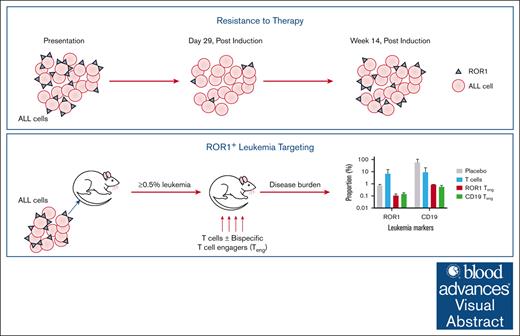
Key Points
ROR1 is overexpressed in pediatric BCP-ALL and T-ALL cells and can be resistant to induction therapy.
Treatment with ROR1 bispecific Teng can reduce disease burden in NSG mice.
Visual Abstract
Receptor tyrosine kinase–like orphan receptor (ROR)1 is overexpressed in some hematological cancers but has low expression in normal tissues, making it a potential therapeutic target. We investigated this therapeutic potential in childhood B-cell precursor (BCP) and T-cell acute lymphoblastic leukemia (T-ALL) cases. The proportion of ROR1+ cells was significantly higher in T-ALL (median, 13.8%; range, 2.9%-87%) than BCP-ALL (6%, 0.3%-83%, P = .02). Antigen density was also lower in BCP-ALL (median, 1027; range, 876-2588) compared to T-ALL (1089, 865-1527). In leukemia propagating cells (LPCs), ROR1 levels were highest in CD34–/CD19+ and CD34–/CD7+ subpopulations. Notably, ROR1+ LPC, in both BCP-ALL and T-ALL, survived induction therapy and their numbers increased post treatment. Subsequently, ROR1 bispecific T-cell engagers (Teng) were tested on primary cases in vitro and in vivo. Addition of ROR1 Teng in vitro reduced ALL survival to 44% in BCP-ALL and 58% in T-ALL, compared to T cells alone (94% and 84%, respectively; P ≤ .01). When NOD.Cg-PrkdcscidIl2rγtm1Wjl/SzJ mice engrafted with primary leukemia were treated with ROR1 Teng, disease burden was reduced by up to 520-fold (from 15.6% to 0.03%) in ROR1+ cells and 68-fold (58% to 0.9%) in CD19+ cells in BCP-ALL. In T-ALL cases, there was a fourfold reduction (from 1.2% to 0.3%) in ROR1+ and 2.3-fold (from 83.7% to 36.7%) in CD7+ levels. This resistance of ROR1+ cells to current therapies makes it an important target. Moreover, as ROR1 Teng were at least comparable to CD19 Teng in vivo, they could be considered for the treatment of refractory BCP-ALL.
Introduction
Progress in the treatmentof childhood acute lymphoblastic leukemia (ALL) has been remarkable in recent decades, with event-free survival rates of over 88% in standard risk B-cell precursor ALL (BCP-ALL) and 85% in T-cell ALL (T-ALL).1,2 However, both treatment resistance and toxicity remain significant hurdles to overcome.3,4 Nevertheless, the field is changing with increased use of immune effector cells and more targeted therapies that aim to reduce adverse effects associated with standard chemotherapy and increase efficacy, especially in refractory disease.5-7
Immunotherapies, such as CD19 bispecific T-cell engagers (Teng) (eg, blinatumomab and ADZ0486) and CD19 chimeric antigen receptor (CAR) T cells, have had a significant impact in pediatric leukemias and more broadly in B-cell malignancies in recent years.6 In children and young adults with refractory or relapsed ALL, blinatumomab has been shown to be more effective and less toxic than intensive chemotherapy.8-11 There is also evidence to support the use of blinatumomab as a replacement for intensive therapy in refractory cases, rather than an additional component.8,10 Although progress in the treatment of BCP-ALL has been remarkable, the same cannot be said for T-ALL, where introduction of new agents has been lacking. In addition, some immunotherapies have severe adverse effects, including cytokine release syndrome and neurotoxicity,12 and crucially, CD19-targeted therapies are ineffective in patients with CD19-negative disease or on CD19– leukemia propagating cells (LPCs).13,14 We have previously shown that some leukemia-associated immunophenotype antigens are not expressed in all LPCs, limiting their potential as therapeutic targets.15,16 Consequently, identifying new potential targets for refractory leukemia is essential.
Receptor tyrosine kinase–like orphan receptor (ROR)1 is a member of the ROR subfamily, belonging to the receptor tyrosine kinases superfamily.17 ROR are receptors for Wnt signaling molecules Wnt5a/b and Wnt16.18 Overexpression of ROR1 in chronic lymphocytic leukemia (CLL), mature B-cell ALL subsets, lung adenocarcinoma, and breast cancer make it a potential therapeutic target.17,19-22 Elevated ROR1 expression in breast cancer and CLL is correlated with severe disease progression and reduced survival.19,22 Previous reports in B-cell ALL have linked overexpression of ROR1 with t(1;19), t(12;21), t(9;22) and KMT2A rearrangements.23,24
Monoclonal antibodies against ROR1 (cirmtuzumab) have shown some promise in phase 1 clinical trials in CLL.25-28 More recently, the development of Teng against ROR1 has shown beneficial preclinical results in CLL and solid tumors, such as pancreatic and breast cancer.29,30 ROR1 Teng were reported to prevent or reduce tumor burden by at least 50% in pancreatic and ovarian tumors in murine xenografts, whereas there was no effect using CD19 Teng. The ROR1 Teng, NVG-111, is currently in phase 1/2 trials for CLL and small lymphocytic lymphoma (ClinicalTrials.gov identifier: NCT04763083).
This study aimed to explore the expression and functional significance of ROR1 in LPC, validated in previous in vivo studies,5,13,15,16,31 in a wide cohort of BCP-ALL and T-ALL cases. The efficacy of ROR1 Teng was subsequently assessed in vitro and in vivo to determine potential for the treatment of childhood ALL.
