Key Points
No treatment-related serious adverse events occurred during either study and all related treatment-emergent adverse events resolved.
Participants with hemophilia B in the 101HEMB01/101HEMB02 studies failed to achieve plasma FIX levels of 20 IU/dL with DTX101 treatment.
Visual Abstract
Hemophilia B is a rare, X-linked bleeding disorder that predominantly affects males and is caused by factor IX (FIX) gene variants, leading to spontaneous bleeding and impaired ability to clot after injury or surgeries. Standard of care is prophylaxis to increase FIX levels. DTX101 is a nonreplicating adeno-associated viral serotype rh10 gene transfer vector containing a codon-optimized wild-type human FIX coding sequence. The phase 1/2 open-label, single-arm, multicenter, dose-finding 101HEMB01 study examined the safety/efficacy of DTX101 in adult males with hemophilia B; the 101HEMB02 follow-up study assessed long-term outcomes. Participants received DTX101 as 1.6 × 1012 (cohort 1; n = 3) or 5.0 × 1012 genome copies/kg (cohort 2; n = 3) at baseline and were monitored through week 44 (cohort 2) or 52 (cohort 1) in 101HEMB01, and 4 additional years in 101HEMB02. The primary end point of 101HEMB01, peak plasma FIX level at week 6, showed median levels of 7.0 (range, 5.0-8.0) and 10.0 IU/dL (range, 6.0-16.0) in cohorts 1 and 2, respectively. Levels failed to reach the 20 IU/dL target criteria; all participants required adjunct FIX replacement therapy based on low FIX activity at intermediate time points. In 101HEMB01, 4 of 6 participants experienced treatment-related adverse events of elevated transaminase levels (n = 3) and fatigue (n = 1), and 1 experienced fatigue in 101HEMB02; none experienced related serious adverse events. Elevated transaminase levels were asymptomatic and resolved with steroids in all participants. The DTX101 program was halted for insufficient treatment response; however, from its completion, lessons can be learned regarding the design and execution of gene therapy clinical trials, including additional optimization of transgene sequence and immunosuppression protocols. The 101HEMB01 and 101HEMB02 studies were registered at www.ClinicalTrials.gov as #NCT02618915 and #NCT02971969, respectively.
Introduction
Hemophilia B is a an X-linked bleeding disorder that predominantly affects males, resulting from a deficiency or dysfunction of coagulation factor IX (FIX), an essential component of hemostasis,1-3 with a prevalence of ∼1 in 30 000 males.4 Insufficient FIX can lead to spontaneous bleeding, particularly into joints (hemarthrosis), as well as impaired ability to properly clot after injury or surgeries.5 Hemophilia B severity is classified based on the residual level of FIX activity as mild (6-40 IU/dL), moderate (1-5 IU/dL), or severe (<1 IU/dL).5,6 Those with severe hemophilia B (<1 IU/dL FIX) can have recurrent spontaneous hemarthroses or deep-muscle bleeding, typically 2 to 5 times per month. Recurrent hemarthroses lead to hypertrophic synovitis, progressive osteochondral degradation, and ultimately chronic hemophilic arthropathy, characterized by chronic pain, joint deformity, and reduced mobility, severely affecting their quality of life.7
The current standard of care for the management of severe hemophilia B is prophylaxis aimed at increasing FIX levels sufficiently to prevent bleeding episodes. Therapeutic options include IV injections with FIX concentrates prophylactically to maintain plasma FIX levels above critical thresholds, at least above 1 to 3 IU/dL for standard replacement therapy, with spontaneous bleeding uncommon at FIX levels >15 IU/dL.8 Although this approach is effective in preventing hemorrhage and has significantly improved the clinical management of hemophilia B, it comes with several important limitations. Standard half-life FIX concentrates have relatively short half-lives, requiring infusions 2 to 3 times per week to maintain effective circulating levels of FIX.8 Currently approved extended half-life recombinant FIX concentrates require IV injections every 7 to 14 days.8 FIX replacement therapy can be complicated by the development of inhibitor antibodies in up to 3% of patients, neutralizing the efficacy of the FIX concentrates, requiring alternative procoagulants, bypassing agents, for bleed control.9 Finally, as of 2020, only a small proportion of the global population has access to prophylactic treatments for hemophilia, resulting in a significant worldwide burden of preventable chronic suffering.10 Together, these limitations highlight the unmet need for a novel treatment option for patients with hemophilia B.
