Key Points
CAR T cells induce a cellular and humoral immunodeficiency resulting in an initial susceptibility for bacterial and later viral infections.
Severe cytokine release syndrome harbors a relevant risk for late-onset infectious complications.
Visual Abstract
Chimeric antigen receptor (CAR) T-cell therapy has demonstrated remarkable efficacy in treating relapsed and refractory (R/R) B-cell neoplasms, such as diffuse large B-cell lymphoma (DLBCL) and multiple myeloma (MM). Despite its success, the long-term effects and sequelae of CAR T cells on the immune system remain underexplored. This study presents a 1-year follow-up analysis of 52 patients (42 with R/R DLBCL and 10 with R/R MM) treated with anti-CD19– and B-cell maturation antigen-targeted CAR T cells, focusing on immune reconstitution and infectious complications. Our findings reveal that CAR T-cell therapy leads to profound depletion of B and T cells. CD4+ T cells and CD19+ B cells exhibited impaired regeneration after treatment. Infections were more frequent during the first 30 days. In the short-term follow-up, density of infections within 100 days at risk was 1.8 in patients with DLBCL and 4.6 in patients with MM, with bacterial infections predominating in this early period after CAR T-cell infusion. In addition, we observed a shift to viral infections in the long-term follow-up, alongside with a decline in infection density to 0.1 in patients with DLBCL and 0.4 infections per 100 days at risk in patients with MM, respectively. Severe cytokine release syndrome was associated with a higher risk of late-onset infections. These findings highlight the importance of close monitoring and prophylactic measures in patients undergoing CAR T-cell therapy to reduce infection risks and enhance immune recovery.
Introduction
Chimeric antigen receptor (CAR) T-cell therapy became a breakthrough for the treatment of relapsed and refractory (R/R) B-cell neoplasia resulting in high response rates and improved long-term disease-free remission.1-3 Owing to their high effectiveness in refractory B-cell neoplasms, the range of indications for CAR T-cell therapy is expanding continuously. There are currently 4 approved anti-CD19 CAR (lisocabtagene maraleucel, tisagenlecleucel, axicabtagene ciloleucel, and brexucabtagene autoleucel) and 2 anti-B-cell maturation antigen (BCMA) CAR T-cell products (idecabtagene vicleucel and ciltacabtagene autoleucel) available in Germany. Beside the classical indications in hematological neoplasms as diffuse large B-cell lymphoma (DLBCL), acute lymphoblastic leukemia, mantle cell lymphoma, follicular lymphoma, and multiple myeloma (MM), new indications in autoimmune diseases, as for example, systemic sclerosis, systemic lupus erythematosus, and antisynthetase syndrome, are rapidly evolving.4,5 These developments have led to rapid increase in patient numbers, but the long-term effects and sequela of CAR T cells on the immune system remain poorly understood. Immune effector cell–associated adverse events such as immune effector cell–associated neurotoxicity syndrome (ICANS), immune effector cell–associated hemophagocytic lymphohistiocytosis-like syndrome, and immune effector cell–associated hematotoxicity (ICAHT), cytokine release syndrome (CRS), and infectious events can cause relevant nonrelapse mortality (NRM) in ∼7% of patients.6 These adverse events are challenging to assess and manage, due to their overlapping symptoms with other pathologies and causes.
The risk of severe infections in these patients is driven by a variety of reasons. Anti-CD19 and BCMA CAR T cells lead as an off-tumor but on-target effect to a profound depletion of B cells, plasma cells, and a subsequent hypogammaglobulinemia.7,8 Furthermore, cytopenia found after CAR T-cell therapy, which is often attributed to an ICAHT, aggravates the underlying immune deficiency in these patients.9,10 Moreover, this immunosuppressed condition is exacerbated by the previous cytotoxic therapies and immunosuppressive agents in the therapy of immune effector cell–associated adverse events, such as interleukin-6 receptor antagonist (eg, tocilizumab) and corticosteroids.11-13 As a result, in a recent study, up to 50% of the NRM is attributed to infectious complications.6
In light of this background, our research aims to explore the short- and long-term immune surveillance against infections after CAR T-cell treatment. Here, we report our results of a 1-year follow-up single-center analysis investigating infectious complications and immune reconstitution after CD19- and BCMA-targeted CAR T-cell therapy in 52 patients with R/R DLBCL and MM.
Methods
Patients and data collection
In this retrospective unicentric observational study, we included all patients with R/R DLBCL and MM who received commercial CAR T-cell products at our institution. No additional exclusion criteria were defined. Overall, we analyzed 42 patients with R/R DLBCL and 10 patients with R/R MM treated at our institution between May 2019 and August 2023. Data were extracted from the electronic medical records. In addition to patient characteristics (Table 1), we analyzed the kinetics of immune reconstitution, including cell counts, lymphocyte subsets (CD19+, CD3+/CD4+, CD3+/CD8+, CD56+, and CAR+), and immunoglobulin levels on days 30, 60, 90, 180, and 360 (supplemental Table 1). We also evaluated characteristics of infectious events, including their foci, pathogens, and onset. The study was approved by the ethical committee of the University of Tübingen (project 771/2023BO2). Patients were censored in case of progressive disease, reaching the end point of a 1-year follow-up or development of a new malignancy. The analysis of absolute neutrophil count (ANC) and immunoglobulin level reconstitution included patients who received granulocyte colony-stimulating factor (G-CSF) or IV immunoglobulins, unless otherwise stated.
