receptor agonists (TPO-RAs), 3 of which are approved in both Europe and the United States (romiplostim, eltrombopag, and avatrombopag), are considered second-line therapy after an insufficient response to first-line treatments.2-9 Although romiplostim, eltrombopag, and avatrombopag have each demonstrated durable platelet responses in clinical trials,10-14 differences among these TPO-RAs remain. For example, eltrombopag chelates polyvalent cations and should be taken either without food or with a low-calcium (≤50 mg) meal.6 In addition, routine liver function monitoring is required for patients taking eltrombopag; patients who are Asian and those with liver impairment require dose adjustments.6 In contrast, romiplostim and avatrombopag do not have these restrictions. Route of administration also differs. Both eltrombopag and avatrombopag are administered orally, whereas romiplostim is administered via subcutaneous injection.
Patient satisfaction is recognized as an important clinical outcome, because many health care systems are pivoting toward patient-centered care.15 Satisfaction with TPO-RA treatment among patients with ITP has not been extensively studied, particularly among patients who are taking avatrombopag. The pan-European online survey (Thrombopoietin-Receptor Agonist Patient experience survey [TRAPeze]), which was conducted before the approval of avatrombopag, included questions related to treatment preference regarding TPO-RAs. Overall findings related to TPO-RA treatment preferences from the UK and Republic of Ireland cohort (TRAPeze UK & IE study), The Netherlands cohort (TRAPeze Netherlands study), and the Italy cohort were that patients preferred oral treatment and treatment without food-type restrictions.16-18 These results highlight individual preferences for different characteristics of eltrombopag and romiplostim when considering satisfaction with treatment.
Patients receiving treatment with 1 TPO-RA may choose to switch to another for several reasons such as limiting adverse events (AEs), lack of effectiveness, convenience, or health care insurance coverage.19,20 Previous retrospective studies have shown that most patients with ITP who switched from romiplostim or eltrombopag to avatrombopag for any reason achieved platelet response after switching and that this response was both durable and stable.20,21 However, switching from romiplostim or eltrombopag to avatrombopag has not yet been evaluated in a prospective trial with a large patient population, and treatment satisfaction associated with the switch to avatrombopag has not been previously studied. This study was conducted to evaluate the safety, PC, and patient-reported medication satisfaction in adults with ITP who switched to avatrombopag from eltrombopag or romiplostim.
Methods
Study design
This was a phase 4, prospective, multicenter, open-label study. Patients switched TPO-RA medication to avatrombopag after ≥90 days of treatment with either eltrombopag or romiplostim. To mimic the real-world clinical situation, there was no protocol-defined washout period; however, avatrombopag was not administered on the same day as the patient’s previous TPO-RA medication. Avatrombopag dose was determined by the treating physician in conjunction with the manufacturer’s recommendations. There was no protocol-defined starting dose. A dosing diary in which patients recorded the date and time of each avatrombopag dose was used to monitor treatment compliance.
Patients were screened within the 28 days before or in combination with the baseline visit. Protocol-required clinic visits were at baseline and on days 15, 30, 60, and 90/end of study (EOS). Safety was assessed throughout the study. Treatment-emergent AEs (TEAEs) and serious TEAEs were coded according to the Medical Dictionary for Regulatory Activities version 26.1. The severity of TEAEs (mild, moderate, and severe) and the relationship to avatrombopag were determined according to predefined criteria by the study investigator. AEs of special interest (AESI) were thromboembolic events and bleeding events (bleeding events with AE severity = “Severe”). PCs were measured at each study visit. The Treatment Satisfaction Questionnaire for Medication (TSQM) version 1.4 is a 14-item, validated, self-administered patient-reported outcome questionnaire that measures medication satisfaction in 4 domains: convenience, effectiveness, global satisfaction, and side effects.22 The scoring for each domain ranges from 0 to 100; a higher score indicates greater satisfaction with a medication for the given domain. The TSQM was administered at baseline (before avatrombopag administration) and on days 30 and 90/EOS to assess treatment satisfaction. The TSQM administered at baseline was reflective of treatment satisfaction while receiving the previous TPO-RA, whereas the TSQMs administered on days 30 and 90/EOS reflected treatment satisfaction while receiving avatrombopag. The study duration was a minimum (min) of 90 days from the screening visit to day 90. If a patient terminated study participation early, assessments were completed before study discharge.
Patients
Patients eligible for participation were aged ≥18 years, had been treated for ITP with eltrombopag or romiplostim for ≥90 days, and had a previous response (2 PCs ≥50 × 109/L) to either eltrombopag or romiplostim. Patients were excluded if they were receiving chemotherapy or radiation therapy, had a condition that was likely to prevent them from accurately and reliably completing study self-assessments (eg, evidence of moderate to severe dementia, severe and progressive medical illness), had previously used avatrombopag, had previously participated in the present study, or were enrolled in another clinical study with any investigational drug or device within 30 days of the baseline visit.
This study was conducted in compliance with the protocol and all regulatory requirements and in accordance with Good Clinical Practice, including the International Council for Harmonisation guidelines and the Declaration of Helsinki. The study protocol was approved by the institutional review board at each study site and is available in the supplemental Materials. All patients provided a written informed consent before study enrollment. This study was registered at www.ClinicalTrials.gov (identifier: NCT04638829).
End points
The primary end point was the occurrence of TEAEs and serious TEAEs. This was selected as the primary end point given that there had been no previous prospective clinical trials evaluating the switch to avatrombopag from another TPO-RA. Secondary end points included the proportion of patients who had a PC between ≥50 × 109/L and ≤200 × 109/L on days 15, 30, 60, and 90 and change from baseline in each of the 4 domains of the self-administered TSQM (convenience, effectiveness, global satisfaction, and side effects) to day 90/EOS.