Because hemophilia B is caused by defects within a single gene, F9, it is an excellent candidate for gene therapy.11 Gene therapy has been shown to have resulted in sustained FIX levels over the long term, thereby preventing bleeding in patients with severe or moderate hemophilia B.8,12-15 Approaches commonly use recombinant adeno-associated viral (AAV) vectors of varying serotypes to reliably and efficiently introduce therapeutic transgenes into hepatocytes.12 AAV vectors are particularly well suited for hemophilia B gene therapies, because they have particular tropism for hepatocytes in the liver; FIX messenger RNA sequence can be packaged within the AAV vector without the need for truncation, unlike FVIII in hemophilia A gene therapies, and its expression can be regulated through hepatocyte-specific promoter elements.16 In November 2022, the US Food and Drug Administration approved the first gene therapy for the treatment of hemophilia B (etranacogene dezaparvovec; Hemgenix; CSL Behring, King of Prussia, PA), a 1-time, single-dose IV infusion of an AAV5 vector containing a highly active variant (Padua variant) of FIX. Moreover, a second gene therapy has been recently approved (April 2024) for the treatment of hemophilia B in the United States (fidanacogene elaparvovec-dzkt; Beqvez; Pfizer, New York, NY). In light of these approvals, we present here the longer-term outcomes from one of the early programs investigating gene therapy for hemophilia B: DTX101.
DTX101 is a nonreplicating AAV serotype rh10 (AAVrh10) gene transfer vector that contains a codon-optimized wild-type human FIX coding sequence. Gene expression in DTX101 is driven by a liver-specific enhancer and promoter. Notably, in murine models, AAVrh10 has demonstrated liver-specific tropism, strong transduction efficiency, and durability of protein expression, with no adverse histopathological findings in the liver in nonclinical studies.17,18
The phase 1/2 open-label, single-arm, multicenter, dose-finding 101HEMB01 study (ClinicalTrials.gov identifier: NCT02618915) examined the safety and efficacy of DTX101 in adult male patients with moderate to severe hemophilia B. 101HEMB01 was followed by the noninterventional 101HEMB02 study (ClinicalTrials.gov identifier: NCT02971969), which assessed the long-term safety, tolerability, and efficacy of DTX101 in patients who completed the 101HEMB01 study. Here, we present the findings from both the 101HEMB01 and 101HEMB02 studies.
Methods
Study population
Participants were eligible for enrollment in 101HEMB01 if they were males aged ≥18 years with moderate/severe or severe hemophilia B with ≤0.02 IU/dL of normal FIX activity, had at least 3 bleeding episodes per year requiring on-demand FIX treatment or received a prophylactic FIX regimen, had ≥100 exposure days to FIX, had no documented history of anti-FIX antibodies, were willing to stop prophylactic treatment with recombinant FIX at specified time points within the study, and were negative for preexisting neutralizing antibodies directed against the AAVrh10 vector capsid. To be eligible for 101HEMB02, participants had to have completed the 101HEMB01 study through the week 44 (cohort 2) or 52 (cohort 1) visit. Participants were ineligible for the study if they had a history of liver disease or had significant hepatic inflammation or cirrhosis (as evidenced by aspartate transaminase [AST] and alanine aminotransferase [ALT] >2× the upper limit of normal, total bilirubin >1.5× the upper limit of normal, alkaline phosphatase >2.5× the upper limit of normal, platelet count <75 × 103/μL, or prothrombin time or international normalized ratio >1.5× the upper limit of normal). In addition, participants were ineligible if they had a history of HIV infection and any of the following: CD4+ cell count <350 cells per mm3, change in antiretroviral therapy regimen within 6 months of study start, or plasma viral load >200 copies/mL on 2 separate occasions, as measured by polymerase chain reaction. All participants provided written informed consent.