Clinical characteristics
| Variable . | N . | DLBCL, N = 42∗ . | MM, N = 10∗ . |
|---|---|---|---|
| Age at CAR T-cell infusion, y | 52 | ||
| Median (IQR) | 65 (48-70) | 69 (65-71) | |
| Number of therapy lines before CAR T-cell infusion | 52 | ||
| 1-3 | 37 (88%) | 2 (20%) | |
| 4-6 | 5 (12%) | 4 (40%) | |
| 7-10 | 0 (0%) | 4 (40%) | |
| Disease status | 52 | ||
| CR | 4 (9.5%) | 0 (0%) | |
| PD | 27 (64%) | 6 (60%) | |
| PR | 7 (17%) | 2 (20%) | |
| SD | 4 (9.5%) | 2 (20%) | |
| ECOG performance status | 52 | ||
| 0 | 18 (43%) | 0 (0%) | |
| 1 | 21 (50%) | 9 (90%) | |
| 2 | 3 (7.1%) | 1 (10%) | |
| CAR T-cell product | 52 | ||
| Idecabtagene vicleucel | 0 (0%) | 9 (90%) | |
| Lisocabtagene maraleucel | 1 (2.4%) | 0 (0%) | |
| Ciltacabtagene autoleucel | 0 (0%) | 1 (10%) | |
| Tisagenlecleucel | 10 (24%) | 0 (0%) | |
| Axicabtagene ciloleucel | 31 (74%) | 0 (0%) | |
| CRS grade | 52 | ||
| Grades 0-1 | 24 (57%) | 5 (50%) | |
| Grade 2 | 14 (33%) | 3 (30%) | |
| Grade 3 | 3 (7.1%) | 2 (20%) | |
| Grade 4 | 1 (2.4%) | 0 (0%) | |
| ICANS grade | 52 | ||
| Grades 0-1 | 28 (67%) | 8 (80%) | |
| Grade 2 | 4 (9.5%) | 1 (10%) | |
| Grade 3 | 7 (17%) | 0 (0%) | |
| Grade 4 | 3 (7.1%) | 1 (10%) | |
| Granulocytes recovery, d | 50 | ||
| Median (IQR) | 13 (10-20) | 11 (10-14) | |
| Not reached (n) | 1 | 1 | |
| Thrombocytes recovery, d | 47 | ||
| Median (IQR) | 0 (0-18) | 7 (0-10) | |
| Not reached (n) | 4 | 1 |
| Variable . | N . | DLBCL, N = 42∗ . | MM, N = 10∗ . |
|---|---|---|---|
| Age at CAR T-cell infusion, y | 52 | ||
| Median (IQR) | 65 (48-70) | 69 (65-71) | |
| Number of therapy lines before CAR T-cell infusion | 52 | ||
| 1-3 | 37 (88%) | 2 (20%) | |
| 4-6 | 5 (12%) | 4 (40%) | |
| 7-10 | 0 (0%) | 4 (40%) | |
| Disease status | 52 | ||
| CR | 4 (9.5%) | 0 (0%) | |
| PD | 27 (64%) | 6 (60%) | |
| PR | 7 (17%) | 2 (20%) | |
| SD | 4 (9.5%) | 2 (20%) | |
| ECOG performance status | 52 | ||
| 0 | 18 (43%) | 0 (0%) | |
| 1 | 21 (50%) | 9 (90%) | |
| 2 | 3 (7.1%) | 1 (10%) | |
| CAR T-cell product | 52 | ||
| Idecabtagene vicleucel | 0 (0%) | 9 (90%) | |
| Lisocabtagene maraleucel | 1 (2.4%) | 0 (0%) | |
| Ciltacabtagene autoleucel | 0 (0%) | 1 (10%) | |
| Tisagenlecleucel | 10 (24%) | 0 (0%) | |
| Axicabtagene ciloleucel | 31 (74%) | 0 (0%) | |
| CRS grade | 52 | ||
| Grades 0-1 | 24 (57%) | 5 (50%) | |
| Grade 2 | 14 (33%) | 3 (30%) | |
| Grade 3 | 3 (7.1%) | 2 (20%) | |
| Grade 4 | 1 (2.4%) | 0 (0%) | |
| ICANS grade | 52 | ||
| Grades 0-1 | 28 (67%) | 8 (80%) | |
| Grade 2 | 4 (9.5%) | 1 (10%) | |
| Grade 3 | 7 (17%) | 0 (0%) | |
| Grade 4 | 3 (7.1%) | 1 (10%) | |
| Granulocytes recovery, d | 50 | ||
| Median (IQR) | 13 (10-20) | 11 (10-14) | |
| Not reached (n) | 1 | 1 | |
| Thrombocytes recovery, d | 47 | ||
| Median (IQR) | 0 (0-18) | 7 (0-10) | |
| Not reached (n) | 4 | 1 |
CR, complete remission; ECOG, Eastern Cooperative Oncology Group; PD, progressive disease; PR, partial remission; SD, stable disease.
n (%).