Statistical analysis
The assumed mean TSQM convenience scores at baseline and EOS were 74 and 82, respectively, with a standard deviation (SD) of 19. Based on this assumption, it was determined that 50 patients would provide 83% power to detect a change from baseline of 8 at a 2-sided significance level of .05 using a paired t test. The sample size was set at 100 patients, which was expected to include ∼50 patients in each group with previous eltrombopag or romiplostim treatment.
The total population was defined as all patients who received at least 1 dose of avatrombopag. Data are also presented by previous TPO-RA treatment (eltrombopag and romiplostim). TEAEs are summarized overall and by preferred term. In addition, TEAEs are summarized by severity and relationship to avatrombopag. AESIs are summarized by event type. PC is summarized descriptively and the proportion of patients with a PC between ≥50 × 109/L and ≤200 × 109/L was tabulated using count and percentage at all study visits. Domain scores of convenience, effectiveness, global satisfaction, and side effects were computed according to the TSQM manual, version 1.4 (score range, 0-100 with a higher score indicating a better outcome). Descriptive statistics are summarized for each domain score and each visit. Change from baseline was calculated as (postbaseline score – baseline score); percent change from baseline was calculated as ([postbaseline score – baseline score]/baseline score). Mean change from baseline score was calculated as (sum of change from baseline score of each patient/number of patients). Mean percent change from baseline score was calculated as (sum of the percent change from baseline score of each patient/number of patients). Change from baseline to day 90/EOS was tested using a paired t test for the total population and by previous TPO-RA treatment (eltrombopag vs romiplostim).
A post hoc analysis of TSQM domain score (convenience, effectiveness, global satisfaction, and side effects) changes from baseline to day 90 and PC changes from baseline to day 90, stratified by baseline dose of the previous TPO-RA (≤25 mg [low dose], 26-50 mg [mid dose], and ≥51 mg [high dose] for eltrombopag switchers; and ≤1.5 μg/kg [low dose], 1.6-3.0 μg/kg [mid dose], and ≥3.1 μg/kg [high dose] for romiplostim switchers) was also conducted.
All statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC).
Results
Patients
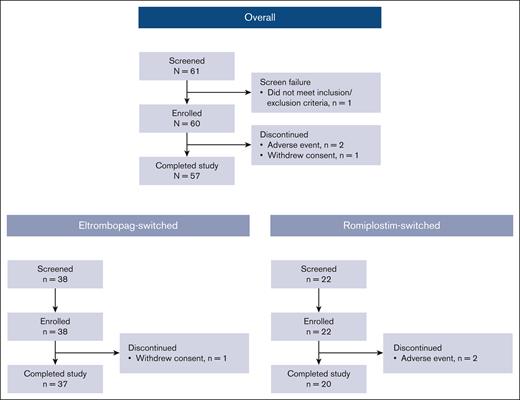
Between 15 March 2021, and 27 September 2023, a total of 61 patients were screened and 60 were enrolled from 19 study sites across the United States (eltrombopag switched, n = 38; romiplostim switched, n = 22; Figure 1). This study was initiated during the COVID-19 pandemic and enrollment was difficult. Enrollment was closed at the discretion of the study sponsor after 60 patients because the objectives of the study had been met. There was no prespecified interim analysis or specific criteria applied to make this decision. Of the 60 enrolled patients, 57 completed the study. Reasons for discontinuation were withdrawal of consent (eltrombopag switched, n = 1) and AE (romiplostim switched, n = 2).
In the total population, the mean age was 58.0 years, 61.7% of patients were female, and most were White (71.7%; Table 1). A history of thromboembolic events was reported for 21.7% of patients. Mean (SD) baseline TSQM domain scores for the total population were 71.8 (21.8), 66.7 (22.3), 69.0 (18.8), and 88.5 (24.1) for convenience, effectiveness, global satisfaction, and side effects, respectively. Most patients (91.7%) had a baseline World Health Organization Bleeding Scale Assessment grade 0 and the median (min-maximum [max]) baseline PC was 138.0 (12-530). The median (min-max) time since ITP diagnosis was 3.58 years (0.2-31.0) and 13.3% had a splenectomy. The mean (SD) duration of previous TPO-RA treatment was 74.3 weeks (87.9) and 135.7 weeks (136.6) for patients who switched from eltrombopag or romiplostim, respectively. Avatrombopag treatment compliance and exposure during the trial are presented in supplemental Table 1. The median (min-max) number of avatrombopag doses was 80.5 (8-180) and the median (min-max) duration of exposure was 90.0 days (20-127). The stable avatrombopag dose (by dose category) and previous TPO-RA treatment are presented in supplemental Table 2.