Study design
The 101HEMB01 study was a phase 1/2 open-label, single ascending dose–finding trial of DTX101 (AAVrh10FIX) in participants with moderate/severe to severe hemophilia B. At the start of the study, participants received a single IV infusion of DTX101 at a dose level of either 1.6 × 1012 genome copies/kg (cohort 1) or 5.0 × 1012 genome copies/kg (cohort 2). Participants in cohorts 1 and 2 were monitored for 52 or 44 weeks, respectively, after DTX101 administration to determine the safety and efficacy of treatment.
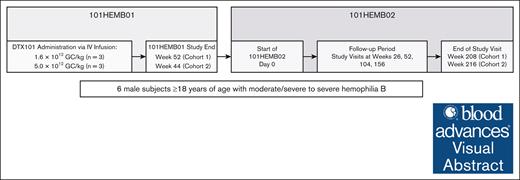
After completing the 101HEMB01 study, participants were eligible to enter the noninterventional follow-up 101HEMB02 study, which evaluated the long-term safety, tolerability, and efficacy of DTX101 in this population. In 101HEMB02, participants were followed for a minimum of 4 additional years, providing a total of 5 years of follow-up after DTX101 administration (Figure 1).
Study design. Design of the phase 1/2 open-label, single ascending dose–finding 101HEMB01 study of DTX101, and the noninterventional follow-up 101HEMB02 study.
Study design. Design of the phase 1/2 open-label, single ascending dose–finding 101HEMB01 study of DTX101, and the noninterventional follow-up 101HEMB02 study.
If, at any point during the study, a participant experienced bleeding episodes frequently, they could return to prophylactic FIX rescue therapy at the investigator’s discretion. However, washout periods preceded data collection visits, during which participants were required to refrain from prophylactic FIX therapy. The duration of these washout periods depended on the type of prophylactic FIX therapy: 21 days for long-acting forms and 7 days for traditional forms.
Stopping criteria leading to suspension of enrollment included the death of a participant considered by the investigator to be related to DTX101, a fivefold increase from baseline in ALT within the first 8 weeks after DTX101 administration, grade 2 toxicity considered related to DTX101 persisting for >7 days, grade 3 toxicity considered related to DTX101, and occurrence of a malignancy considered related to DTX101. Participants were monitored and followed for vector-induced immune hepatitis. If a >1.5-fold increase in ALT from the participant’s baseline level was observed and considered related to DTX101 treatment, the participant was treated with prednisone per a slightly modified American Association for the Study of Liver Disease guidelines protocol. Participants were given prednisone 60 mg per day for week 1, 40 mg per day for week 2, and 30 mg per day for weeks 3 and 4. Starting at week 5, prednisone was tapered by 5 mg per week until liver enzymes reached baseline levels. Abnormal clinically significant laboratory values, including hematology, coagulation panel, and clinical chemistry, considered related to DTX101 would lead to suspension of the study. If study enrollment was suspended, all participants enrolled would remain in the study and continue to be monitored through their completion or withdrawal from the study.
These studies were designed, conducted, recorded, and reported in accordance with the principles established by the World Medical Association Declaration of Helsinki Ethical Principles for Medical Research Involving Human Patients. Institutional review boards at each site approved the protocols. Investigators obtained written informed consent from each participant. Principal investigators and the sponsor, Dimension Therapeutics (acquired by Ultragenyx Pharmaceutical Inc in November 2017), designed the studies. The sponsors and authors/investigators collected, analyzed, and interpreted the data. Authors vouch for the completeness and accuracy of the data. This article was written by the authors with medical writing support from the sponsors. All authors had access to the data and agreed to publish the article.
Outcome measures
The objective of 101HEMB01 was to determine the dose of DTX101 that achieved or was closest to achieving the target peak FIX activity of ≥20% of normal. The primary efficacy end point of the study was the mean peak plasma FIX level 6 weeks after DTX101 administration, as measured by activated partial thromboplastin time clot-based assay. In 101HEMB02, the primary efficacy end point was the change from baseline (before receiving DTX101) in FIX activity at week 208 (cohort 1) or week 216 (cohort 2), both ±14 days, as determined by activated partial thromboplastin time clot-based assay. In both studies, the primary safety end points were the incidence of treatment-emergent adverse events (AEs; TEAEs) and serious adverse events (SAEs) within each cohort. Key secondary end points of the studies included transaminase levels (AST and ALT) and the development of FIX inhibitors.