We assessed early-onset (≤30 days) and late-onset (>30 days) infectious complications.8,14,15 CRS and ICANS grading was assessed according to the American Society for Transplantation and Cellular Therapy (ASTCT) consensus grading.16 ICAHT grading was based on the European Hematology Association/European Society for Blood and Marrow Transplantation (EHA/EBMT) consensus grading.9 Outpatient follow-up appointments after CAR T-cell therapy were conducted twice a week for 4 to 6 weeks and then every 2 weeks. During the first 3 months, appointments were scheduled at least once every 4 weeks. After this period, follow-up appointments were extended to once every 3 months.
Definition of infection
We defined infections as pathological conditions with typical symptoms, supported by laboratory, radiographic, or microbiological evidence. On the basis of this definition, microbiological evidence was not necessary for the diagnosis of an infection if the symptoms and clinical tests were interpreted and treated by the physicians as an infectious complication. Similarly, bacterial infections were evaluated. For example, radiographically confirmed lower respiratory tract infections with a typical bacterial appearance, but lacking microbiological evidence, were included. In contrast, cases of febrile neutropenia without any indication of an infectious cause were not considered infectious complications. Early infections that extended beyond day 30 were also counted as late infections, as the infections persisted despite the usual clearance time or because new additional pathogens were identified. Infection onset was defined as the day on which the diagnostic test was performed, or first typical symptoms occurred. Medical records were analyzed on the evidence for outpatient and inpatient infection treatments outside our institution. Multiple pathogens identified at one time were counted as different events. Fungal infections were assessed in cases of proven or probable invasive disease, which led to an antifungal therapy.17 Colonization or residential flora were not assessed as an active infection, if the patients had no typical infectious symptoms or laboratory or radiographic evidence, which led to an anti-infective therapy. Central line-associated bloodstream infections were defined as microbiologically confirmed primary bloodstream infections in patients with a central line. Incidental bloodstream infections without clinical correlation or without a subsequent microbiological confirmation were not evaluated.
Lymphodepletion
Lymphodepletion was administered in 1 cycle according to manufacturer’s recommendations. Fludarabine 30 mg/m2 (in case of tisagenlecleucel 25 mg/m2) and cyclophosphamide 300 mg/m2 (in cases of axicabtagene ciloleucel 500 mg/m2 and tisagenlecleucel 250 mg/m2) were administered from day −5 to −3 before CAR T-cell application.
Infection prophylaxis and supportive care
Management of prophylactic measures was handled according to the EBMT/EHA CAR T-cell handbook.18 Antimicrobial prophylaxis consisted of acyclovir 400 mg twice daily and trimethoprim/sulfamethoxazole 160/800 mg 3 times a week from start of lymphodepletion. Pneumocystis jirovecii pneumonia and varicella-zoster virus (VZV) prophylaxis was usually continued for 6 months or until the CD4+ count was >200/μL. In case of a high-risk HEMATOTOX score, fluconazole 400 mg daily was administered until the patient was discharged and the ANC was >500/μL. In case of prolonged neutropenia (>3 weeks) or use of high-dose steroids, antifungal prophylaxis was switched to posaconazole. According to our in-house standard, antibacterial prophylaxis was started with ciprofloxacin if the ANC fell <500/μL. The standard empiric treatment for febrile neutropenia was piperacillin and tazobactam. In case of an insufficient recovery of the ANC (<500/μL), G-CSF was administered. Neutrophil recovery was defined, according to the standardized definitions of the ASTCT, as the first day an ANC exceeded 500/μL for 3 consecutive days.19 Serum immunoglobulin G (IgG) levels were evaluated monthly after the CAR T-cell therapy. Immunoglobulin substitution was administered in case of frequent infections requiring antibiotic therapy and/or at IgG concentrations <400 mg/dL.
Tocilizumab was administered in case of a CRS grade >1 or a persistence for >24 hours of a CRS grade 1. The standard dose was 8 mg/kg body weight (maximum 800 mg per application, maximum 4 applications). Corticosteroids were administered in cases of a CRS grade 3 or an ICANS grade 2.
Statistical analysis
Statistical analysis was performed with SPSS Statistics 29 (IBM Corp, Armonk, NY), GraphPad Prism 10 (San Diego, CA), and R (version 4.4.1). For descriptive statistical analysis, absolute and relative frequencies are presented. To identify risk factors for infectious complications, odds ratios (ORs) and 95% confidence intervals (95% CIs) were calculated using logistic regression (univariate and multivariate analyses). End points were defined as an infectious event within the first 30 days and an infectious event between days 30 and 360. To compare CAR T cell, CD4+, and ANC expansion after CAR T-cell treatment, an unpaired 2-sided t test was performed. Cellular reconstitution was analyzed for risk factors using full mixed effects models. Statistical significance was defined as P < .05.
Results
Patient cohort and treatment characteristics
A total of 42 adult patients with a diagnosis of R/R DLBCL (80.7%) and 10 patients with R/R MM (19.3%) were included in this study. The median age of the patients with a DLBCL was 65 years and those with MM was 69 years. Although almost 90% of patients with DLBCL had ≤3 prior therapies, 40% of patients with myeloma had 7 to 10 prior therapy lines (Table 1). In both groups, ∼60% of the patients had progressive disease before lymphodepletion. Higher grade (grade >1) CRS was observed in 42.5% of patients with DLBCL and 50% of patients with MM, whereas ICANS grade ≥1 was found in 33.6% and 20% of these cohorts, respectively (Table 1). The median neutrophil recovery was 13 days in DLBCL and 11 days in patients with MM. In each group, 1 patient did not reach an ANC of 500/μL. Before lymphodepletion, 5.7% of patients had an ANC <500/μL (median ANC in DLBCL [n = 42], 2805/μL [range, 440/μL-10 940/μL]; and median ANC in MM [n = 10], 2695/μL [range, 330/μL-12 370/μL]) and 53.5% had an absolute CD4+ count <200/μL (median CD4+ count in DLBCL [n = 38], 199/μL [range, 24/μL-806/μL]; median CD4+ count in MM [n = 5], 73/μL [range, 37/μL-274/μL]; supplemental Table 1). Besides this cellular immunodeficiency, we observed a deficient humoral immune response in our DLBCL cohort before lymphodepletion. Approximately one-third of the patients had a serum IgG level <400 mg/dL (median IgG in DLBCL [n = 40], 1518 mg/dL; range, 246-1490 mg/dL; supplemental Table 1).