Demographic and clinical characteristics
| . | Total population (N = 60) . | Eltrombopag switched (n = 38) . | Romiplostim switched (n = 22) . |
|---|---|---|---|
| Age, mean (SD), y | 58.0 (21.7) | 60.5 (21.2) | 53.7 (22.4) |
| Sex, n (%) | |||
| Female | 37 (61.7) | 22 (57.9) | 15 (68.2) |
| Male | 23 (38.3) | 16 (42.1) | 7 (31.8) |
| Race, n (%) | |||
| Asian | 2 (3.3) | 1 (2.6) | 1 (4.5) |
| Black or African American | 5 (8.3) | 3 (7.9) | 2 (9.1) |
| Native Hawaiian or other Pacific Islander | 1 (1.7) | 0 | 1 (4.5) |
| White | 43 (71.7) | 27 (71.1) | 16 (72.7) |
| Multiracial | 1 (1.7) | 0 | 1 (4.5) |
| Unknown | 3 (5.0) | 3 (7.9) | 0 |
| Not reported | 1 (1.7) | 1 (2.6) | 0 |
| Other | 4 (6.7) | 3 (7.9) | 1 (4.5) |
| Ethnicity, n (%) | |||
| Hispanic or Latino | 13 (21.7) | 10 (26.3) | 3 (13.6) |
| Not Hispanic or Latino | 46 (76.7) | 27 (71.1) | 19 (86.4) |
| Not reported | 1 (1.7) | 1 (2.6) | 0 |
| Baseline TSQM domain score, mean (SD) | |||
| Convenience | 71.8 (21.8) | 74.0 (19.4) | 67.9 (25.2) |
| Effectiveness | 66.7 (22.3) | 62.3 (23.3) | 74.0 (18.7) |
| Global satisfaction | 69.0 (18.8) | 66.4 (19.0) | 73.4 (18.1) |
| Side effects | 88.5 (24.1) | 86.7 (27.3) | 91.5 (17.8) |
| Baseline WHO Bleeding Scale,∗n (%) | |||
| Grade 0 | 55 (91.7) | 34 (89.5) | 21 (95.5) |
| Grade 1 | 5 (8.3) | 4 (10.5) | 1 (4.5) |
| Baseline PC, mean (SD), ×109/L | 157.5 (118.2) | 137.7 (112.3) | 191.0 (122.9) |
| Time since ITP diagnosis (on the date of enrollment), median (min-max), y | 3.58 (0.2-31.0) | 3.63 (0.2-31.0) | 2.71 (0.4-21.3) |
| No. of platelet transfusions in the previous 1 y, mean (SD) | 0.85 (2.71) | 0.42 (0.95) | 1.59 (4.25) |
| No. of previous hospitalizations for ITP, mean (SD) | 1.55 (3.71) | 1.08 (1.55) | 2.36 (5.78) |
| No. of previous significant bleeding events | |||
| Mean (SD) | 0.55 (1.65) | 0.29 (0.61) | 1.00 (2.58) |
| No significant bleeds, n (%) | 44 (73.3) | 30 (78.9) | 14 (63.6) |
| 1 significant bleeding event, n (%) | 10 (16.7) | 5 (13.2) | 5 (22.7) |
| 2 significant bleeding events, n (%) | 4 (6.7) | 3 (7.9) | 1 (4.5) |
| 3 significant bleeding events, n (%) | 1 (1.7) | 0 | 1 (4.5) |
| 12 significant bleeding events, n (%) | 1 (1.7) | 0 | 1 (4.5) |
| History of splenectomy | |||
| Yes | 8 (13.3) | 5 (13.2) | 3 (13.6) |
| No | 52 (86.7) | 33 (86.8) | 19 (86.4) |
| History of thromboembolic events | |||
| Yes | 13 (21.7) | 8 (21.1) | 5 (22.7) |
| No | 47 (78.3) | 30 (78.9) | 17 (77.3) |
| Duration of previous TPO-RA treatment, weeks | |||
| Mean (SD) | – | 74.3 (87.9) | 135.7 (136.6) |
| Most recent dose level,† n (%) | |||
| Low | – | 10 (26.3) | 14 (63.6) |
| Medium | – | 20 (52.6) | 4 (18.2) |
| High | – | 7 (18.4) | 1 (4.5) |
| Other | – | 1 (2.6) | 3 (13.6) |
| . | Total population (N = 60) . | Eltrombopag switched (n = 38) . | Romiplostim switched (n = 22) . |
|---|---|---|---|
| Age, mean (SD), y | 58.0 (21.7) | 60.5 (21.2) | 53.7 (22.4) |
| Sex, n (%) | |||
| Female | 37 (61.7) | 22 (57.9) | 15 (68.2) |
| Male | 23 (38.3) | 16 (42.1) | 7 (31.8) |
| Race, n (%) | |||
| Asian | 2 (3.3) | 1 (2.6) | 1 (4.5) |
| Black or African American | 5 (8.3) | 3 (7.9) | 2 (9.1) |
| Native Hawaiian or other Pacific Islander | 1 (1.7) | 0 | 1 (4.5) |
| White | 43 (71.7) | 27 (71.1) | 16 (72.7) |
| Multiracial | 1 (1.7) | 0 | 1 (4.5) |
| Unknown | 3 (5.0) | 3 (7.9) | 0 |
| Not reported | 1 (1.7) | 1 (2.6) | 0 |
| Other | 4 (6.7) | 3 (7.9) | 1 (4.5) |
| Ethnicity, n (%) | |||
| Hispanic or Latino | 13 (21.7) | 10 (26.3) | 3 (13.6) |
| Not Hispanic or Latino | 46 (76.7) | 27 (71.1) | 19 (86.4) |
| Not reported | 1 (1.7) | 1 (2.6) | 0 |
| Baseline TSQM domain score, mean (SD) | |||
| Convenience | 71.8 (21.8) | 74.0 (19.4) | 67.9 (25.2) |
| Effectiveness | 66.7 (22.3) | 62.3 (23.3) | 74.0 (18.7) |
| Global satisfaction | 69.0 (18.8) | 66.4 (19.0) | 73.4 (18.1) |
| Side effects | 88.5 (24.1) | 86.7 (27.3) | 91.5 (17.8) |
| Baseline WHO Bleeding Scale,∗n (%) | |||
| Grade 0 | 55 (91.7) | 34 (89.5) | 21 (95.5) |
| Grade 1 | 5 (8.3) | 4 (10.5) | 1 (4.5) |
| Baseline PC, mean (SD), ×109/L | 157.5 (118.2) | 137.7 (112.3) | 191.0 (122.9) |
| Time since ITP diagnosis (on the date of enrollment), median (min-max), y | 3.58 (0.2-31.0) | 3.63 (0.2-31.0) | 2.71 (0.4-21.3) |
| No. of platelet transfusions in the previous 1 y, mean (SD) | 0.85 (2.71) | 0.42 (0.95) | 1.59 (4.25) |
| No. of previous hospitalizations for ITP, mean (SD) | 1.55 (3.71) | 1.08 (1.55) | 2.36 (5.78) |
| No. of previous significant bleeding events | |||
| Mean (SD) | 0.55 (1.65) | 0.29 (0.61) | 1.00 (2.58) |
| No significant bleeds, n (%) | 44 (73.3) | 30 (78.9) | 14 (63.6) |
| 1 significant bleeding event, n (%) | 10 (16.7) | 5 (13.2) | 5 (22.7) |
| 2 significant bleeding events, n (%) | 4 (6.7) | 3 (7.9) | 1 (4.5) |
| 3 significant bleeding events, n (%) | 1 (1.7) | 0 | 1 (4.5) |
| 12 significant bleeding events, n (%) | 1 (1.7) | 0 | 1 (4.5) |
| History of splenectomy | |||
| Yes | 8 (13.3) | 5 (13.2) | 3 (13.6) |
| No | 52 (86.7) | 33 (86.8) | 19 (86.4) |
| History of thromboembolic events | |||
| Yes | 13 (21.7) | 8 (21.1) | 5 (22.7) |
| No | 47 (78.3) | 30 (78.9) | 17 (77.3) |
| Duration of previous TPO-RA treatment, weeks | |||
| Mean (SD) | – | 74.3 (87.9) | 135.7 (136.6) |
| Most recent dose level,† n (%) | |||
| Low | – | 10 (26.3) | 14 (63.6) |
| Medium | – | 20 (52.6) | 4 (18.2) |
| High | – | 7 (18.4) | 1 (4.5) |
| Other | – | 1 (2.6) | 3 (13.6) |
WHO, World Health Organization.