Methods
Samples
Bone marrow (BM) cells from children (median age, 8 years; range, 1-17) with BCP-ALL (n = 19) or T-ALL (n = 10) were collected at diagnosis on day 29 or week 14 of treatment, with informed consent and approval of University Hospitals Bristol and Weston National Health Service Foundation Trust. The use of human samples was approved by London Brent Research Ethics Committee (12/LO/1193). This study was conducted in accordance with the Declaration of Helsinki. Patient characteristics are shown in Table 1. Patients were treated on the UKALL 2011 protocol and classified as low risk (<0.005% measurable residual disease [MRD] at day 29) or risk (≥0.005% MRD or with KMT2A rearrangements, near/low haploidy, iAMP21 or t[17;19]).32 Patients with postconsolidation MRD levels of ≥0.5% were deemed high risk and those with <0.5% as intermediate risk. Patient 23 (Pt 23) was treated on UKALL2003 and classified as high risk (MRD ≥0.01%, at day 29).33 Normal BM (NBM) and peripheral blood (PB) samples were obtained from healthy donors. Mononuclear cells (MNCs) from normal and ALL samples were isolated by density gradient centrifugation and used either fresh for in vivo assays or cryopreserved prior to use, as previously described.16
Patient characteristics
| Pt . | Subtype . | Karyotype . | Age at diagnosis, y . | Sex . | MRD level at day 29 . | MRD level at week 14 . | MRD risk∗ . |
|---|---|---|---|---|---|---|---|
| 1 | Pre B | t(12;21) | 12 | F | N/A | N/A | Low |
| 2 | Pre B | Hyperdiploid | 3 | F | <0.01% | N/A | Low |
| 3 | Pre B | Hyperdiploid | 1 | F | <0.01% | N/A | Low |
| 4 | Pre B | t(9;14), −20,+21, +3mar | 2 | F | <0.01% | N/A | Low |
| 5 | Pre B | t(12;21) | 6 | M | N/A | N/A | Low |
| 6 | Pre B | 46xx | 17 | F | <0.01% | N/A | Low |
| 7 | Pre B | t(8;11),+1,+7,+9,+12,+21,–3,–14 | 3 | M | <0.01% | <0.01% | Int |
| 8 | Pre B | iAMP21 | 11 | M | <0.01% | <0.01% | Int |
| 9 | Pro B | KMT2A rearr | 17 | F | <0.01% | <0.01% | Int |
| 10 | Pre B | t(12;21),–10,+12,–13,+21,+3mar | 5 | M | 0.14% | N/A | Int |
| 11 | c-ALL | t(9;22) | 5 | M | N/A | N/A | Int |
| 12 | Pre B | 46xx | 16 | F | 0.24% | <0.01% | Int |
| 13 | Pre B | der21, +21 | 11 | F | 0.01% | N/A | Int |
| 14 | Pre B | 46xy | 15 | M | N/A | N/A | Int |
| 15 | Pre B | PDGFRB rearr, −12p | 8 | F | 19.0% | <0.01% | Int |
| 16 | Pre B | t(1;12),+1,der12,+12,+mar | 4 | M | N/A | N/A | Low |
| 17 | Pre B | t(1;19) | 12 | M | N/A | N/A | Low |
| 18 | Pre B | t(1;19) | 15 | F | N/A | N/A | Low |
| 19 | Pre B | t(1;19) | 12 | M | <0.05% | N/A | Low |
| 20 | T-ALL | −6 | 13 | M | 0.03% | <0.01% | Int |
| 21 | T-ALL | der11,+7 | 8 | F | 18.0% | N/A | High |
| 22 | T-ALL | +ABL1 | 10 | M | <0.01% | N/A | Int |
| 23 | T-ALL | 46xy | 13 | M | N/A | N/A | High |
| 24 | T-ALL | RUNX1 rearr | 3 | M | N/A | N/A | ND |
| 25 | T-ALL | t(8;14),–9,i9 | 3 | M | <0.01% | N/A | Low |
| 26 | T-ALL | −4, −9 | 9 | M | <0.01% | N/A | Low |
| 27 | T-ALL | 46xy | 6 | M | 0.06% | <0.01% | Low |
| 28 | T-ALL | t(11;14) | 4 | M | <0.01% | N/A | Low |
| 29 | T-ALL | ND | 13 | M | <0.01% | N/A | Low |
| Pt . | Subtype . | Karyotype . | Age at diagnosis, y . | Sex . | MRD level at day 29 . | MRD level at week 14 . | MRD risk∗ . |
|---|---|---|---|---|---|---|---|
| 1 | Pre B | t(12;21) | 12 | F | N/A | N/A | Low |
| 2 | Pre B | Hyperdiploid | 3 | F | <0.01% | N/A | Low |
| 3 | Pre B | Hyperdiploid | 1 | F | <0.01% | N/A | Low |
| 4 | Pre B | t(9;14), −20,+21, +3mar | 2 | F | <0.01% | N/A | Low |
| 5 | Pre B | t(12;21) | 6 | M | N/A | N/A | Low |
| 6 | Pre B | 46xx | 17 | F | <0.01% | N/A | Low |
| 7 | Pre B | t(8;11),+1,+7,+9,+12,+21,–3,–14 | 3 | M | <0.01% | <0.01% | Int |
| 8 | Pre B | iAMP21 | 11 | M | <0.01% | <0.01% | Int |
| 9 | Pro B | KMT2A rearr | 17 | F | <0.01% | <0.01% | Int |
| 10 | Pre B | t(12;21),–10,+12,–13,+21,+3mar | 5 | M | 0.14% | N/A | Int |
| 11 | c-ALL | t(9;22) | 5 | M | N/A | N/A | Int |
| 12 | Pre B | 46xx | 16 | F | 0.24% | <0.01% | Int |
| 13 | Pre B | der21, +21 | 11 | F | 0.01% | N/A | Int |
| 14 | Pre B | 46xy | 15 | M | N/A | N/A | Int |
| 15 | Pre B | PDGFRB rearr, −12p | 8 | F | 19.0% | <0.01% | Int |
| 16 | Pre B | t(1;12),+1,der12,+12,+mar | 4 | M | N/A | N/A | Low |
| 17 | Pre B | t(1;19) | 12 | M | N/A | N/A | Low |
| 18 | Pre B | t(1;19) | 15 | F | N/A | N/A | Low |
| 19 | Pre B | t(1;19) | 12 | M | <0.05% | N/A | Low |
| 20 | T-ALL | −6 | 13 | M | 0.03% | <0.01% | Int |
| 21 | T-ALL | der11,+7 | 8 | F | 18.0% | N/A | High |
| 22 | T-ALL | +ABL1 | 10 | M | <0.01% | N/A | Int |
| 23 | T-ALL | 46xy | 13 | M | N/A | N/A | High |
| 24 | T-ALL | RUNX1 rearr | 3 | M | N/A | N/A | ND |
| 25 | T-ALL | t(8;14),–9,i9 | 3 | M | <0.01% | N/A | Low |
| 26 | T-ALL | −4, −9 | 9 | M | <0.01% | N/A | Low |
| 27 | T-ALL | 46xy | 6 | M | 0.06% | <0.01% | Low |
| 28 | T-ALL | t(11;14) | 4 | M | <0.01% | N/A | Low |
| 29 | T-ALL | ND | 13 | M | <0.01% | N/A | Low |
F, female; Int, intermediate; M, male; N/A, not available; ND, not determined.