Bacterial infections are the predominant early-onset infectious complications
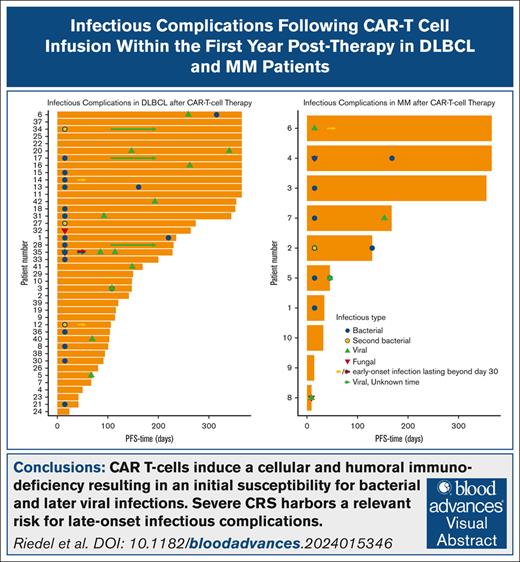
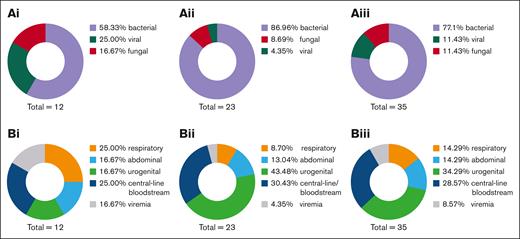
Exactly 80% of patients with MM developed an infection within the first 30 days. Most of these infections were attributed to bacteria (58.3%), followed by viral infections (25.0%; Figure 1Ai). Infection rates in patients with DLBCL seemed to be less frequent with a rate of only 42.9%. Almost all early infections after anti-CD19 CAR T-cell therapy were bacterial (87%; Figure 1Aii). This corresponds to an infection density during the first 30 days after therapy of 1.8 infections per 100 days at risk in patients with DLBCL and 4.6 infections per 100 days at risk in patients with MM. Early-onset infections (<30 days) occurred in 50% of all patients. Most of these infectious complications were attributed to bacteria (approximately 80%; Figure 1Aiii), likely due to the lymphodepletion and ICAHT causing a significant cytopenia within the first 30 days. Most common infection foci after anti-BCMA CAR T-cell therapy were the respiratory tract (25%) and central-line/bloodstream–associated infections (25%; Figure 1Bi). Interestingly, the leading infection foci after anti-CD19 CAR T-cell therapy differ from anti-BCMA CAR T cells. In the anti-CD19–targeted cohort, the most frequent infection foci were urogenital (43.4%) and central line associated (30.4%; Figure 1Bii). According to these results, in the overall cohort, most infections occurred in the urogenital tract (34.3%), followed by central-line infections (28.6%; Figure 1Biii). Frequently identified bacteria were Staphylococcus epidermidis, followed by Enterococcus faecium and Klebsiella pneumoniae. In 2 patients with MM, a cytomegalovirus reactivation was detected, with both developed a higher grade (grade >1) CRS. In total, 4 invasive fungal infections occurred in 4 different patients within the first 30 days after CAR T-cell treatment. We observed 2 patients with DLBCL with evidence of Pichia kudriavzevii in blood cultures and pleural effusion, but without significant antifungal resistance. Furthermore, 2 patients tested positive for Candida antigen, prompting antifungal therapy with caspofungin. Of them, 3 were under antifungal prophylactic measures with fluconazole due to high-risk HEMATOTOX scoring. All these patients developed a CRS or ICANS and were treated with tocilizumab and steroids as well. None of these patients succumbed from the fungal infection.
Early-onset pathogens and foci following CAR T-cell therapy. (A) Early-onset infections and (B) infection foci after (i) anti-BCMA CAR T-cell therapy (n = 10), (ii) anti-CD19 CAR T-cell therapy (n = 42), and (iii) overall cohort receiving either CAR T-cell therapy (n = 52).
Early-onset pathogens and foci following CAR T-cell therapy. (A) Early-onset infections and (B) infection foci after (i) anti-BCMA CAR T-cell therapy (n = 10), (ii) anti-CD19 CAR T-cell therapy (n = 42), and (iii) overall cohort receiving either CAR T-cell therapy (n = 52).