Statistical analysis
All analyses are descriptive in nature. Continuous variables are summarized by the number of participants, mean, standard deviation, median, minimum, and maximum values. Categorical variables are summarized by frequency counts and percentages in each category.
Results
Baseline demographics and characteristics
Baseline characteristics of the 6 men (aged ≥18 years) who enrolled in the 101HEMB01 study, are summarized in Table 1. In 101HEMB01, participants were followed for a mean of 408.3 (standard deviation [SD], 23.3) and 342.0 days (SD, 21.6) in cohorts 1 and 2, respectively. All 6 participants continued on to the 101HEMB02 follow-up study, in which they were followed for a mean of 1466.0 (SD, 4.4) and 1517.0 days (SD, 19.1) in cohorts 1 and 2, respectively.
Baseline characteristics and demographics
| Parameter . | Cohort 1 n = 3 . | Cohort 2 n = 3 . | Total N = 6 . |
|---|---|---|---|
| Sex, male, n (%) | 3 (100) | 3 (100) | 6 (100) |
| Age, mean (SD), y | 59.3 (9.3) | 41.3 (11.5) | 50.3 (13.6) |
| Range | 53-70 | 28-48 | 28-70 |
| White, n (%) | 3 (100) | 3 (100) | 6 (100) |
| FIX activity, mean (SD), IU/dL | 1.7 (5.8) | 0.9 (0.7) | 1.3 (0.7) |
| Range | 1-2 | 0.1-1.5 | 0.1-2 |
| Receiving FIX therapy, n (%) | |||
| On demand | 1 (33.3) | 1 (33.3) | 2 (33.3) |
| Prophylactic | 2 (66.6) | 2 (66.6) | 4 (66.6) |
| Annualized bleeding rate, mean (SD) | 11.0 (12.2) | 4.7 (5.5) | 7.8 (9.1) |
| Range | 3-25 | 1-11 | 1-25 |
| Parameter . | Cohort 1 n = 3 . | Cohort 2 n = 3 . | Total N = 6 . |
|---|---|---|---|
| Sex, male, n (%) | 3 (100) | 3 (100) | 6 (100) |
| Age, mean (SD), y | 59.3 (9.3) | 41.3 (11.5) | 50.3 (13.6) |
| Range | 53-70 | 28-48 | 28-70 |
| White, n (%) | 3 (100) | 3 (100) | 6 (100) |
| FIX activity, mean (SD), IU/dL | 1.7 (5.8) | 0.9 (0.7) | 1.3 (0.7) |
| Range | 1-2 | 0.1-1.5 | 0.1-2 |
| Receiving FIX therapy, n (%) | |||
| On demand | 1 (33.3) | 1 (33.3) | 2 (33.3) |
| Prophylactic | 2 (66.6) | 2 (66.6) | 4 (66.6) |
| Annualized bleeding rate, mean (SD) | 11.0 (12.2) | 4.7 (5.5) | 7.8 (9.1) |
| Range | 3-25 | 1-11 | 1-25 |
Primary efficacy end points
At 101HEMB01 study baseline, the median plasma FIX levels were 2.0 (range, 1.0-2.0) and 1.0 IU/dL (range, 0.1-1.5) in cohorts 1 and 2, respectively. By week 6, the primary efficacy end point of the study was not met, with median levels in these groups improving to 7.0 (range, 5.0-8.0) and 10.0 IU/dL (range, 6.0-16.0), respectively, both below the target criteria of 20 IU/dL. At week 208 of the 101HEMB02 study, FIX activity level was 66.0 IU/dL in cohort 1 (n = 1) and a median of 6.0 IU/dL (range, 3.0-14.0) in cohort 2 (n = 3), representing a change from baseline of 64.0 and 5.9 IU/dL, respectively. However, it should be noted that all participants had returned to receiving standard-of-care FIX replacement therapy, starting at weeks 1, 1, and 11 (cohort 1) and 1, 18, and 18 (cohort 2), based on low measured FIX activity at intermediate time points. Detailed synopses of the individual participants, including the timing of FIX replacement therapy, are included as Figure 2. FIX activity level was not assessed at week 156 for 1 participant in cohort 1 and at week 208 for 2 participants in cohort 1, because the participants were unable to return to the study site due to COVID-19 restrictions.
