TO THE EDITOR:
The POLARIX study demonstrated a sustainable improvement in progression-free survival (PFS) with Pola-R-CHP (polatuzumab vedotin in combination with rituximab, cyclophosphamide, doxorubicin, and prednisone) vs R-CHOP (rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone), with a similar tolerability profile in previously untreated diffuse large B-cell lymphoma (DLBCL). This report provides additional details regarding peripheral neuropathy (PN) events from both a physician and patient perspective. Clinician- and patient-reported outcomes (ClinROs and PROs, respectively) similarly reported no increase in the incidence rate or severity of PN events in patients treated with Pola-R-CHP compared with R-CHOP.
Microtubule inhibitors, such as vincristine, have long been a component of the various regimens used to treat DLBCL and other hematological malignancies, including R-CHOP and R-CHOP–like therapies.1,2 Newer treatment options in DLBCL include antibody-drug conjugates, such as polatuzumab vedotin, which are also given in combination with chemotherapy.1,2 Polatuzumab vedotin contains the potent microtubule inhibitor monomethyl auristatin E as its cytotoxic payload, conjugated by a protease-cleavable linker to an anti-CD79b monoclonal antibody.3
In the phase 3 randomized, double-blind, placebo-controlled POLARIX study (ClinicalTrials.gov identifier: NCT03274492), Pola-R-CHP was compared with R-CHOP in patients with previously untreated DLBCL.2 Results of the study demonstrated improved PFS with Pola-R-CHP vs R-CHOP, which was sustained with longer follow-up,4 with a similar tolerability profile.2
PN is a well-established dose-limiting side effect of vincristine- and monomethyl auristatin E–containing treatments,5-7 with the severity and incidence of PN related to the dose and number of treatment cycles.7,8 To avoid their overlapping toxicities, polatuzumab vedotin replaced vincristine in the R-CHOP regimen in POLARIX. Additionally, age has been reported to influence treatment tolerability.9 Thus, in this curative setting, maintaining an efficacious dose of vincristine or polatuzumab vedotin balanced with the safety of the patient is critical. Additionally, although microtubule inhibitor–induced PN can be reversible, it can result in long-term impairments in irreversible cases.10 Therefore, understanding the pattern of PN appearance and resolution is important for clinicians and patients.
Here, we evaluate the impact of Pola-R-CHP vs R-CHOP on the rates and severity of PN during the POLARIX study using ClinROs and PROs.
Detailed methodology of POLARIX, a double-blind, placebo-controlled, international phase 3 study, has been reported previously.2 Briefly, the study enrolled 879 patients with previously untreated DLBCL and an International Prognostic Index score of 2 to 5; the median age was 65 (range, 19-80) years. Patients with preexisting grade >1 PN or known Charcot-Marie-Tooth disease were not permitted. Patients were randomized (1:1) to 6 cycles of either Pola-R-CHP or R-CHOP; all patients also received 2 additional cycles of rituximab (reported as cycles 7 and 8).2
PN was assessed in the safety population (ie, all randomized patients who received ≥1 dose of study treatment; Pola-R-CHP, n = 435; R-CHOP, n = 438) using ClinROs (National Cancer Institute Common Terminology Criteria for Adverse Events [NCI CTCAE] v4.0) and PROs (Functional Assessment of Cancer Therapy/Gynecologic Oncology Group–Neurotoxicity questionnaire [FACT-GOG/Ntx]) at baseline, during treatment, and at follow-up visits for up to 24 months after treatment completion/early discontinuation. FACT-GOG/Ntx subscale scores range from 0 to 44, with higher scores reflecting fewer symptoms (minimal clinically important difference, 1.38-3.68).11 Incidence of PN was also compared by age (<60 and ≥60 years). This descriptive analysis was not powered to statistically evaluate differences between treatment arms; no formal hypothesis testing was performed.