Pathogen spectrum changes in the long-term follow-up to viral infections
To describe late-onset infectious complications, infections from day 30 to disease progression or a 1-year follow-up were monitored. We detected a shift from bacterial toward viral infections in all 3 cohorts (MM: bacterial infections and viral infections, 50%; DLBCL: bacterial infections, 29.2%, and viral infections, 66.6%; overall: bacterial infections, 33.3%, and viral infections, 63.3%; Figure 2Ai-iii). Infection density during the period from days 30 to 360 declined to 0.1 infections per 100 days in patients with DLBCL and 0.4 infections per 100 days at risk in patients with MM. Moreover, the primary focus in these patients became the respiratory tract with 50% of all infections (Figure 2Bi-iii). VZV was observed in 13% of late-onset infections in patients with DLBCL, whereas severe acute respiratory syndrome coronavirus 2 was evident in 26.0%. All VZV reactivations occurred in patients after cessation of prophylactic acyclovir. These findings highlight the importance of prophylactic measures such as vaccines and medicinal prophylaxis in this patient cohort.
Late-onset pathogens and foci following CAR T-cell therapy. (A) Late-onset infections and (B) long-term foci after (i) anti-BCMA CAR T-cell therapy, (ii) anti-CD19 CAR T-cell therapy, and (iii) overall cohort receiving either CAR T-cell therapy. Respiratory infections increase in the long-term follow-up, whereas urogenital and abdominal foci were observed less frequently. A switch from bacterial infections in the short-term follow-up to mostly viral infections from day 30 to day 360 was observed.
Late-onset pathogens and foci following CAR T-cell therapy. (A) Late-onset infections and (B) long-term foci after (i) anti-BCMA CAR T-cell therapy, (ii) anti-CD19 CAR T-cell therapy, and (iii) overall cohort receiving either CAR T-cell therapy. Respiratory infections increase in the long-term follow-up, whereas urogenital and abdominal foci were observed less frequently. A switch from bacterial infections in the short-term follow-up to mostly viral infections from day 30 to day 360 was observed.
Analysis of risk factors for early- and late-onset infectious complications
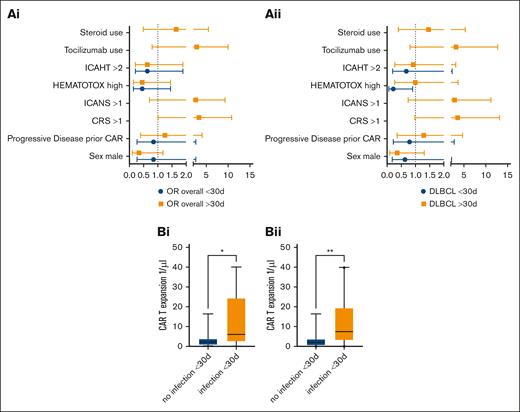
Severe CRS (grade >1) was associated with late infectious complications in the overall cohort (univariate analysis: OR, 3.31; 95% CI, 1.01-10.83; Figure 3Ai). Strikingly, ICANS grading >1, tocilizumab use, and steroid use were not significantly associated with a higher risk of infection in the long-term follow-up. CRS and ICANS occurred in every case before late-onset infectious complications. In the DLBCL group, statistical significance was not reached for CRS grading >1 (OR, 3.59; 95% CI, 0.98-13.16; Figure 3Aii). Multivariate analysis did not reveal independent prognostic risk factors. Patients who developed an infection within the first 30 days had a significantly higher CAR expansion at day 30, both in the overall cohort comprising patients with MM and patients with DLBCL (t test; mean, 3.53/μL vs 11.97/μL; P = .009; Figure 3Bi) and in the DLBCL-only cohort (t test; mean, 3.88/μL vs 11.76/μL; P = .04; Figure 3Bii). CRS incidence was balanced in both groups. Neither the ANC (DLBCL: P = .13; overall, P = .71) nor the CD4+ count (DLBCL: P = .65; overall, P = .22) on day 30 had a significant difference depending on previous infections in either group (supplemental Figure 1).
Risk factors for early- and late-onset infectious complications after CAR T-cell therapy. (A) ORs (univariate analysis) for early- and late-onset infectious complications for (i) the overall cohort receiving either CAR T-cell therapy and (ii) the anti-CD19 CAR T-cell cohort. Whiskers indicate 5th to 95th percentile. (Bi) CAR expansion at day 30 in the MM and DLBCL cohort (t test; mean, 3.53 vs 11.97; P = .009). Whiskers indicate 5th to 95th percentile. (Bii) CAR expansion in patients with DLBCL depending on infectious events within the first 30 days (t test; mean, 3.73 vs 12.58; P = .02).
Risk factors for early- and late-onset infectious complications after CAR T-cell therapy. (A) ORs (univariate analysis) for early- and late-onset infectious complications for (i) the overall cohort receiving either CAR T-cell therapy and (ii) the anti-CD19 CAR T-cell cohort. Whiskers indicate 5th to 95th percentile. (Bi) CAR expansion at day 30 in the MM and DLBCL cohort (t test; mean, 3.53 vs 11.97; P = .009). Whiskers indicate 5th to 95th percentile. (Bii) CAR expansion in patients with DLBCL depending on infectious events within the first 30 days (t test; mean, 3.73 vs 12.58; P = .02).