MRD risk status at day 29 for Pt 23 (UKALL 2003 protocol) and at day 29 or week 14 for all other patients (UKALL 2011).
High numbers of fresh MNCs (up to 1 × 109 cells) were cultured in the presence of 100 IU/mL interleukin-2 (IL-2) (Miltenyi Biotec, Bisley, United Kingdom) for 5 days, to enrich T-cell content, as assessed by flow cytometry (CD3-FITC, clone BW264/56; Miltenyi Biotec). Enriched T-cell products were also assessed for their activation state (CD69-PE, clone FN50) and natural killer cells (CD56-PEVio770, clone AF12-7H3; both from Miltenyi Biotec). The T-cell products used for in vivo treatment were those with the highest CD3 expression (median, 80%; range, 73%-87%), lowest activation state to minimize T-cell exhaustion (CD69+/CD3+, 10%; range, 4%-15%) and low natural killer cells (6%; range, 3%-7%).
ROR1 quantification
Normal and ALL cells were stained with anti-ROR1-PE (clone 2A2) and propidium iodide (both from Miltenyi Biotec). Leukemia samples were also stained with anti-CD34-APC (clone 8G12) and either anti-CD19-FITC (clone 4G7) or anti-CD7-FITC (clone, M-T701; all from BD Biosciences, Wokingham, United Kingdom) and analyzed using a MACSQuant Analyzer 10 flow cytometer (Miltenyi Biotec), acquiring at least 10 000 events. The proportion of ROR1+ cells was measured in viable MNC and in gated LPC populations (CD34+/CD19+, CD34+/CD19–, CD34–/CD19+ and CD34–/CD19– for BCP-ALL samples; and CD34+/CD7+, CD34+/CD7–, CD34–/CD7+ and CD34–/CD7– for T-ALL samples; supplemental Figures 1 and 2). ROR1 density was measured on ROR1+ cells, using a fluorescence quantification kit (Quantibrite; BD Biosciences) and depicted as number of ROR1 binding sites.
Proliferation of ROR1+ cells
To investigate proliferative capacity, ALL cells were incubated with CellTrace violet (CTV; ThermoFisher Scientific, Altrincham, United Kingdom), according to manufacturer’s instructions, and seeded at 5 × 105 cells per mL in Iscoves modified Dulbecco’s medium with Glutamax (Invitrogen, Paisley, United Kingdom) supplemented with rhIL-3 (recombinant human interleukin-3; 20 ng/mL), rhIL-7 (20 ng/mL), stem cell factor (50 ng/mL; all from R&D Systems, Abingdon, United Kingdom), and human insulin transferrin serum substitute (20% volume-to-volume ratio, human serum albumin, insulin, and transferrin; StemCell Technologies, Cambridge, United Kingdom). Cells were maintained at 37°C (5% CO2 and 5% O2, in a humidified atmosphere) for 7 days. Absolute cell counts, viability, and proliferation (measured by reduction of CTV median fluorescence intensity) were assessed by flow cytometry.
Cytotoxicity assay
MNC were isolated from normal PB by density gradient centrifugation. T cells were isolated by negative selection using a pan T-cell isolation kit (Miltenyi Biotec) and stored in liquid nitrogen until use. T cells were incubated with CTV for 20 minutes prior to mixing in a ratio of 1:1 with ALL cells in the presence or absence of 1 μg/mL of ROR1-bispecific Teng (provided by NovalGen, Northwood Hills, United Kingdom), then incubated for 24 hours at 37°C (5% CO2 and 5% O2, humidified atmosphere). BCP-ALL cases were cocultured with CD19 Teng (1 μg/mL, NovalGen), as described above. After incubation, the supernatants were analyzed with an OptEIA ELISA Kit II for interferon gamma (IFN-γ) detection (BD Biosciences) according to manufacturer’s instructions. Cells were stained with propidium iodide to assess viability by flow cytometry. CTV-stained T cells were excluded to distinguish donor T cells from ALL cells.