At baseline, most patients receiving Pola-R-CHP or R-CHOP had no history of PN (94% and 93%, respectively). During the study, the overall incidence of clinician-reported PN was comparable between Pola-R-CHP and R-CHOP (52.9% and 53.9%, respectively), with most events reported as grade 1 in severity (Table 1). Incidences of grade 2 (12.2% and 15.5%) and grade 3 events (1.6% and 1.1%) were also similar with Pola-R-CHP and R-CHOP, respectively. When outcomes were evaluated according to patient age, the incidence of grade 2 to 3 events was slightly higher in patients aged ≥60 years than those aged <60 years (Table 1). Rates of PN with Pola-R-CHP are in line with those reported in other polatuzumab vedotin clinical1,6,12,13 and real-world14 studies. Similarly, the currently reported rates of PN with R-CHOP were comparable with those reported in other studies of patients with DLBCL.15-17
Overall PN safety profile
| . | Pola-R-CHP (n = 435) . | R-CHOP (n = 438) . | ||
|---|---|---|---|---|
| Patients with ≥1 PN event | 230 (52.9) | 236 (53.9) | ||
| Grade 1 | 170 (39.1) | 163 (37.2) | ||
| Grade 2 | 53 (12.2) | 68 (15.5) | ||
| Grade 3 | 7 (1.6) | 5 (1.1) | ||
| Incidence of PN event by age group | <60 y (n = 129) | ≥60 y (n = 306) | <60 y (n = 121) | ≥60 y (n = 317) |
| Patients with ≥1 PN event | 65 (50.4) | 165 (53.9) | 75 (62.0) | 161 (50.8) |
| Grade 1 | 54 (41.9) | 116 (37.9) | 58 (47.9) | 105 (33.1) |
| Grade 2 | 11 (8.5) | 42 (13.7) | 17 (14.0) | 51 (16.1) |
| Grade 3 | 0 | 7 (2.3) | 0 | 5 (1.6) |
| PN events leading to | ||||
| Study discontinuation | 0 | 0 | ||
| Treatment discontinuation | ||||
| Polatuzumab vedotin/vincristine | 3 (0.7) | 9 (2.1) | ||
| Dose reduction | ||||
| Polatuzumab vedotin/vincristine | 17 (3.9) | 35 (8.0) | ||
| Dose interruption | ||||
| Polatuzumab vedotin/vincristine | 3 (0.7) | 3 (0.7) | ||
| . | Pola-R-CHP (n = 435) . | R-CHOP (n = 438) . | ||
|---|---|---|---|---|
| Patients with ≥1 PN event | 230 (52.9) | 236 (53.9) | ||
| Grade 1 | 170 (39.1) | 163 (37.2) | ||
| Grade 2 | 53 (12.2) | 68 (15.5) | ||
| Grade 3 | 7 (1.6) | 5 (1.1) | ||
| Incidence of PN event by age group | <60 y (n = 129) | ≥60 y (n = 306) | <60 y (n = 121) | ≥60 y (n = 317) |
| Patients with ≥1 PN event | 65 (50.4) | 165 (53.9) | 75 (62.0) | 161 (50.8) |
| Grade 1 | 54 (41.9) | 116 (37.9) | 58 (47.9) | 105 (33.1) |
| Grade 2 | 11 (8.5) | 42 (13.7) | 17 (14.0) | 51 (16.1) |
| Grade 3 | 0 | 7 (2.3) | 0 | 5 (1.6) |
| PN events leading to | ||||
| Study discontinuation | 0 | 0 | ||
| Treatment discontinuation | ||||
| Polatuzumab vedotin/vincristine | 3 (0.7) | 9 (2.1) | ||
| Dose reduction | ||||
| Polatuzumab vedotin/vincristine | 17 (3.9) | 35 (8.0) | ||
| Dose interruption | ||||
| Polatuzumab vedotin/vincristine | 3 (0.7) | 3 (0.7) | ||
The overall safety profile was assessed according to NCI CTCAE v4.0. Data are presented as n (%).