Grade 0, no bleeding; grade 1, petechial bleeding.
Eltrombopag: low dose, ≤25 mg; medium dose, 50 mg; high dose, ≥75 mg. Romiplostim: low dose, ≤3 μg/kg; medium dose, >3 to <7 μg/kg; high dose, ≥7 μg/kg.
Safety
In the total population, 58.3% of patients (n = 35) experienced at least 1 TEAE and 10.0% (n = 6) experienced at least 1 serious TEAE (related to avatrombopag, n = 1 [thrombocytopenia that resolved]; unrelated to avatrombopag, n = 5; Table 2). One death (sudden death not otherwise specified), which was unrelated to avatrombopag, was reported. AESIs (thromboembolic events and bleeding events) were reported in 1.7% of patients (n = 1). TEAEs related to avatrombopag were reported in 25% of patients (n = 15) and TEAEs that led to treatment discontinuation were reported in 3.3% of patients (n = 2; 1 incidence each of thrombocytopenia and headache).
Summary of safety findings
| . | Total population (N = 60) . | Eltrombopag switched (n = 38) . | Romiplostim switched (n = 22) . |
|---|---|---|---|
| TEAE | 35 (58.3) | 20 (52.6) | 15 (68.2) |
| Serious TEAE | 6 (10.0) | 4 (10.5) | 2 (9.1) |
| Thrombocytopenia | 2 (3.3) | 2 (5.3) | 0 |
| Rectal hemorrhage | 1 (1.7) | 0 | 1 (4.5) |
| Sudden death | 1 (1.7) | 0 | 1 (4.5) |
| Osteoarthritis | 1 (1.7) | 1 (2.6) | 0 |
| Nephrolithiasis | 1 (1.7) | 1 (2.6) | 0 |
| AESI | 1 (1.7) | 0 | 1 (4.5) |
| Thrombotic events | 0 | 0 | 0 |
| Bleeding events | 1 (1.7) | 0 | 1 (4.5) |
| TEAE related to avatrombopag | 15 (25.0) | 9 (23.7) | 6 (27.3) |
| Thrombocytopenia | 2 (3.3) | 1 (2.6) | 1 (4.5) |
| Dyspepsia | 1 (1.7) | 0 | 1 (4.5) |
| Nausea | 1 (1.7) | 1 (2.6) | 0 |
| Vomiting | 1 (1.7) | 1 (2.6) | 0 |
| Fatigue | 2 (3.3) | 0 | 2 (9.1) |
| Influenza-like illness | 1 (1.7) | 0 | 1 (4.5) |
| Edema peripheral | 1 (1.7) | 1 (2.6) | 0 |
| Contusion | 2 (3.3) | 2 (5.3) | 0 |
| PC decreased | 1 (1.7) | 1 (2.6) | 0 |
| PC increased | 1 (1.7) | 1 (2.6) | 0 |
| Dizziness | 1 (1.7) | 0 | 1 (4.5) |
| Headache | 4 (6.7) | 3 (7.9) | 1 (4.5) |
| Hypoesthesia | 1 (1.7) | 0 | 1 (4.5) |
| Paresthesia | 1 (1.7) | 0 | 1 (4.5) |
| Insomnia | 1 (1.7) | 1 (2.6) | 0 |
| Heavy menstrual bleeding | 1 (1.7) | 1 (2.6) | 0 |
| Severe TEAE | 8 (13.3) | 4 (10.5) | 4 (18.2) |
| Thrombocytopenia | 3 (5.0) | 2 (5.3) | 1 (4.5) |
| Rectal hemorrhage | 1 (1.7) | 0 | 1 (4.5) |
| Sudden death | 1 (1.7) | 0 | 1 (4.5) |
| Osteoarthritis | 1 (1.7) | 1 (2.6) | 0 |
| Headache | 1 (1.7) | 0 | 1 (4.5) |
| Nephrolithiasis | 1 (1.7) | 1 (2.6) | 0 |
| TEAE leading to avatrombopag discontinuation | 2 (3.3) | 1 (2.6) | 1 (4.5) |
| Thrombocytopenia | 1 (1.7) | 1 (2.6) | 0 |
| Headache | 1 (1.7) | 0 | 1 (4.5) |
| . | Total population (N = 60) . | Eltrombopag switched (n = 38) . | Romiplostim switched (n = 22) . |
|---|---|---|---|
| TEAE | 35 (58.3) | 20 (52.6) | 15 (68.2) |
| Serious TEAE | 6 (10.0) | 4 (10.5) | 2 (9.1) |
| Thrombocytopenia | 2 (3.3) | 2 (5.3) | 0 |
| Rectal hemorrhage | 1 (1.7) | 0 | 1 (4.5) |