Individual patient outcomes. (A-F) Individualized patient outcomes including the timing of FIX replacement therapy (blue marker), steroid administration (red square), FIX levels (orange square/line), and ALT levels (green circle/line).
Individual patient outcomes. (A-F) Individualized patient outcomes including the timing of FIX replacement therapy (blue marker), steroid administration (red square), FIX levels (orange square/line), and ALT levels (green circle/line).
Primary safety end points
During the 101HEMB01 study, 6 of 6 participants (100%) experienced at least 1 TEAE, with 1 participant experiencing an SAE (Table 2). Of these, 4 participants experienced TEAEs (elevated transaminase levels and fatigue) considered related to DTX101 treatment by study investigators (Table 3). No SAEs reported during the 101HEMB01 study were considered by investigators to be related to DTX101 treatment. Similarly, all participants (100%) experienced at least 1 TEAE during the 101HEMB02 study (Table 2). Of these, 1 TEAE (fatigue) and no SAE were found to be related to DTX101 (Table 3). No participants discontinued from either study due to a TEAE, and no TEAEs resulted in death.
Summary of TEAEs reported during 101HEMB02
| . | Cohort 1 n = 3 . | Cohort 2 n = 3 . | Overall N = 6 . |
|---|---|---|---|
| 101HEMB01 | |||
| TEAE | 3 (100) | 3 (100) | 6 (100) |
| Related TEAE | 1 (33.3) | 3 (100) | 4 (66.7) |
| SAE | 1 (33.3) | 0 | 1 (16.7) |
| Related SAE | 0 | 0 | 0 |
| TEAE leading to discontinuation | 0 | 0 | 0 |
| TEAE leading to death | 0 | 0 | 0 |
| 101HEMB02 | |||
| TEAE | 3 (100) | 3 (100) | 6 (100) |
| Related TEAE | 1 (33) | 0 | 1 (17) |
| SAE | 1 (33) | 1 (33) | 2 (33) |
| Related SAE | 0 | 0 | 0 |
| TEAE leading to discontinuation | 0 | 0 | 0 |
| TEAE leading to death | 0 | 0 | 0 |
| . | Cohort 1 n = 3 . | Cohort 2 n = 3 . | Overall N = 6 . |
|---|---|---|---|
| 101HEMB01 | |||
| TEAE | 3 (100) | 3 (100) | 6 (100) |
| Related TEAE | 1 (33.3) | 3 (100) | 4 (66.7) |
| SAE | 1 (33.3) | 0 | 1 (16.7) |
| Related SAE | 0 | 0 | 0 |
| TEAE leading to discontinuation | 0 | 0 | 0 |
| TEAE leading to death | 0 | 0 | 0 |
| 101HEMB02 | |||
| TEAE | 3 (100) | 3 (100) | 6 (100) |
| Related TEAE | 1 (33) | 0 | 1 (17) |
| SAE | 1 (33) | 1 (33) | 2 (33) |
| Related SAE | 0 | 0 | 0 |
| TEAE leading to discontinuation | 0 | 0 | 0 |
| TEAE leading to death | 0 | 0 | 0 |
Data are presented as n (%).