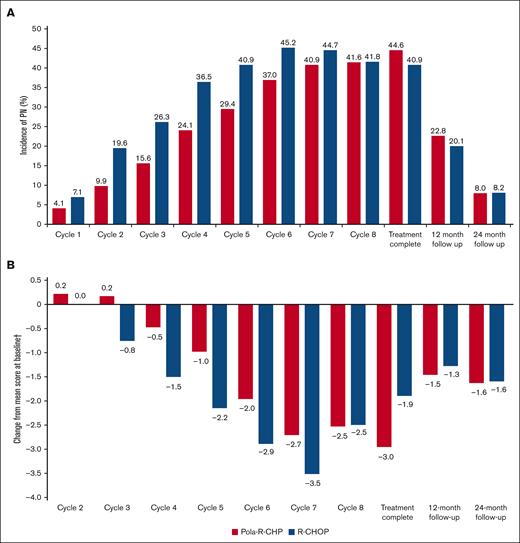
When evaluated by cycle, the incidence of clinician-reported PN adverse events was lower with Pola-R-CHP than with R-CHOP during cycles 1 to 7 (Figure 1A). More patients experienced earlier onset of PN with R-CHOP than with Pola-R-CHP, with a median time to onset of 1.9 months in the R-CHOP arm and 2.3 months in the Pola-R-CHP arm. Rates of clinician-reported PN were 10.0% to 12.4% lower with Pola-R-CHP than with R-CHOP during cycles 2 to 5, with similar rates of PN between treatment groups from cycle 8 onward, including treatment completion visit rates of 44.6% and 40.9%, respectively. The duration of PN was similar in both treatment arms, with a median time to resolution of PN events of 4.0 months in the Pola-R-CHP arm and 4.6 months in the R-CHOP arm. These results show that Pola-R-CHP is associated with a delayed onset of PN events than R-CHOP, particularly during early treatment cycles. There were fewer dose reductions and drug discontinuations due to PN with Pola-R-CHP vs R-CHOP (Table 1). This may have an impact on the ability to deliver vincristine as part of the treatment regimen, as evidenced by the lower rates of dose reductions (3.9% vs 8.2%) and drug discontinuations (0.7% vs 2.1%) due to PN events with Pola-R-CHP vs R-CHOP (Table 1). Importantly, although there was a delayed onset of PN from polatuzumab vedotin compared with vincristine, the overall incidence of PN at 12-month and 24-month follow-up was comparable between treatment arms (Figure 1A).
Rates and severtity of PN. (A) Incidence of PN by visit, assessed by ClinRO (NCI CTCAE v4.0, any grade). (B) Mean change in PN symptom score by visit, assessed by PRO (FACT-GOG/Ntx∗). ∗FACT-GOG/Ntx is scored from 0 to 44, with higher scores reflecting fewer symptoms (minimal clinically important difference, 1.38-3.68); †Mean score at baseline was 39.8 for Pola-R-CHP and 39.5 for R-CHOP.
Rates and severtity of PN. (A) Incidence of PN by visit, assessed by ClinRO (NCI CTCAE v4.0, any grade). (B) Mean change in PN symptom score by visit, assessed by PRO (FACT-GOG/Ntx∗). ∗FACT-GOG/Ntx is scored from 0 to 44, with higher scores reflecting fewer symptoms (minimal clinically important difference, 1.38-3.68); †Mean score at baseline was 39.8 for Pola-R-CHP and 39.5 for R-CHOP.
Compliance for the FACT/GOG-Ntx questionnaire was high throughout the study, with a completion rate of 96% at baseline and >80% at subsequent assessments. Both treatment arms showed low PN symptom scores at baseline (mean, 39.8 with Pola-R-CHP and 39.5 with R-CHOP). Similar to ClinROs, patient-reported onset of PN symptoms after initial exposure to treatment occurred later in those receiving Pola-R-CHP than in those receiving R-CHOP (cycle 4 vs cycle 3). Beyond cycle 4, PN symptoms increased in both treatment arms, peaking at cycle 7 (Figure 1B). However, changes from baseline in mean FACT-GOG/Ntx scores were smaller with Pola-R-CHP than with R-CHOP, with an approximately +1-point difference between treatment arms during cycles 3 to 6. At cycle 8, and at 12- and 24-month follow-up, patient-reported symptoms and incidence of PN were comparable between treatment arms. Overall, rates of clinician- and patient-reported PN demonstrated the temporal relationship of PN symptoms and their resolution. During follow-up, PN scores returned to near baseline, consistent with the resolution of most neuropathy events.