In vivo efficacy
NOD.Cg-PrkdcscidIl2rγtm1Wjl/SzJ (NSG) mice were bred and maintained at the University of Bristol Animal Service Unit. All experiments were conducted under license from the United Kingdom Home Office. Primary BCP-ALL and T-ALL cells were resuspended in 300 μL Iscoves modified Dulbecco’s medium, supplemented with 5% human serum albumin (Bio Products Laboratory, Elstree, United Kingdom) and injected into the lateral tail vein. Once the level of human cells in murine PB was ≥0.5%, animals were given ROR1 or CD19 Teng (10 μg/kg) daily for 7 days and 3 doses of T cells, grown in culture from healthy donor MNC, on day 0 (107), day 3 (5 × 106), and day 6 (5 × 106) (supplemental Figure 3). Mouse weights were monitored daily because a sudden drop could indicate the onset of cytokine release syndrome.34 Human cell engraftment was assessed with weekly PB aspirates and animals were maintained for up to 4 weeks or until they showed symptoms of disease. On termination, femoral BM was assessed by flow cytometry for human cell engraftment using antibodies against CD34, CD45, CD7, and CD19 (all from BD Biosciences) and ROR1 (Miltenyi biotec). Plasma from cardiac punctures was assayed using an OptEIA ELISA Kit II for IFN-γ detection (BD Biosciences).
Statistical analyses
One-way analysis of variance followed by Tukey’s post hoc testing was used to investigate ROR1 expression between BCP-ALL, T-ALL, and respective LPC and NBM. IFN-γ production and the effect of Teng on cell viability in vitro and in vivo were also compared using this method. CTV median fluorescence intensity of ungated ROR1+ and ROR1– cells and subpopulations were compared and analyzed by the Kruskal-Wallis test.
Results
ROR1 expression in ALL and normal BM
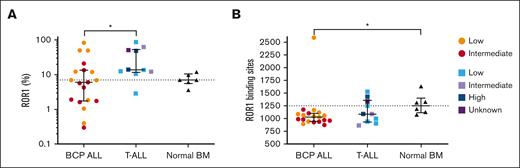
The proportion of ROR1+ cells and the number of antigen binding sites were determined in BCP-ALL (Pts 1-19), 10 T-ALL (Pts 20-29), and 6 NBM samples. The proportion of ROR1+ cells was significantly higher in T-ALL (median, 13.8%; range, 2.9%-87%) than in BCP-ALL (median, 6%; range, 0.3%-83%; P = .02). Similarly, the number of ROR1 binding sites was higher in T-ALL (median, 1089; range, 865-1527) than BCP-ALL (median, 1027; range, 876-2588), although not significant. The number of ROR-1 binding sites in BCP-ALL and T-ALL were both lower than NBM (median, 1250; range, 1076-1631; Figure 1), although only significantly so in BCP-ALL cases (P = .03). There were no significant differences in ROR1 expression between MRD low, intermediate, or high-risk groups in BCP-ALL and T-ALL (supplemental Figure 4). When ROR1 expression in NBM was further investigated, it was observed that all ROR1+ cells were CD19+ B cells (supplemental Figure 5). It was of interest to analyze ROR1 gene expression in a published data set of a separate cohort of 5 BCP-ALL, 5 T-ALL, and 5 NBM samples (supplemental Figure 6).5 NBM and T-ALL samples had similar log2 ROR1 median expressions of 0.44 (range, −0.67 to 0.98) and 0.41 (−0.81 to 2.56), whereas BCP-ALL had the lowest at 0.08 (range, −0.094 to 0.64). Two T-ALL cases had a higher ROR1 expression compared to all other samples.
ROR1 is expressed in both BCP-ALL and T-ALL. (A) Proportion of ROR1+ cells and (B) number of ROR1 binding sites in 19 BCP-ALL, 10 T-ALL cases, and 6 NBM samples. Each symbol can be distinguished by MRD status (low-, intermediate-, or high-risk). Solid horizontal lines represent medians, error bars represent the interquartile ranges, and interrupted lines represent the NBM median. ∗P ≤ .05.
ROR1 is expressed in both BCP-ALL and T-ALL. (A) Proportion of ROR1+ cells and (B) number of ROR1 binding sites in 19 BCP-ALL, 10 T-ALL cases, and 6 NBM samples. Each symbol can be distinguished by MRD status (low-, intermediate-, or high-risk). Solid horizontal lines represent medians, error bars represent the interquartile ranges, and interrupted lines represent the NBM median. ∗P ≤ .05.
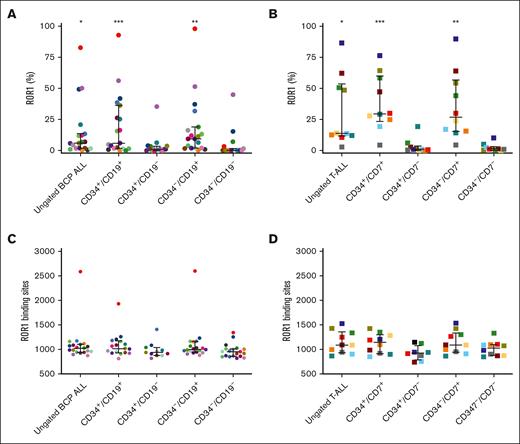
The expression of ROR1 was investigated in LPC populations (CD34+/CD19+, CD34+/CD19–, CD34–/CD19+, and CD34–/CD19–) in BCP-ALL (Pts 1-19) and in T-ALL (CD34+/CD7+, CD34+/CD7–, CD34–/CD7+, and CD34–/CD7–) (Pts 20-29). Higher proportions of ROR1+ cells were observed in ungated, CD19+, and CD7+ subpopulations (P < .05 when compared to CD19– and CD7– subpopulations, Figure 2A-B), with the highest in CD34–/CD19+ BCP LPC (median, 9.4%; range, 0.3%-98.1%) and in CD34+/CD7+ T-ALL LPC (median, 29.6%; range, 4.3%-76.5%). However, higher proportions of ROR1+ cells were not associated with an increased number of ROR1 binding sites, which were similar across the LPC subpopulations (Figure 2C-D).