Related TEAEs
| TEAE, n (%) . | Cohort 1 n = 3 . | Cohort 2 n = 3 . | Overall N = 6 . |
|---|---|---|---|
| 101HEMB01 | |||
| Elevated ALT | 0 | 2 (66.7) | 2 (33.3) |
| Elevated aspartate aminotransferase | 0 | 1 (33.3) | 1 (16.7) |
| Elevated transaminases | 0 | 1 (33.3) | 1 (16.7) |
| Fatigue | 1 (33.3) | 0 | 1 (16.7) |
| Total | 1 (33.3) | 3 (100) | 4 (66.7) |
| 101HEMB02 | |||
| Fatigue | 1 (33.3) | 0 | 1 (16.7) |
| Total | 1 (33.3) | 0 | 1 (16.7) |
| TEAE, n (%) . | Cohort 1 n = 3 . | Cohort 2 n = 3 . | Overall N = 6 . |
|---|---|---|---|
| 101HEMB01 | |||
| Elevated ALT | 0 | 2 (66.7) | 2 (33.3) |
| Elevated aspartate aminotransferase | 0 | 1 (33.3) | 1 (16.7) |
| Elevated transaminases | 0 | 1 (33.3) | 1 (16.7) |
| Fatigue | 1 (33.3) | 0 | 1 (16.7) |
| Total | 1 (33.3) | 3 (100) | 4 (66.7) |
| 101HEMB02 | |||
| Fatigue | 1 (33.3) | 0 | 1 (16.7) |
| Total | 1 (33.3) | 0 | 1 (16.7) |
Additional secondary end points
Mean ALT and AST levels rose throughout the 101HEMB01 study after DTX101 administration in both cohorts (Figure 3A-B) and were considered TEAEs in some participants. Numerically greater rises in transaminase levels were observed in cohort 2, in which participants received the higher dose of DTX101. Steroid treatment was initiated in participants with TEAEs of elevated transaminases, leading to resolution. Notably, participants with elevated liver enzymes remained asymptomatic through normalization. Transaminase levels remained within their respective normal ranges throughout the 101HEMB02 study (Figure 3C-D). Notably, the ALT levels of 1 participant in cohort 2 rose sharply after DTX101 administration, peaking at 914 U/L on day 30 (Figure 3F), triggering one of the predefined stopping criteria. This participant remained asymptomatic, was treated with prednisone 60 to 100 mg orally once per day, and their ALT levels returned to normal by day 279.
Measures of liver enzymes (ALT/AST). Mean ALT and AST levels across the 101HEMB01 (A-B) and 101HEMB02 (C-D) studies. Gray markers indicate cohort 1 (DTX101; 1.6 × 1012 genome copies/kg), and orange markers indicate cohort 2 (DTX101; 5.0 × 1012 genome copies/kg).
Measures of liver enzymes (ALT/AST). Mean ALT and AST levels across the 101HEMB01 (A-B) and 101HEMB02 (C-D) studies. Gray markers indicate cohort 1 (DTX101; 1.6 × 1012 genome copies/kg), and orange markers indicate cohort 2 (DTX101; 5.0 × 1012 genome copies/kg).
No clinically meaningful changes were observed in clinical chemistry, hematology, coagulation, or urinalysis parameters or in measurements of vital signs throughout either study (data not shown). Levels of inhibitor to FIX remained low (<0.3 Bethesda units) for all participants at all time points (data not shown), indicating no or minimal immune response to the transgene.
Discussion
The 101HEMB01 study was a phase 1/2, open-label, single-arm, multicenter, dose-finding study designed to determine the safety, tolerability, and efficacy of DTX101 gene therapy in adult men with moderate/severe to severe hemophilia B. All 6 participants completed either 44 or 52 weeks of the 101HEMB01 study before continuing to the 101HEMB02 follow-up study, in which they were monitored for at least 4 additional years.
After DTX101 administration, participants in both the lower- (cohort 1) and higher-dose groups (cohort 2) saw initial improvements in FIX activity levels from predose levels. However, over time, improvements waned, and FIX activity levels were variable for the remainder of the 101HEMB01 study and throughout the 101HEMB02 follow-up study. All participants eventually required a recombinant FIX treatment to maintain FIX activity to sufficiently manage their disease.
Overall safety findings from both studies suggest that DTX101 was generally well tolerated for at least 5 years among these participants. Most reported TEAEs were mild (grade 1) or moderate (grade 2) in severity and included symptoms typical of hemophilia. Four participants in the 101HEMB01 study had TEAEs considered related to DTX101 treatment, consisting of elevated transaminases and fatigue, and 1 participant had a related TEAE of fatigue in 101HEMB02. All related TEAEs resolved by study end. No participants had SAEs considered related to treatment in either study.