CAR T-cell therapy leads to profound depletion of B and T cells
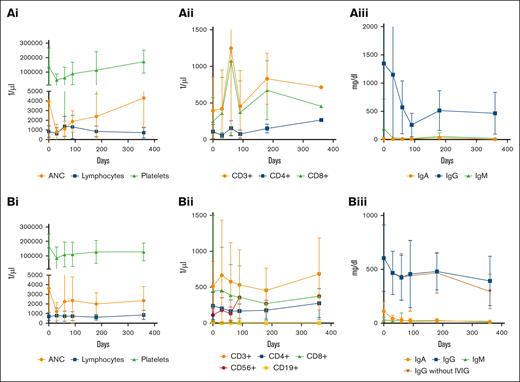
Median neutrophil counts demonstrated an adequate recovery at day 90 (DLBCL median [interquartile range (IQR)], 1710/μL [1170-2410/μL]; MM median [IQR], 1930 [970-2370/μL]), day 180 (DLBCL median [IQR], 1980/μL [1210-2600/μL]; MM median [IQR], 1180/μL [900-5080/μL]), and day 360 (DLBCL median [IQR], 1900/μL [1520-2870/μL]; MM median [IQR], 4305 [2110-6500/μL]), whereas absolute lymphocyte counts remained deficient at these time points (DLBCL: 660/μL [510-820/μL], 520/μL [395-835/μL], and 655/μL [490-1320/μL]; MM: 1040/μL [320-1850/μL], 900/μL [260-1350/μL], and 705/μL [310-1100/μL], respectively; supplemental Table 1; Figure 4Ai,Bi). These results are reflected in the composition of lymphocyte subsets after anti-CD19 CAR T-cell therapy. We observed a persistent deficiency of CD4+ T cells (median [IQR]: 162/μL [101-214/μL], 126/μL [83-270/μL], and 208/μL [110-483/μL]) and CD19+ B cells (median, 0/μL [0/μL]) at 90, 180, and 360 days after infusion, respectively (supplemental Table 1; Figure 4Bi-ii). Surprisingly, CD8+ T-cell and CD56+ lymphocyte counts did not demonstrate deficient values. Median CD8+ T-cell counts were 234.5/μL (IQR, 74-496/μL), 230/μL (105-375μL), and 262/μL (119-627/μL) at 3, 6, and 12 months, respectively (supplemental Table 1; Figure 4Aii,Bii). We also observed a persistent deficiency in IgA, IgM, and IgG levels without substitution (supplemental Table 1; Figure 4Biii). Despite frequent preemptive IV immunoglobulin substitution in 50% of the patients 6 months after treatment, IgG levels were below 400 mg/dL in 25% of the patients with DLBCL (median, 510 mg/dL; IQR, 377-608 mg/dl; supplemental Table 1; Figure 4Aiii,Biii).
Cellular and humoral reconstitution within the first year after CAR T-cell treatment. Reconstitution of the hematopoiesis after CAR T-cell therapy for (Ai) MM and (Bi) DLBCL (whiskers indicate SD). A deficiency of CD4+, CD8+, and CD19+ cells was observed in the first year in patients treated with (Aii) anti-BCMA and (Bii) anti-CD19 CAR T cells. Course of immunoglobulin reconstitution after CAR T-cell therapy (whiskers indicate SD) for (Aiii) MM and (Biii) DLBCL. Patients were included in the analysis until progressive disease or reaching the end point of 1 year after CAR T-cell therapy. All data were included in the analysis, containing patients who received G-CSF or IVIG. IVIG, IV immunoglobulin.
Cellular and humoral reconstitution within the first year after CAR T-cell treatment. Reconstitution of the hematopoiesis after CAR T-cell therapy for (Ai) MM and (Bi) DLBCL (whiskers indicate SD). A deficiency of CD4+, CD8+, and CD19+ cells was observed in the first year in patients treated with (Aii) anti-BCMA and (Bii) anti-CD19 CAR T cells. Course of immunoglobulin reconstitution after CAR T-cell therapy (whiskers indicate SD) for (Aiii) MM and (Biii) DLBCL. Patients were included in the analysis until progressive disease or reaching the end point of 1 year after CAR T-cell therapy. All data were included in the analysis, containing patients who received G-CSF or IVIG. IVIG, IV immunoglobulin.
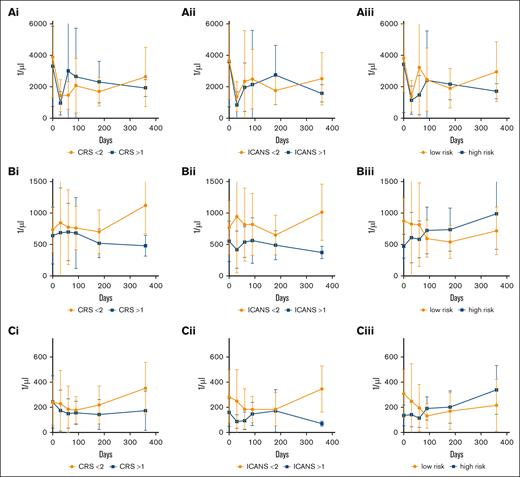
Severe ICANS is associated with lower lymphocyte and CD4+ T-cell counts
To investigate whether patient characteristics or preconditions have an influence on immune reconstitution in patients treated with anti-CD19 CAR T cells, we analyzed the course of ANC, lymphocytes, CD4+, and CD8+ T cells, and CD56+ lymphocyte counts. Interestingly, neither sex, >2 previous therapy lines, or CRS had a significant influence on the cellular reconstitution within the first year after treatment (mixed effects model, as shown in Figure 5Ai-Ci). ICANS grading >1 is associated with lower lymphocyte and CD4+ T-cell counts (mixed effects model, column factor: P = .02 and P = .01), but not with lower ANC (Figure 5Aii-Cii). HEMATOTX grading was not associated with the long-term ANC (Figure 5Aiii; P = .19). Unexpectedly, low HEMATOTOX grading was associated with lower lymphocyte and CD4+ counts in the long-term follow-up but not in the short-term follow-up (Figure 5Biii,Ciii; time × column factor; P = .03 and P = .01).