ROR1 is coexpressed with BCP-ALL and T-ALL lineage markers. Proportion of ROR1+ cells in (A) BCP-ALL (Pts 1-19) or (B) T-ALL (Pts 20-29) and their respective LPC subpopulations. (C-D) Number of ROR1 binding sites in BCP-ALL and T-ALL and subpopulations. Each symbol represents an individual patient sample. Horizontal lines represent medians and error bars represent the interquartile ranges. ∗P ≤ .05, ∗∗P ≤ .01, and ∗∗∗P ≤ .001 when directly compared to CD19– and CD7– subpopulations and analyzed by Kruskal-Wallis test.
ROR1 is coexpressed with BCP-ALL and T-ALL lineage markers. Proportion of ROR1+ cells in (A) BCP-ALL (Pts 1-19) or (B) T-ALL (Pts 20-29) and their respective LPC subpopulations. (C-D) Number of ROR1 binding sites in BCP-ALL and T-ALL and subpopulations. Each symbol represents an individual patient sample. Horizontal lines represent medians and error bars represent the interquartile ranges. ∗P ≤ .05, ∗∗P ≤ .01, and ∗∗∗P ≤ .001 when directly compared to CD19– and CD7– subpopulations and analyzed by Kruskal-Wallis test.
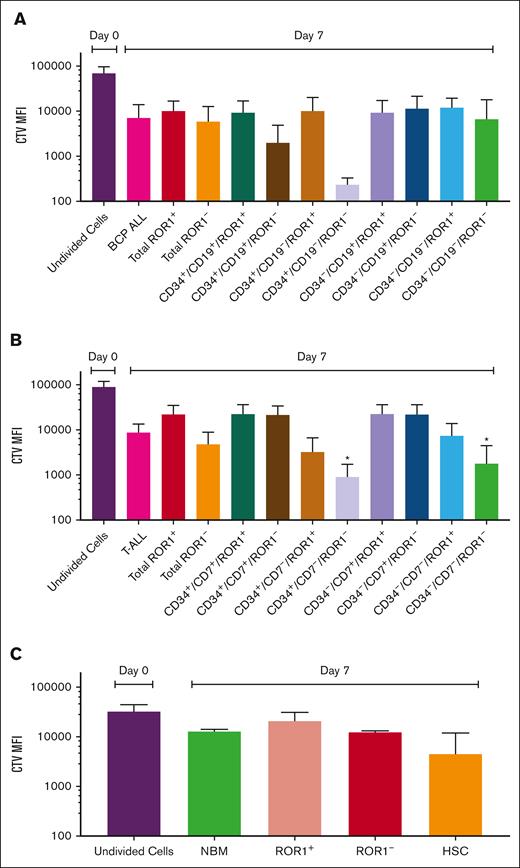
Proliferation of ALL cells and LPC
ROR1+ and ROR1– BCP-ALL cells had similar proliferation profiles in culture. CD34+/CD19+/ROR1– and CD34+/CD19–/ROR1– cells were the most proliferative with 34- and 260-fold decreases in the CTV, respectively. In T-ALL, ROR1– cells proliferated most, with CTV decreasing 19-fold by day 7, compared to ROR1+ cells (fourfold decrease). Most cell divisions were observed in CD34+/CD7–/ROR1– and CD34–/CD7–/ROR1– populations (100- and 49-fold decreases, both P = .04, compared to day 0; Figure 3). ROR1+ and ROR1– populations in NBM had modest proliferation over 7 days (1.5- and 2.6-fold decrease in CTV), whereas normal hematopoietic stem cell had more divisions (sevenfold decrease).
Proliferation of ROR1+ and ROR1– cell populations. Cells from BCP-ALL (Pts 16, 17, and 19) (A), T-ALL (Pts 20, 22, and 27) (B), and 3 NBM cases (C) were stained with proliferation marker, CTV before being cultured for 7 days. CTV MFI of ungated, ROR1+ and ROR1– cells and subpopulations was compared between day 0 (undivided) and day 7 cells and analyzed by Kruskal-Wallis test. ∗P = .04. Data are expressed as mean ± standard deviation (SD). MFI, median fluorescence intensity.
Proliferation of ROR1+ and ROR1– cell populations. Cells from BCP-ALL (Pts 16, 17, and 19) (A), T-ALL (Pts 20, 22, and 27) (B), and 3 NBM cases (C) were stained with proliferation marker, CTV before being cultured for 7 days. CTV MFI of ungated, ROR1+ and ROR1– cells and subpopulations was compared between day 0 (undivided) and day 7 cells and analyzed by Kruskal-Wallis test. ∗P = .04. Data are expressed as mean ± standard deviation (SD). MFI, median fluorescence intensity.
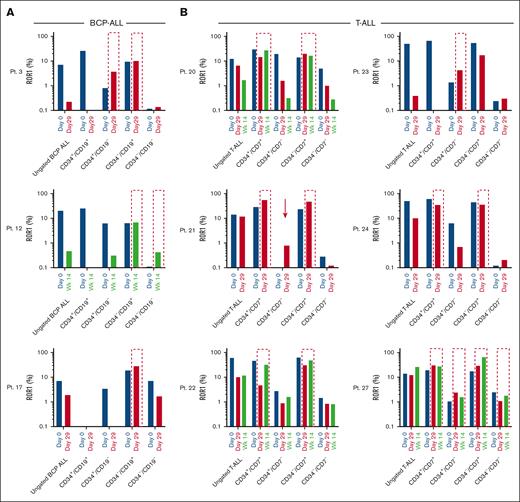
Effect of induction therapy on ROR1 expression
The effect of induction therapy on ROR1 was assessed in samples from key time points; at presentation, day 29, and, in some cases, week 14 after treatment. Induction therapy resulted in a decrease in the proportion of ROR1+ cells in both BCP-ALL and T-ALL cases (Figure 4A-B, respectively). Notably, ROR1+ cells survived treatment, in certain LPC populations. In all BCP-ALL cases, expression of ROR1 was unchanged or increased in CD34–/CD19+ cells after induction (Pts 3, 12, and 17), albeit at modest levels. The proportion of CD34+/CD19–/ROR1+ cells was also increased in Pt 3 and in Pt 12, an increase in the proportion of CD34–/CD19–/ROR1+ cells was observed (Figure 4A). Survival of ROR1+ cells was more pronounced in CD34+/CD7+ and CD34–/CD7+ T-ALL subpopulations (Pts 20-22, 24, and 27). Interestingly, a ROR1+ population emerged (Pt 21) or increased (Pts 23 and 27) in CD34+/CD7– LPC after induction (Figure 4B). The number of ROR1 binding sites increased in BCP-ALL cases in CD34–/CD19+ and CD34–/CD19– LPC (Figure 4C). Increased binding site numbers were observed in all 6 T-ALL cases after treatment, with Pts 20 and 21 demonstrating an increase in all 4 LPC subpopulations (Figure 4D).