Prior studies evaluating patients with lymphoma receiving R-CHOP–based regimens have shown that FACT-GOG/Ntx scores and NCI CTCAE capture clinically relevant PN without invasive electromyography/nerve conduction velocity procedures.18,19 The POLARIX study provided ClinRO and PRO PN assessments in one of the largest populations of patients with lymphoma who were evaluated using the FACT-GOG/Ntx questionnaire and NCI CTCAE. As noted above, because vincristine and polatuzumab vedotin are both microtubule inhibitors and have similar toxicities, including PN, polatuzumab vedotin replaced vincristine in the R-CHOP regimen in the POLARIX study. Most patients received all 6 doses of the active agents of polatuzumab vedotin or vincristine in the Pola-R-CHP (91.7%) and R-CHOP arms (88.5%), respectively, and the median relative dose intensities of all study drugs were >99% in both treatment arms.2 Exposure-response analyses have suggested that lowering polatuzumab vedotin dose below 1.8 mg/kg will reduce the likelihood of PN but also reduce its efficacy;20 therefore, in POLARIX, the risk of PN was further minimized by reducing the dose of polatuzumab vedotin/vincristine in the event of grade 2 sensory neuropathy, withholding these agents in the event of grade 3 sensory neuropathy or grade 2 to 3 motor neuropathy, and permanently discontinuing these agents in the event of grade 4 PN. Using these measures, these results suggest that PN can be adequately managed without unduly compromising the efficacy of Pola-R-CHP in patients with previously untreated DLBCL.
In conclusion, Pola-R-CHP did not result in different rates or severity of PN compared with R-CHOP during the POLARIX study. After initial exposure, PN occurred later with Pola-R-CHP than with R-CHOP and resulted in fewer dose modifications. Rates of PN in the follow-up period were similar between treatment arms. Overall, the risk of PN was manageable in the context of increased PFS benefit with Pola-R-CHP vs R-CHOP.
The POLARIX study was approved by the institutional review board or ethics committee at each participating institution.
Acknowledgments: The authors thank Veronica Craine for her contribution to this research. Third-party medical writing assistance, under the direction of the authors, was provided by Andrea Bothwell and Anna Nagy, on behalf of Ashfield MedComms, an Inizio company, and was funded by F. Hoffmann-La Roche Ltd.
C.R.F. is a scholar in Cancer Research at the Cancer Prevention and Research Institute of Texas.
This study was sponsored by F. Hoffmann-La Roche Ltd and Genentech, Inc.
Contribution: J.H., C.L., M.C., and G.M. conceptualized and designed the study and analyzed and interpreted data; J.H., C.L., M.C., and F.G. are accountable for all aspects of work; and all authors contributed to manuscript writing and provided the final approval of manuscript.
Conflict-of-interest disclosure: M.T. reports consultancy fees from Takeda, Bristol-Myers Squibb (BMS), Incyte, AbbVie, Amgen, Roche, Gilead Sciences, Janssen, MorphoSys, Novartis, and Zentiva; research funding from Roche, Takeda, and Novartis; and honoraria from Takeda, BMS, Incyte, AbbVie, Amgen, Roche, Gilead Sciences, Janssen, MorphoSys, Novartis, and Zentiva. I.F. reports consultancy fees (all payments made to Sarah Cannon Research Institute) from BeiGene, Century Therapeutics, Genentech, Genmab, Hutchison MediPharma, InnoCare Pharma, Kite Pharma, Myeloid Therapeutics, Novartis, Secura Bio, Servier Pharmaceuticals, TG Therapeutics, Vincerx Pharma, and Xencor; research grants (all payments made to Sarah Cannon Research Institute) from AbbVie, Acerta Pharma, Agios, ArQule, AstraZeneca, BeiGene, Biopath, BMS, CALIBR, CALGB, Celgene, City of Hope National Medical Center, Constellation Pharmaceuticals, Curis, CTI Biopharma, Epizyme, Fate Therapeutics, Forma Therapeutics, Forty Seven, Genentech, Gilead Sciences, InnoCare