Prognostic factors of cellular reconstituion after CAR T-cell treatment. Reconstitution of (A) ANC, (B) lymphocytes, and (C) CD4+ T cells depending on (i) CRS grading, (ii) ICANS grading, and (iii) HEMATOTOX risk in patients with DLBCL. Squares indicate mean values and whiskers indicate standard deviation. Data were analyzed with a mixed effects model. ICANS grading >1 is associated with lower lymphocyte and CD4+ T-cell counts (column factor, P = .02 and P = .01, respectively). Low HEMATOTOX grading was associated with lower lymphocyte and CD4+ counts in the long-term follow-up (time × column factor, P = .03 and P = .01, respectively).
Prognostic factors of cellular reconstituion after CAR T-cell treatment. Reconstitution of (A) ANC, (B) lymphocytes, and (C) CD4+ T cells depending on (i) CRS grading, (ii) ICANS grading, and (iii) HEMATOTOX risk in patients with DLBCL. Squares indicate mean values and whiskers indicate standard deviation. Data were analyzed with a mixed effects model. ICANS grading >1 is associated with lower lymphocyte and CD4+ T-cell counts (column factor, P = .02 and P = .01, respectively). Low HEMATOTOX grading was associated with lower lymphocyte and CD4+ counts in the long-term follow-up (time × column factor, P = .03 and P = .01, respectively).
Discussion
In this single-center cohort study, we have analyzed 42 patients with R/R DLBCL and 10 patients with R/R MM, all treated with anti-CD19 or anti-BCMA CAR T cells, to investigate infection patterns and characterize immune reconstitution. Early-onset infectious complications were more frequent than late-onset infections in both cohorts, with bacterial infections predominating in the early period after CAR T-cell infusion. In contrast, late-onset infections were characterized by a higher proportion of viral pathogens. Across both timeframes, patients with MM exhibited higher infection densities compared with patients with DLBCL. To assess immune reconstitution, we performed flow cytometric analysis of lymphocyte subpopulations, revealing a profound impairment in CD4+ T-cell and CD19+ B-cell regeneration after treatment. In addition, severe CRS was associated with an increased risk of late-onset infections. These factors can contribute to the critical role of infections as the main driver for NRM. Studies by Cordas Dos Santos et al6 and Lemoine et al20 report that infections account for 50% to 60% of all patient deaths caused not by a relapse of the underlying disease after CAR T-cell therapy. Therefore, more evidence on infection patterns, immune recovery duration, and optimal antimicrobial prophylaxis is urgently needed.
The incidence rates of infections in patients undergoing anti-CD19 CAR T-cell therapy have varied in prospective studies, ranging from 18% to 56%, and in retrospective studies, from 20% to 60%.2,14,15,21-23 Our results align with the upper range of these previously published values, revealing infection rates of 45% in the short-term and 40% in the long-term follow-up. When compared with the study by Hill et al,14 the infection density 30 days after CAR T-cell infusion in our cohort was 1.5 times higher, at 1.8, compared with their reported 1.2 infections per 100 days at risk. However, comparisons between studies investigating infectious complications after CAR T-cell therapy must be approached cautiously, as definitions of infectious events often vary. Furthermore, in the short-term follow-up, CRS remains the most critical differential diagnosis for infectious complications. Distinguishing between these conditions poses a significant challenge in therapy due to their broadly overlapping symptoms.
Studies investigating infection patterns after anti-BCMA CAR T-cell therapy are still limited. Reported incidence rates are ∼50% in a follow-up of 180 days and 360 days, respectively.24,25 In these cohorts, incidence rates seem to be comparable to anti-CD19 CAR T cells. In contrast, our findings indicate higher infection rates, with >70% observed in both the short- and long-term follow-up, resulting in a higher infection density as well. These findings seem well in line with an observed high real-world NRM of almost 15% in the anti-BCMA-targeted CAR T-cell product ciltacabtagene autoleucel.6 Although comparisons are limited due to the small cohort size and the different CAR T-cell constructs used, our study highlights potential differences between CAR T cells targeting different antigens.6
Hematological short- and long-term toxicity of CAR T-cell therapy is expected to differ significantly. Both Hill et al14 and Josyula et al24 reported a shift in infection patterns after CAR T-cell therapies, from bacterial infections in short-term follow-up to viral infections during long-term follow-up. These findings are consistent with the observed deficient reconstitution of CD4+ T cells, CD19+ B cells, and hypogammaglobulinemia at 6 and 12 months after treatment, as previously described by Strati et al.26 Bacterial infections typically occur within the first 30 days after infusion, most likely due to the combination of lympho-depleting chemotherapy and the acute hematotoxicity caused by expanding CAR T cells. Over time, the pathogen spectrum shifts predominantly to viral infections. Our findings support this assumption, as we observed higher CAR T-cell expansion in patients with DLBCL who developed infections within 30 days after infusion. Moreover, we identified CRS grading ≥2 as a risk factor for late-onset infections and ICANS grading ≥2 as a risk factor for a deficient reconstitution of lymphocytes and CD4+ T cells, underlining this theory. However, it is important to mention that hypogammaglobulinemia is also frequently associated with sinopulmonary infections caused by encapsulated bacteria, in patients with primary B-cell deficiencies and patients who underwent allogeneic transplantation.27,28 We suggest that this risk constellation should be also addressed during the follow-up of patients undergoing CAR T-cell therapy.