ROR1+ LPC are resistant to induction therapy. Proportion of ROR1 positive cells (A-B) and number of binding sites (C-D) detected on samples at presentation (day 0), day 29, and wk 14 from BCP-ALL Pts 3, 12, and 17 (A,C) and T-ALL Pts 20 to 24 and 27 (B,D). Interrupted outlines indicate survival of ROR1+ LPC populations after induction therapy. Arrows indicate the emergence of ROR1+ in LPC. HSC, hematopoietic stem cell. Wk14, week 14.
ROR1+ LPC are resistant to induction therapy. Proportion of ROR1 positive cells (A-B) and number of binding sites (C-D) detected on samples at presentation (day 0), day 29, and wk 14 from BCP-ALL Pts 3, 12, and 17 (A,C) and T-ALL Pts 20 to 24 and 27 (B,D). Interrupted outlines indicate survival of ROR1+ LPC populations after induction therapy. Arrows indicate the emergence of ROR1+ in LPC. HSC, hematopoietic stem cell. Wk14, week 14.
Effects of bispecific Teng
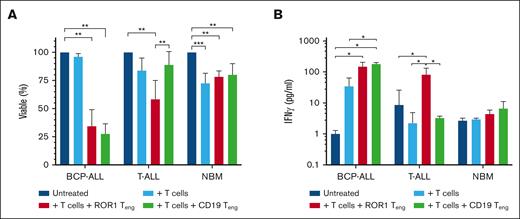
Cytotoxicity assays were established by incubating ALL cells with T cells, T cells + ROR1 Teng, or with T cells + CD19 Teng. Treating BCP-ALL samples with T cells ± ROR1 Teng significantly reduced viability to 34% ± 15%, which was comparable to 28 ± 9% observed using T cells ± CD19 Teng (P < .0001). Using T cells alone had minimal effect (96% ± 3% viable) (Figure 5A). Likewise, in T-ALL cases, viability was significantly reduced with the addition of T cells + ROR1 Teng (58%, P = .01), albeit more modest than in BCP-ALL. The addition of T cells alone only reduced T-ALL viability to 84%, whereas the T cells + CD19 Teng combination had less of an effect (89% ± 12%). In NBM controls, viability was significantly reduced to 73% ± 9% (P = .0007) using T cells alone. However, the addition of ROR1 or CD19 Teng did not increase this effect (78% ± 5% viable, P = .005; and 80% ± 10%, P = .008, respectively).
ROR1-bispecific Teng reduce ALL viability. (A) BCP-ALL cells (Pts 16 and 19), T-ALL cells (Pts 22-24 and 26), and NBM cells (n = 4) were incubated 1:1 with T cells ± ROR1 Teng or CD19 Teng for 24 hours and cell viability assessed by flow cytometry. (B) IFN-γ release was measured in supernatants of cytotoxicity assays. Data represent mean ± SD. ∗P ≤ .05, ∗∗P ≤ .01, and ∗∗∗P ≤ .001.
ROR1-bispecific Teng reduce ALL viability. (A) BCP-ALL cells (Pts 16 and 19), T-ALL cells (Pts 22-24 and 26), and NBM cells (n = 4) were incubated 1:1 with T cells ± ROR1 Teng or CD19 Teng for 24 hours and cell viability assessed by flow cytometry. (B) IFN-γ release was measured in supernatants of cytotoxicity assays. Data represent mean ± SD. ∗P ≤ .05, ∗∗P ≤ .01, and ∗∗∗P ≤ .001.
IFN-γ production was measured as an indicator of immune response (Figure 5B). There was only a 34-fold increase in IFN-γ when T cells were cocultured with BCP-ALL cells, but this increased to 148-fold with the addition of ROR1 Teng and 179-fold with CD19 Teng. In T-ALL cases, the addition of T cells + ROR1 Teng produced significant increases in IFN-γ compared to untreated cells (2.3-fold; P = .02) and those treated with T cells only (18.3-fold; P = .02). IFN-γ was reduced using T cells only or T cells + CD19 Teng. There was no significant change in IFN-γ production in NBM cells.