Pharma, IGM Biosciences, Incyte, Infinity Pharmaceuticals, Janssen, Kite Pharma, Loxo, Marker Therapeutics, Merck, Millennium Pharmaceuticals, MorphoSys, Myeloid Therapeutics, Novartis, Nurix, Pfizer, Pharmacyclics, Portola Pharmaceuticals, Rhizen Pharmaceuticals, Roche, Seattle Genetics, Step Pharma, Tessa Therapeutics, TG Therapeutics, Trillium Therapeutics, Triphase Research & Development Corp, Unum Therapeutics, Verastem, Vincerx Pharma, and 2seventy bio, Inc. C.H. reports honoraria from F. Hoffmann-La Roche Ltd, Janssen-Cilag, Gilead Sciences, Miltenyi Biotec, Amgen, Takeda, and Celgene. M.C. is an employee of La Paz University Hospital; reports consultancy fees from BeiGene, BMS, Kite, Incyte, Janssen, Karyopharm, Kyowa, Novartis, Roche, Sanofi, and Takeda; and speaker’s bureau fees from Amgen, Kite, Janssen, Kyowa, Novartis, Roche, Sandoz, and Takeda. H.G. reports honoraria from AbbVie, Sanofi, Chugai, Symbio, Novartis, Gilead Sciences, Esai, BMS, Janssen, and Kyowa Kirin. R.H. reports honoraria from Takeda, Amgen, Oncopeptides, Sanofi, Janssen, Novartis, and Celgene; research funding from Celgene, Novartis, Amgen, Takeda, Janssen, and BMS; and advisory board fees from BMS, Takeda, Amgen, Oncopeptides, Sanofi, and Janssen. G.S. reports consultancy fees from AbbVie, BeiGene, BMS/Celgene, Genentech/Roche, Genmab, Innate Pharma, Incyte, Ipsen, Janssen, Kite/Gilead, Merck, Modex, Novartis, Orna Therapeutics, Pfizer, and Treeline. M.Y., D.S. and V.C. are employees of F. Hoffmann-La Roche Ltd and receive F. Hoffmann-La Roche Ltd stocks/stock options. A.S., J.H., R.K., G.M., and C.L. are employees of Genentech, Inc; and receive F. Hoffmann-La Roche Ltd. stocks/stock options. C.R.F. reports consultant fees from AbbVie, Bayer, BeiGene, Celgene, Denovo Biopharma, Foresight Diagnostics, Genentech, Inc/ F. Hoffmann-La Roche Ltd, Genmab, Gilead, Karyopharm, N-Power Medicine, Pharmacyclics/Janssen, SeaGen, and Spectrum; stock options in Foresight Diagnostics and N-Power Medicine; research funding from 4D, AbbVie, Acerta, Adaptimmune, Allogene, Amgen, Bayer, Celgene, Cellectis EMD, Gilead, Genentech, Inc/F. Hoffmann-La Roche Ltd, Guardant, Iovance, Janssen, Kite, MorphoSys, Nektar, Novartis, Pfizer, Pharmacyclics, Sanofi, Takeda, TG Therapeutics, Xencor, Ziopharm, Burroughs Wellcome Fund, Eastern Cooperative Oncology Group, National Cancer Institute, V Foundation, and Cancer Prevention and Research Institute of Texas. H.T. reports consulting or advisory role with F. Hoffmann-La Roche Ltd, Incyte, Celgene/BMS, and ADC Therapeutics; travel, accommodation, and expenses from Janssen and Gilead; honoraria from BMS and F. Hoffmann-La Roche Ltd; and research funding from Genentech, Inc/F. Hoffmann-La Roche Ltd. The remaining authors declare no competing financial interests.
The current affiliation for J.H. is BeOne Medicine, San Carlos, CA.
The current affiliation for C.L. is Gilead Sciences, Foster City, CA.
Correspondence: Marek Trněný, Charles University General Hospital, U Nemocnice 499/2, 128 08 Prague, Czech Republic; email: trneny@cesnet.cz.
References
Author notes
Presented as a poster at the 2022 American Society of Clinical Oncology Annual Meeting, Chicago, IL, 3 to 7 June 2022 and at the 2022 European Hematology Association Congress, Vienna, Austria, 9 to 17 June 2022.
For eligible studies, qualified researchers may request access to individual patient-level clinical data through the clinical study data request platform. At the time of writing this request, the platform is Vivli (https://vivli.org/ourmember/roche/). Up-to-date details on Roche’s Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents are available at https://go.roche.com/data_sharing. Anonymized records for individual patients across more than one data source external to Roche cannot, and should not, be linked owing to a potential increase in risk of patient reidentification.