ICANS grading >1, tocilizumab use, and steroid use were not significantly associated with a higher risk for long-term infectious complications. The discrepancy in risk profiles between CRS and the use of tocilizumab can be attributed to the fact that patients with a prolonged CRS grade 1 (longer than 24 hours) were treated with tocilizumab as well. Overall, 23 patients experienced a severe CRS (grade >1), whereas 33 patients were treated with tocilizumab (Table 1). Owing to the small sample size in this study, significance was not reached for CRS grading as risk factor for impaired immune reconstitution and ICANS grading as risk factor for late infectious complications.
Antifungal prophylaxis for patients undergoing CAR T-cell therapy is a subject of debate.29-32 Although the EBMT guidelines recommend considering antifungal prophylaxis only in cases of severe or prolonged neutropenia, the Spanish Group recommends its use during any period of neutropenia, whereas the ASTCT guideline recommends the use of fluconazole with the beginning of lymphodepletion.29,32,33 Fungal infections occurred less frequently than viral or bacterial complications in our cohort. All 4 early-onset fungal infections were yeast infections (Candida albicans and Pichia kudriavzevii), and 3 of these patients had a high-risk score according to the HEMATOTOX score before lymphodepletion and were prophylactically treated with fluconazole. This suggests that not all patients undergoing CAR T-cell therapy are at risk for fungal infections. We believe that clinical tools such as the HEMATOTOX score and ICAHT grading are valuable for identifying patients who may significantly benefit from antifungal prophylaxis.
Although national and European guidelines exist, the application of prophylaxis varies across transplant centers and is often based on experience from allogeneic hematopoietic cell transplantation.30,33-35 We observed frequent severe acute respiratory syndrome coronavirus 2 infections and VZV reactivations in our long-term follow-up, indicating that prophylactic measures are essential for patients undergoing CAR T-cell therapy. The EHA/EBMT recommendations include VZV prophylaxis with valacyclovir or acyclovir from the start of lymphodepletion to 1 year after CAR T-cell infusion and until the CD4+ count is >200/μL.35 In contrast, best practice guidelines from the ASTCT recommend antiviral prophylaxis against VZV/HSV for at least 6 months.33 Notably, we observed VZV reactivation in 2 of 3 cases after 6 months, and in all these cases, CD4+ counts were >200/μL at the time of the nearest flow cytometric analysis. These findings highlight ongoing uncertainties regarding infection prophylaxis in patients undergoing CAR T-cell therapy. However, this study has several limitations, particularly the retrospective design and the small patient cohort of 10 patients with MM, which should be critically assessed. We believe that prospective and larger studies are needed to better define patient risk groups, optimize prophylactic strategies in this patient cohort, and harmonize prophylactic measures.
This study links immunity markers, immune effector cell–associated adverse events, and CAR expansion to the pathogen spectrum and infection patterns during short- and long-term follow-up. Furthermore, it highlights differences in infection rates associated with different target antigens. In conclusion, patients with R/R DLBCL and R/R MM are at high risk for infections after CAR T-cell treatment. Our study found higher infection rates than previous reports, particularly among patients treated with anti-BCMA CAR T cells. However, a key limitation of our study is the single-center approach with a subsequent small patient number. Infection patterns shift from bacterial, in the short-term, to viral, in the long-term, correlating with a profoundly suppressed immune recovery. Despite existing prophylactic guidelines, uncertainties remain, highlighting the need for further research to better define infection risks and optimize preventive strategies for patients undergoing CAR T-cell therapy.
Acknowledgments
A.R. was supported by the Junior Clinician Scientist Program of the University of Tübingen (524-0-0). J.C.S. is supported by the Medical Innovation through Interdisciplinarity-Clinician Scientist program of the Medical Faculty Tübingen, funded by the Deutsche Forschungsgemeinschaft (DFG; German Research Foundation; grant 493665037). C.L. received funding from the DFG (grant 467578951).
Authorship
Contribution: A.R., L.P., A.M.P.S., and J.F.W. collected the data; A.R., J.F.W., and W.B. analyzed the data; C.L. and W.B. developed the project; A.R., C.L., J.F.W., and W.B. wrote the manuscript; and all authors edited the manuscript.
Conflict-of-interest disclosure: W.B. reports consulting for Janssen. A.R. and B.B. report receiving honoraria from Janssen. The remaining authors declare no competing financial interests.
Correspondence: Andreas Riedel, Department of Internal Medicine II, Hematology, Oncology, Clinical Immunology and Rheumatology, University Hospital Tübingen, Otfried-Müller Str. 10, 72076 Tübingen, Germany; email: andreas.riedel@med.uni-tuebingen.de.
References
Author notes
J.F.W. and W.B. contributed equally to this study.
Data are available on request from the corresponding author, Andreas Riedel (andreas.riedel@med.uni-tuebingen.de).
The full-text version of this article contains a data supplement.