Use of bispecific Teng in vivo
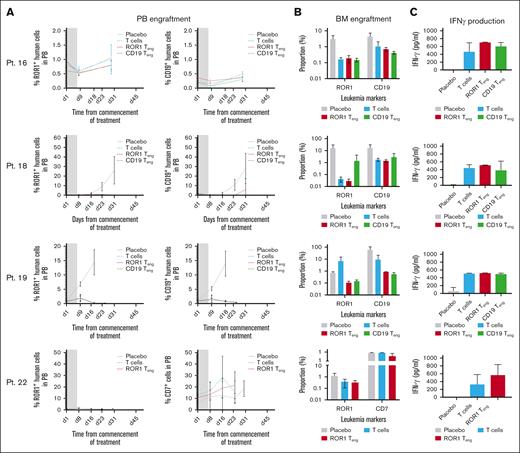
NSG mice were inoculated with cells from BCP-ALL (Pts 16-19) and T-ALL (Pt 22) samples and treated with ROR1 or CD19 Teng, once disease was established (n = 3-4 mice in each group). The effects on disease burden were measured in PB and BM aspirates and IFN-γ production was measured in plasma. Treating mice with Teng or with T cells alone delayed disease progression in most cases (Figure 6A). Treatment with ROR1 or CD19 Teng reduced CD19+ levels in mice engrafted with cells from Pt 18, to <0.5%, which was maintained for at least 24 days. In contrast, disease progressed in the placebo group and reached 28.11% ± 15.6% on termination at day 31. Similarly, disease levels in mice engrafted with Pt 19 remained as low as 0.39% ± 0.1% during treatment and up to day 25, whereas placebo treated mice were terminated at day 18. ROR1+ levels were also lower in both Teng-treated groups (0.8% ± 0.1% and 0.8% ± 0.2%) at day 29 compared to placebo (1.1% ± 0.1%) in Pt 16, albeit lower engraftment levels were observed in this case. ROR1 Teng were comparable with (Pts 16 and 19) or more effective (Pt 18) than CD19 Teng at reducing ROR1+ cells in BM at termination (Figure 6B). Both ROR1+ and CD19+ cells were reduced following treatment with ROR1 Teng (up to 520-fold and up to 68-fold, respectively) and with CD19 Teng (up to 20-fold and 105-fold, respectively). These observations were accompanied with elevated IFN-γ production, in some cases, signifying increased immune responses (Figure 6C). In the T-ALL case, more modest effects were observed (fourfold and 2.3-fold reductions in ROR1+ and CD7+ cells, respectively), following treatment with T cells and Teng, alongside a large increase in IFN-γ production (70%).
Teng reduce leukemia burden in vivo. NSG mice were inoculated with BCP-ALL (Pts 16, 18, and 19) and T-ALL (Pt 22) samples, before being treated with Teng and T cells. (A) Engraftment levels in PB, monitored weekly by flow cytometry. Gray boxes represent duration of treatment. Data in PB graphs represent mean ± SD (n = 3-4 mice per treatment condition). (B) Proportion of ROR1+ and CD19+ cells in BM at termination. (C) IFN-γ levels in murine plasma at termination. Bars represent mean ± SD (n = 3-4).
Teng reduce leukemia burden in vivo. NSG mice were inoculated with BCP-ALL (Pts 16, 18, and 19) and T-ALL (Pt 22) samples, before being treated with Teng and T cells. (A) Engraftment levels in PB, monitored weekly by flow cytometry. Gray boxes represent duration of treatment. Data in PB graphs represent mean ± SD (n = 3-4 mice per treatment condition). (B) Proportion of ROR1+ and CD19+ cells in BM at termination. (C) IFN-γ levels in murine plasma at termination. Bars represent mean ± SD (n = 3-4).
Discussion
This study investigated ROR1 as a potential therapeutic target in childhood BCP-ALL and T-ALL using the ROR1 Teng, NVG-111.35,36 ROR1 expression was assessed in a mixed cohort of BCP-ALL and T-ALL cases and demonstrated that 37% of cases had higher proportions of ROR1+ cells compared with NBM cells. Expression in T-ALL cases was significantly higher than BCP-ALL cases. These findings were also reflected in messenger RNA data in a separate sample cohort. Higher ROR1 expression was not limited to any particular subtype or specific genotype, unlike some previous reports,23,24 potentially making it a more viable candidate for targeting in both BCP-ALL and T-ALL. Proliferation profiles were similar among ROR1+ and ROR1– cells in BCP, T-ALL, and NBM samples, indicating that ROR1 expressing cells would not be particularly responsive to agents targeting actively cycling cells. Notably, ROR1+ populations persisted after induction therapy, indicating that current therapies may not be effective against ROR1+ cells. Furthermore, 4 of 6 cases, where follow-up data was available, required hematopoietic stem cell transplants within 2 to 4 years of diagnosis. The persistence of ROR1+ cells after induction therapy was particularly evident in T-ALL cases, where progress with the introduction of new therapeutic agents has been limited. Consequently, targeting ROR1 may lead to better outcomes. Phase 1/2 trials using ROR1 Teng (NVG-111) have reported antitumor activity with durable responses in relapsed/refractory CLL, demonstrating proof of concept for selective ROR1 targeting.36
High expression of the target antigen and density are important factors for effective cell-surface targeting, as cells with lower target densities have been shown to be less impacted and may be a contributory factor in treatment resistance.37,38 Majzner et al,38 demonstrated that efficacy of CD19 CAR T cells was proportional to target antigen density. However, the endodomain components also play an important role in efficacy, with CD28 surpassing 4-1BB against targets with low antigen density in cell line models.39 Watanabe et al39 showed that the threshold antigen density for lytic activity of CD20 CAR T cells, in a transduced cell model, was ∼200 molecules per target cell, whereas the antigen density required for cytokine production of CAR T cells was ∼10-fold higher. Lack of target antigen in some LPC subpopulations is another limiting factor in efficacy against ALL. We and others have shown that some LPC lack expression of CD19 (BCP) or CD7 (T-ALL) and that these subpopulations are chemoresistant in vivo.5,31 Consequently, CD19– LPC may contribute to blinatumomab resistance and CD19– relapse following CAR T-cell therapy. In the current sample cohort, the expression of ROR1 was associated with CD19 or CD7 expression in BCP-ALL and T-ALL cases, respectively. However, antigen density was similar among all LPC subpopulations investigated, including populations we have shown to be resistant, indicating the target is not restrictive. Moreover, ROR1 density increased after treatment in the majority of LPC in both BCP-ALL and T-ALL, not only in those with higher expression at diagnosis. Such a phenomenon has also been reported in patients with breast cancer after chemotherapy, where ROR1 expression had increased in 64% of samples.40 Therefore, targeting ROR1 could potentially affect more LPC subpopulations and consequently, reduce the risk of relapse.