Elevated transaminase levels (ALT and AST) are commonly used as biomarkers of hepatotoxicity after liver-targeting gene therapies.19 In this study, mean transaminase levels rose after DTX101 administration in both dose groups, with numerically higher levels in cohort 2 than cohort 1. This observed difference is likely explained, in part, by the higher dose level in these participants but also by a single participant in cohort 2 who had particularly elevated transaminase levels. By day 279, after steroid treatment, the AE of elevated transaminase levels was considered resolved. Despite elevated levels, this participant was asymptomatic and was never hospitalized for the AE. Medical history review did not identify any factors that could have predicted this ALT response, and it is unknown why this occurred. These findings of elevated transaminases are similar to other early studies of gene therapies for hemophilia and other indications in which participants also developed elevated transaminase levels.20-22 These participants also remained asymptomatic, and elevated transaminase levels similarly resolved with steroid use.20-22
Due to the limited ability to raise FIX levels to the target, the DTX101 program was ultimately discontinued after the 101HEMB01 study. Importantly, although one of the safety-stopping criterion was met, the elevated transaminase levels did not produce symptoms in participants and resolved with prednisone administration, and the discontinuation of the program was not due to safety concerns over the therapy. Although it is truly unknown why DTX101 failed to produce sufficient FIX to ameliorate the symptoms of hemophilia B, there are several factors that we hypothesize may have affected its success.
First, the FIX sequence used in DTX101 was designed with a fairly-high CpG content (96 copies). Cell culture studies have suggested that higher CpG content can increase transgene expression by influencing transcriptional activity.23 However, other studies using AAV vector-based transgene expression, including other FIX gene therapies, report loss of transgene expression due to CpG-induced innate immune response.24 Here, vector DNA activates the TLR9-MyD88 pathway, and vector capsid-derived peptides are presented by major histocompatibility complex class 2 molecules, recruiting capsid-specific CD4+ T cells.25 In turn, dendritic cells and capsid-specific CD8+ cytotoxic T lymphocytes eliminate transduced hepatocytes in the liver, reducing transgene expression.25 Indeed, murine studies have suggested that lowering or depleting CpG sequences from vector genome can evade the AAV-directed immune response, resulting in persistent transgene expression.26 Similar studies report increased transgene expression by as much as twofold to threefold in the 24 hours after vector administration.27 Therefore, we hypothesize that the high CpG content in the DTX101 FIX sequence may have resulted in a stronger immune response than expected, thereby limiting transgenic efficiency. It is possible that a revised sequence with lower CpG content would have performed better.
Additionally, elevated transaminase levels, such as those observed within AAV-mediated liver-directed gene therapy for hemophilia, can be accompanied by a reduction in transgene expression. It has been hypothesized that the infusion of the transgenic vector induces the formation of AAV capsid–specific CD8+ T cells, which in turn target transduced hepatocytes for destruction.20 This could reduce the number of transduced cells, which, in turn, leads to reduced transgene expression accompanying the increase in transaminase levels.20 Indeed, participants in this study experienced concurrent decreases in FIX activity approaching baseline levels as transaminase levels rose. The observed decreases in FIX activity were such that they warranted resumption of prophylaxis with recombinant FIX in all participants from both cohorts to ensure that their disease was well controlled. These findings are consistent with previous studies of gene therapy for hemophilia, in which an inverse relationship was reported between FIX activity and transaminase levels.20-22 Participants in these other studies also required the use of prophylactic recombinant FIX, to which they responded well with no reported signs of inhibitor formation.20,22
Although it is not known what truly led to the lack of efficacy of DTX101, there are several measures that could be implemented in future trials to improve treatment efficacy.