| Sudden death | 1 (1.7) | 0 | 1 (4.5) |
| Osteoarthritis | 1 (1.7) | 1 (2.6) | 0 |
| Nephrolithiasis | 1 (1.7) | 1 (2.6) | 0 |
| AESI | 1 (1.7) | 0 | 1 (4.5) |
| Thrombotic events | 0 | 0 | 0 |
| Bleeding events | 1 (1.7) | 0 | 1 (4.5) |
| TEAE related to avatrombopag | 15 (25.0) | 9 (23.7) | 6 (27.3) |
| Thrombocytopenia | 2 (3.3) | 1 (2.6) | 1 (4.5) |
| Dyspepsia | 1 (1.7) | 0 | 1 (4.5) |
| Nausea | 1 (1.7) | 1 (2.6) | 0 |
| Vomiting | 1 (1.7) | 1 (2.6) | 0 |
| Fatigue | 2 (3.3) | 0 | 2 (9.1) |
| Influenza-like illness | 1 (1.7) | 0 | 1 (4.5) |
| Edema peripheral | 1 (1.7) | 1 (2.6) | 0 |
| Contusion | 2 (3.3) | 2 (5.3) | 0 |
| PC decreased | 1 (1.7) | 1 (2.6) | 0 |
| PC increased | 1 (1.7) | 1 (2.6) | 0 |
| Dizziness | 1 (1.7) | 0 | 1 (4.5) |
| Headache | 4 (6.7) | 3 (7.9) | 1 (4.5) |
| Hypoesthesia | 1 (1.7) | 0 | 1 (4.5) |
| Paresthesia | 1 (1.7) | 0 | 1 (4.5) |
| Insomnia | 1 (1.7) | 1 (2.6) | 0 |
| Heavy menstrual bleeding | 1 (1.7) | 1 (2.6) | 0 |
| Severe TEAE | 8 (13.3) | 4 (10.5) | 4 (18.2) |
| Thrombocytopenia | 3 (5.0) | 2 (5.3) | 1 (4.5) |
| Rectal hemorrhage | 1 (1.7) | 0 | 1 (4.5) |
| Sudden death | 1 (1.7) | 0 | 1 (4.5) |
| Osteoarthritis | 1 (1.7) | 1 (2.6) | 0 |
| Headache | 1 (1.7) | 0 | 1 (4.5) |
| Nephrolithiasis | 1 (1.7) | 1 (2.6) | 0 |
| TEAE leading to avatrombopag discontinuation | 2 (3.3) | 1 (2.6) | 1 (4.5) |
| Thrombocytopenia | 1 (1.7) | 1 (2.6) | 0 |
| Headache | 1 (1.7) | 0 | 1 (4.5) |
Data are presented as n (%).
PCs
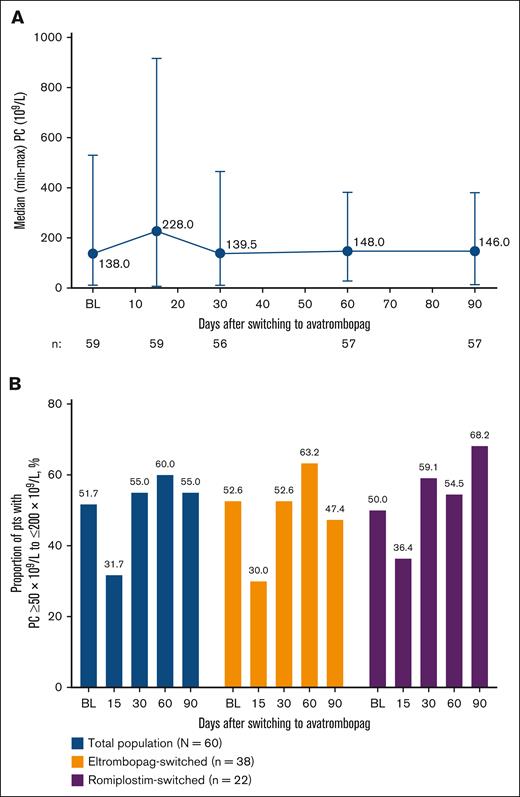
The median (min-max) PC substantially increased from baseline on day 15 (228 × 109/L [8 × 109/L-917 × 109/L]) and then dipped to baseline levels on day 30 (139.5 × 109/L [11 × 109/L-465 × 109/L]) and increased to slightly above baseline on days 60 (148.0 × 109/L [28 × 109/L-382 × 109/L]) and 90 (146.0 × 109/L [14 × 109/L-380 × 109/L]; Figure 2A). Regardless of whether patients were on a low dose, mid dose, or high dose of eltrombopag at baseline before switching, PCs were ≥50 × 109/L at baseline and increased to ≥100 × 109/L on day 90; median (min-max) PCs on day 90 were 182.5 × 109/L (96 × 109/L-297 × 109/L), 155.5 × 109/L (14 × 109/L-347 × 109/L), and 138.0 × 109/L (46 × 109/L-228 × 109/L) for the low-, mid-, and high-dose groups, respectively (supplemental Figure 1A). By baseline dose of romiplostim, all groups had a PC ≥100 × 109/L at baseline. PCs fluctuated over time but maintained ≥75 × 109/L for all groups on day 90, with median (min-max) PCs of 184.0 × 109/L (101 × 109/L-250 × 109/L), 153.0 × 109/L (75 × 109/L-193 × 109/L), and 75.0 × 109/L (27 × 109/L-266 × 109/L) for the high-, mid-, and low-dose groups, respectively (supplemental Figure 1B).
Changes in PCs after switching to avatrombopag. (A) Median PCs from baseline (BL) to day 90 after switching to avatrombopag (total population) and (B) percentage of pts with a PC ≥50 × 109/L to ≤200 × 109/L at BL and on days 15, 30, 60, and 90. pts, patients.