To investigate this novel target, ROR1 Teng (ClinicalTrials.gov identifier: NCT04763083) were used to treat primary BCP-ALL, T-ALL, and NBM cells and the results compared to CD19 Teng or T cells alone. Exposure of BCP-ALL cells to T cells + ROR1 Teng in vitro significantly reduced ALL survival to only 34%. This was similar to that observed using CD19 Teng, where BCP-ALL survival was reduced to 28%. The addition of T cells + ROR1 Teng also significantly reduced viability of T-ALL (58%), something that was not seen with the addition of T cells alone. To our knowledge, this is the first report of ROR1-targeted killing in primary pediatric T-ALL samples. The same Teng were previously reported to induce killing of primary CLL cells (68% at 3:1 effector-to-target ratio) and PANC-1 cells (97.3% at 1:1 effector-to-target ratio).29,41 The number of cases investigated was too low for reliable correlation assessment of ROR1 density and ROR1 Teng efficacy in this study but should be addressed in future investigations. Importantly, ROR1 Teng had limited effects on NBM cells, similar to those observed with CD19 Teng, which is clinically acceptable. Furthermore, not all CD19+ cells expressed ROR1, so effects on normal B cells may be lower than with CD19 Teng. Activated T cells release large numbers of IFN-γ among other cytokines, such as IL-10 and IL-6. Addition of ROR1 Teng increased cytokine production by fourfold for BCP-ALL and 18.3-fold for T-ALL in vitro, compared to using T cells alone. Gohil et al29 reported a 15-fold increase in IFN-γ compared to T cells alone in CLL. Our findings are comparable and indicate potential for use of ROR1 Teng in acute leukemias.
To further investigate efficacy, ROR1 Teng were used to treat NSG mice engrafted with a subset of BCP-ALL and T-ALL cases. Disease progression was prevented in 1 BCP-ALL case and delayed in the others. Furthermore, ROR1 Teng (NVG-11) were at least comparable to blinatumomab treating BCP-ALL in vivo and had a larger impact on ROR1+ cells. In the T-ALL case, ROR1 Teng did not prevent disease progression, however there was a fourfold reduction in CD7+ leukemia cells in BM. Additional studies will be required to determine the potential of targeting ROR1 in T-ALL cases. Gohil et al29 compared the effects of these ROR1 Teng on SKOV3 cancer cells in Hsd:Athymic Nude-Foxn1nu mice, where there was a significant reduction in tumor size at 12 days after treatment, compared to the CD19 Teng-treated group. The shorter follow-up and the use of a different cancer model does not permit a direct comparison with our study. However, both studies demonstrate the potential of ROR1-targeting in vivo.
Relapse with target-negative disease remains a leading cause of treatment failure following treatment with immune effector cells.42-45 Although this has been attributed to loss/downregulation of the target marker, it could also be due to clonal proliferation of LPC that do not express the target. Dual target CD19/CD22 CAR T-cell therapy is currently being investigated, as a means of preventing relapse,46,47 but issues with antigen-negative relapse remain.48 Because ROR1 was expressed on most investigated LPCs, relapse rates may be reduced by using ROR1 Teng. The potential of targeting ROR1 in T-ALL certainly warrants further investigation, because there has been limited progress in the treatment of this subtype. It may also be possible to combine ROR1 with additional antigenic targets. We have previously shown that CD200 expression was essential for engraftment and serial transplantation of LPC in BCP-ALL cases and that this could be targeted using the monoclonal antibody TT-CD200.15 Investigating dual targeting of ROR1 and CD200 for BCP-ALL is warranted and the risk of antigen-negative release could be lower as CD200– ALL cells were not capable of establishing disease in NSG mice.15
In conclusion, this study has demonstrated for the first time that ROR1 is overexpressed in a considerable proportion of BCP-ALL and T-ALL cases and expression was not restricted to specific subgroups or LPC populations, making it a viable candidate for targeting. The demonstration that ROR1 Teng were as effective as blinatumomab is very encouraging and could be an alternative for patients who are refractory or have CD19– disease. Emergence of ROR1+ cells after therapy was an important finding and warrants further investigation to determine its potential as a measure of residual disease and its utility in risk stratification.
Acknowledgments
The authors thank Paul Archer and Jeremy Hancock, Bristol Genetics Laboratory, the oncology staff at Bristol Royal Hospital for Children. The authors also thank Vincent Muczynski, David Granger, and Marie Brockwell, NovalGen. The authors are grateful to the patients and their families who gave permission for their cells to be used for research.
This article presents independent research commissioned by the National Institute for Health Research (NIHR) under its Programme Grants scheme (RP-PG-0310-1003) and National Health Service (NHS) Blood and Transplant project grants.
The views expressed in this article are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health.
Authorship
Contribution: P.D. conceived study, designed and performed experiments, and wrote the report; B.K.B. performed experiments; O.E.I. performed experiments; J.P.M. facilitated sample collection, collated the clinical data information, and commented on the report; A.C.N. provided NVG-111 and CD19 T-cell engagers, advised on experimental design, and commented on the report; and A.B. conceived study, designed and performed experiments, and wrote the report.
Conflict-of-interest disclosure: A.C.N. holds patent rights for ROR1-based immunotherapies; is an employee of NovalGen Therapeutics; holds equity in NovalGen Therapeutics, which has licensed ROR1-based immunotherapies from University College London; and is founder and CEO of NovalGen Therapeutics. The company is developing an ROR1 T-cell engager. The remaining authors declare no competing financial interests.
Correspondence: Allison Blair, Cellular and Molecular Therapies, National Health Service Blood and Transplant, North Bristol Park, Bristol BS34 7QH, United Kingdom; email: allison.blair@nhsbt.nhs.uk.
References
Author notes
Microarray data are available at www.ebi.ac.uk/arrayexpress, accession number E-MTAB-4006.
The data that support the findings of this study are available upon reasonable request from the corresponding author, Allison Blair (allison.blair@nhsbt.nhs.uk).
The full-text version of this article contains a data supplement.