One common approach to mute the immune response to gene therapy and enhance efficacy is the use of immunomodulatory agents.19 In this study, the observed spike in FIX activity levels after DTX101 administration was followed closely by elevated transaminase levels. Participants were reactively started on steroid regimens (prednisone) after the detection of elevated transaminase levels, which succeeded in reducing transaminase levels, but could not restore lost transgenic efficacy. Some studies have shown that beginning a steroid regimen within the 48 hours of loss of transgenic FIX activity can prevent further loss of efficacy, but it cannot restore what has already been lost.21,28 Because participants in this study were assessed every 2 weeks for elevated transaminase levels, steroid administration may have come too late to adequately mute an immune response and sustain transgenic activity. Other gene therapy studies conducted after this study used a prophylactic steroid approach with a goal to attenuate the viral-induced hepatitis seen in many AAV-based gene therapies, as summarized by Prasad et al.29 Across these studies, prophylactic steroid administration ahead of or concomitantly with AAV therapy was associated with minimal, if any, impact on transgenic expression, whereas a reactive approach was associated with elevated liver enzymes.29 Future studies could further evaluate a prophylactic approach to immunomodulation, which could potentially maximize the survival and efficacy of transduced hepatocytes.19
Finally, the specific sequence of the FIX gene used in DTX101 is that of the wild type (WT) gene. Research into FIX variants has shown that transgenic sequences with specific amino acid substitutions can confer greater FIX activity than WT.30,31 Perhaps the most heavily researched is the Padua variant (FIX-R338L), which has been shown to produce sixfold to eightfold higher specific activity than WT FIX.31 Notably, the 2 recently approved gene therapies for hemophilia B both use the Padua variant, and despite the amino acid substitution, there were no FIX inhibitors observed in any participants.14
The 101HEMB01 and 101HEMB02 studies were limited in part by the small sample size of 6 participants divided between the 2 dosing groups. Due to the limited efficacy observed in these studies, all participants received additional treatment with recombinant FIX to manage their disease, which altered the measures of FIX activity. Finally, due to the discontinuation of the DTX101 program after the 101HEMB01 study, several additional efficacy end points were not pursued in the 101HEMB02 study. The discontinuation of DTX101 also limited the collection of safety data, namely the longer-term effects of this gene therapy on liver health through hepatic imaging or other methodologies. However, an international registry has now been launched that can enroll participants from past, current, and future gene therapy studies to accumulate continued safety and efficacy data outside of clinical trials.32
Although the loss of efficacy observed in the 101HEMB01 and 101HEMB02 studies ultimately contributed to the discontinuation of the DTX101 program for the treatment of hemophilia, the studies provided several key insights to aid in the development of future gene therapy initiatives.
Acknowledgments
The authors thank Kimo Stine and Charles Hay for their contributions to the DTX101 HEMB01 and HEMB02 studies.
Medical writing support was provided by Jack Pike of Ultragenyx.
Authorship
Contribution: E.C., J.C., and J.A. designed or performed research, analyzed data, and wrote the manuscript; S.S. analyzed data; and S.P., A.P., A.R., and T.E. designed or performed research and wrote the manuscript.
Conflict-of-interest disclosure: S.P. reports consultancy fees from Apcintex/Centessa, ASC Therapeutics, Bayer, BioMarin, CSL Behring, HEMA Biologics, Freeline, LFB, Novo Nordisk, Pfizer, Precision Biosciences, Regeneron/Intellia, Roche/Genentech, Sanofi, Takeda, Spark Therapeutics, and uniQure; research funding from Siemens and YewSavin, Inc.; and scientific advisory board fees from GeneVentiv and Equilibra Bioscience. A.R. reports research support to institution from BioMarin, Dimension Therapeutics, Janssen Momenta Pharmaceuticals, Takeda, and Tremeau Pharmaceuticals. S.S., J.A., J.C., and E.C. are employees/shareholders of Ultragenyx Pharmaceutical Inc. The remaining authors declare no competing financial interests.
The current affiliation for A.P. is Zenas BioPharma, Waltham, MA.
The current affiliation for J.A. is Vertex Pharmaceuticals, Boston, MA.
Correspondence: Steven Pipe, Departments of Pediatrics and Pathology, University of Michigan, D4207 MPB 1500 E Medical Center Dr, Ann Arbor, MI 48109-5718; email: ummdswp@med.umich.edu.
References
Author notes
Data are available upon reasonable request from the corresponding author, Steven Pipe (ummdswp@med.umich.edu).