Changes in PCs after switching to avatrombopag. (A) Median PCs from baseline (BL) to day 90 after switching to avatrombopag (total population) and (B) percentage of pts with a PC ≥50 × 109/L to ≤200 × 109/L at BL and on days 15, 30, 60, and 90. pts, patients.
At baseline, 51.7% of patients had a PC ≥50 × 109/L to ≤200 × 109/L; this decreased to 31.7% on day 15 but increased to 55.0% on day 30 and remained stable through EOS (day 60, 60.0%; day 90, 55.0%; Figure 2B). Findings were similar for the eltrombopag-switched and romiplostim-switched subpopulations. In general, the proportion of patients with a PC ≥50 × 109/L to ≤200 × 109/L by baseline eltrombopag or romiplostim dose followed a similar pattern to that of the total population (supplemental Figure 2A-B, respectively).
TSQM
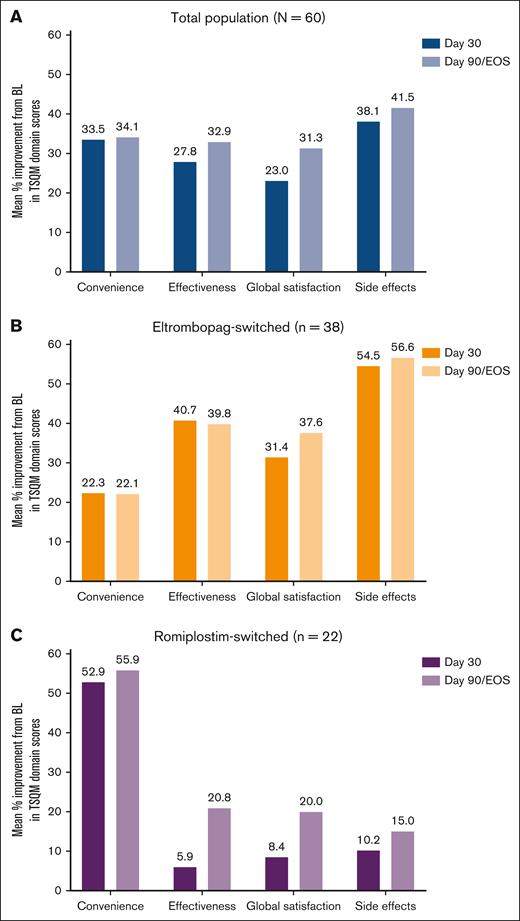
Individual TSQM domain scores at baseline and on days 30 and 90/EOS and change from baseline on days 30 and 90/EOS are presented in Table 3; mean percent improvement from baseline on days 30 and 90/EOS is presented in Figure 3. Although scores at baseline were generally high, increases in TSQM scores were observed across all domains after the switch to avatrombopag. These increases were most pronounced in the effectiveness, convenience, and global satisfaction domains. Improvements began on day 30 and were maintained through day 90/EOS. The increase in satisfaction was greater in the eltrombopag-switched subpopulation for all domains except convenience, which showed a greater increase in satisfaction in the romiplostim-switched subpopulation.
TSQM individual domain baseline, day 30, and day 90/EOS mean values and percent change from baseline after switch to avatrombopag
| . | Total population (N = 60)∗ . | Eltrombopag switched (n = 38)† . | Romiplostim switched (n = 22)‡ . | |||
|---|---|---|---|---|---|---|
| Domain score . | Change from baseline . | Domain score . | Change from baseline . | Domain score . | Change from baseline . | |
| Convenience | ||||||
| Baseline§ | 71.8 (21.8) | – | 74.0 (19.4) | – | 67.9 (25.2) | – |
| Day 30 | 84.9 (15.5) | 13.1 (24.8) | 83.5 (15.4) | 9.5 (23.0) | 87.5 (15.6) | 19.4 (27.2) |
| Day 90 | 85.7 (15.6) | 13.5 (24.7) | 84.2 (14.9) | 9.7 (20.8) | 88.3 (17.0) | 20.3 (29.9) |
| Effectiveness | ||||||
| Baseline§ | 66.7 (22.3) | – | 62.3 (23.3) | – | 74.0 (18.7) | – |
| Day 30 | 76.0 (19.2) | 10.1 (28.5) | 77.9 (18.8) | 16.2 (27.7) | 72.5 (19.9) | −0.6 (27.5) |
| Day 90 | 81.1 (20.4) | 14.4 (27.7) | 80.3 (21.8) | 17.1 (29.5) | 82.5 (18.0) | 9.4 (24.2) |
| Global satisfaction | ||||||
| Baseline§ | 69.0 (18.8) | – | 66.4 (19.0) | – | 73.4 (18.1) | – |
| Day 30 | 77.6 (17.4) | 8.6 (21.8) | 79.6 (17.5) | 12.2 (21.1) | 73.9 (17.1) | 2.1 (22.1) |
| Day 90 | 82.6 (18.6) | 14.2 (20.0) | 82.2 (16.8) | 15.7 (19.3) | 83.2 (22.0) | 11.4 (21.4) |
| Side effects | ||||||
| Baseline§ | 88.5 (24.1) | – | 86.7 (27.3) | – | 91.5 (17.8) | – |
| Day 30 | 93.5 (15.9) | 5.0 (24.8) | 93.1 (16.4) | 6.4 (25.1) | 94.4 (15.2) | 2.5 (24.7) |
| Day 90 | 96.2 (12.3) | 8.3 (22.9) | 94.8 (14.6) | 9.0 (24.8) | 98.8 (5.6) | 6.9 (19.7) |
| . | Total population (N = 60)∗ . | Eltrombopag switched (n = 38)† . | Romiplostim switched (n = 22)‡ . | |||
|---|---|---|---|---|---|---|
| Domain score . | Change from baseline . | Domain score . | Change from baseline . | Domain score . | Change from baseline . | |
| Convenience | ||||||
| Baseline§ | 71.8 (21.8) | – | 74.0 (19.4) | – | 67.9 (25.2) | – |
| Day 30 | 84.9 (15.5) | 13.1 (24.8) | 83.5 (15.4) | 9.5 (23.0) | 87.5 (15.6) | 19.4 (27.2) |
| Day 90 | 85.7 (15.6) | 13.5 (24.7) | 84.2 (14.9) | 9.7 (20.8) | 88.3 (17.0) | 20.3 (29.9) |
| Effectiveness | ||||||
| Baseline§ | 66.7 (22.3) | – | 62.3 (23.3) | – | 74.0 (18.7) | – |
| Day 30 | 76.0 (19.2) | 10.1 (28.5) | 77.9 (18.8) | 16.2 (27.7) | 72.5 (19.9) | −0.6 (27.5) |
| Day 90 | 81.1 (20.4) | 14.4 (27.7) | 80.3 (21.8) | 17.1 (29.5) | 82.5 (18.0) | 9.4 (24.2) |
| Global satisfaction | ||||||
| Baseline§ | 69.0 (18.8) | – | 66.4 (19.0) | – | 73.4 (18.1) | – |
| Day 30 | 77.6 (17.4) | 8.6 (21.8) | 79.6 (17.5) | 12.2 (21.1) | 73.9 (17.1) | 2.1 (22.1) |
| Day 90 | 82.6 (18.6) | 14.2 (20.0) | 82.2 (16.8) | 15.7 (19.3) | 83.2 (22.0) | 11.4 (21.4) |
| Side effects | ||||||
| Baseline§ | 88.5 (24.1) | – | 86.7 (27.3) | – | 91.5 (17.8) | – |
| Day 30 | 93.5 (15.9) | 5.0 (24.8) | 93.1 (16.4) | 6.4 (25.1) | 94.4 (15.2) | 2.5 (24.7) |
| Day 90 | 96.2 (12.3) | 8.3 (22.9) | 94.8 (14.6) | 9.0 (24.8) | 98.8 (5.6) | 6.9 (19.7) |
Data are shown as mean (SD). The scoring scale for each domain of the TSQM ranges from 0 to 100.
Baseline, n = 59; day 30, n = 56; day 90, n = 57.
Baseline, n = 37; day 30, n = 36; day 90, n = 37.
Baseline, n = 22; day 30, n = 20; day 90, n = 20.
Baseline is defined as the last nonmissing assessment done on or before the start of avatrombopag on day 1.
Improvement in baseline TSQM scores in patients that switched to avatrombopag from eltrombopag or romiplostim. Mean percent improvement from BL in TSQM domain scores on days 30 and 90/EOS in the total population (A), eltrombopag-switched subpopulation (B), and romiplostim-switched subpopulation (C).
Improvement in baseline TSQM scores in patients that switched to avatrombopag from eltrombopag or romiplostim. Mean percent improvement from BL in TSQM domain scores on days 30 and 90/EOS in the total population (A), eltrombopag-switched subpopulation (B), and romiplostim-switched subpopulation (C).
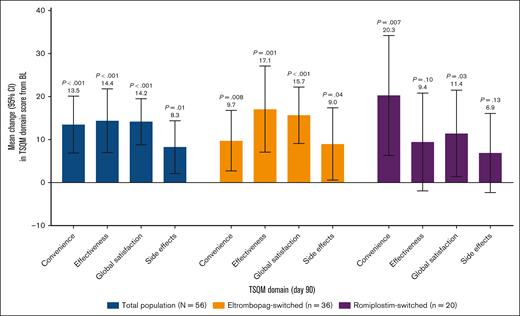
In the total population and eltrombopag-switched subpopulation, scores for all domains had improved significantly from baseline on day 90/EOS (Figure 4). For the romiplostim-switched subpopulation, domain scores for convenience and global satisfaction improved significantly, but not for effectiveness or side effects.
Mean difference in TSQM domain score from BL to day 90 of switching to avatrombopag. The scoring scale for each domain of the TSQM ranges from 0 to 100. Three pts (eltrombopag switched, n = 1; romiplostim switched, n = 2) discontinued the study before day 90 and were excluded from this analysis, and 1 eltrombopag-switched pt was excluded because their BL assessment occurred after switching to avatrombopag. 95% CI, 95% confidence interval.
Mean difference in TSQM domain score from BL to day 90 of switching to avatrombopag. The scoring scale for each domain of the TSQM ranges from 0 to 100. Three pts (eltrombopag switched, n = 1; romiplostim switched, n = 2) discontinued the study before day 90 and were excluded from this analysis, and 1 eltrombopag-switched pt was excluded because their BL assessment occurred after switching to avatrombopag. 95% CI, 95% confidence interval.
Individual TSQM domain scores at baseline and on days 30 and 90/EOS and change from baseline on day 30 and day 90/EOS are presented in supplemental Table 3; mean percent improvement from baseline on day 90/EOS is presented in supplemental Figure 3. Patients who switched from eltrombopag to avatrombopag had higher scores on day 90 than baseline for convenience, global satisfaction, and effectiveness regardless of their baseline dose of eltrombopag. Among patients who switched from romiplostim to avatrombopag, those who had a low dose of romiplostim at baseline had an increased convenience, effectiveness, and global satisfaction score on day 90 whereas the side effects score was unchanged (the score was 100 at baseline and on day 90). Scores for all TSQM domains increased from baseline to day 90 in the mid- and high-dose subgroups.
Discussion
This phase 4, prospective, multicenter, open-label study in adults with ITP who switched to avatrombopag from eltrombopag or romiplostim demonstrates that switching was well tolerated, with a low incidence of treatment discontinuation owing to TEAEs. No patients withdrew for lack of efficacy. After the switch to avatrombopag, the proportion of patients who had a PC ≥50 × 109/L to ≤200 × 109/L decreased from baseline on day 15 but was either improved or maintained compared with baseline on days 30, 60, and 90 of treatment. The decrease on day 15 was likely caused by the increased mean PC of 256.2 × 109/L, which put some patients outside of the higher range. In addition, switching to avatrombopag resulted in higher treatment satisfaction than baseline on day 90 across all domains of the TSQM, despite a high level of preswitch satisfaction. Effectiveness and treatment satisfaction improved from baseline to day 90 regardless of the baseline dose of previous eltrombopag or romiplostim, further reinforcing the sustained effectiveness and enhanced treatment satisfaction after a switch to avatrombopag.
The safety findings of this study were in line with the established safety profile of avatrombopag, with no new safety signals and no previously unreported TEAEs or severe TEAEs. This suggests that there are no unexpected safety concerns with switching to avatrombopag from another TPO-RA in patients with ITP.
Although not a real-world study, the design was intended to mimic the real-world clinical setting where patients would switch immediately between TPO-RAs, without a washout period in between. To date, only retrospective studies of switching between TPO-RAs have been reported.19,20,23-27 A multicenter US study of TPO-RA switching included patients with a ≤1-month gap between treatment with eltrombopag or romiplostim and avatrombopag.20 In general, patients in that study who switched from eltrombopag did so immediately (similar to the present study), whereas those who switched from romiplostim did so ∼7 days after their last dose. In addition, this study included a broader patient population than previous avatrombopag clinical studies, given that patients with previous thromboembolic events were allowed to enroll and there were no exclusions for severe heart disease or concomitant medications.
Previous switching studies have included a proportion of patients who switched TPO-RA treatment because they experienced a lack of response/efficacy with their previous medication.19,20 In the present study, patients were only included if they had a previous response (2 PCs ≥50 × 109/L) to either eltrombopag or romiplostim. Thus, the study findings demonstrate that patients generally responding to their TPO-RA treatment experience continued effectiveness after a switch to avatrombopag.
TSQM domain scores were quite high at baseline (convenience, 71.8; effectiveness, 66.7; global satisfaction, 69.0; side effects, 88.5), indicating that patients were quite satisfied with their previous TPO-RA treatment. Despite this, significant improvement in each of the domain scores was observed 90 days after switching to avatrombopag, indicating enhanced treatment satisfaction with avatrombopag.
This study had a few limitations that should be considered when interpreting the findings. Avatrombopag safety and treatment response were evaluated for 90 days, limiting assessment of the duration of response. However, a previous switching study (from eltrombopag or romiplostim to avatrombopag) found that, among the 93% of patients who responded to avatrombopag, response was maintained for 84% of the time on avatrombopag (median exposure, 9.2 months).20 Patients with no or an inadequate response to eltrombopag or romiplostim were not included. Although it was initially planned to enroll 100 patients in this study, it was conducted during the COVID-19 pandemic and enrollment was closed after 60 patients because the objectives of the study had been met. Currently, there is no defined minimal clinically important difference for the TSQM. As such, the study relied on statistical testing to determine whether changes in the TSQM score were meaningful. Data on dose increases, decreases, or holds were not collected.
Overall, these findings demonstrate that it is safe for patients with ITP to switch to avatrombopag from eltrombopag or romiplostim and that their PCs improve or maintain after the switch. Patients in this study experienced a significant improvement in all 4 TSQM domains, indicating an increase in treatment satisfaction after the switch. Furthermore, in a population of patients who were generally responding to TPO-RA treatment before switching, the improvement or maintenance of PCs, along with the improvement in patient satisfaction across each domain of the TSQM, after the switch to avatrombopag suggests that some patients may experience sustained effectiveness paired with enhanced overall treatment satisfaction when switching from eltrombopag or romiplostim. Patients switching from other TPO-RAs to avatrombopag should start with the label-recommended starting dose (20 mg/d for most patients).8
Acknowledgments
This study was funded by Sobi, Inc (Morrisville, NJ). Medical writing support was provided by Sarah S. Bubeck of BioCentric, Inc (Collingswood, NJ), and funded by Sobi, Inc.
Authorship
Contribution: M.V. and B.D.J. contributed to the study design; B.D.J. was involved in study execution; M.D.T. and K.M. performed the research; J.Z. analyzed the data; M.D.T. and S.K. designed and drafted the manuscript; and all authors contributed equally to the revisions and finalization of this study and read and approved the final manuscript.
Conflict-of-interest disclosure: M.D.T. is the chief medical and chief executive officer of the Bleeding & Clotting Disorders Institute; is the owner and president of Michael D. Tarantino, MD SC, a private medical practice; serves on speakers’ bureaus for Amgen, Grifols, Octapharma, Sanofi, Sobi, and Takeda; and serves as a consultant for Amgen, Genentech, Octapharma, Principia, Sanofi, Sobi, and Takeda. S.K., J.Z., B.D.J., and M.V. are employees of Sobi, Inc (Morrisville, NC). K.M. declares no competing financial interests.
Correspondence: Michael D. Tarantino, Bleeding & Clotting Disorders Institute, 427 W Northmoor Rd, Peoria, IL 61615; email: mtarantino@ilbcdi.org.
References
Author notes
Sobi is committed to responsible and ethical sharing of data on participant level and summary data for medicines and indications approved by European Medicines Agency and/or US Food and Drug Administration, while protecting individual participant integrity and compliance with applicable legislation. With publication, data access will be granted in response to qualified research requests. All requests are evaluated by a cross-functional panel of experts within Sobi, and a decision on sharing will be based on the scientific merit and feasibility of the research proposal, maintenance of personal integrity, and commitment to publication of the results. To request access to study data, a data sharing request form (available at www.sobi.com) should be sent to medical.info@sobi.com. Further information on Sobi’s data sharing policy and process for requesting access can be found at: https://www.sobi.com/en/policies.
The study protocol is included as a data supplement available with the online version of this article.
The full-text version of this article contains a data supplement.