Key Points
Major microbiome disruption was associated with toxicities following ide-cel administration in patients with multiple myeloma.
Bacterial diversity decreased after ide-cel infusion, and bacterial composition was associated with ide-cel response and toxicities.
Visual Abstract
Increasing evidence suggests that the gut microbiome may influence the responses and toxicities associated with chimeric antigen receptor T-cell (CAR-T) therapy. We conducted whole-genome shotgun sequencing on stool samples (N = 117) collected at various times from patients with multiple myeloma (n = 33) who underwent idecabtagene vicleucel (ide-cel) anti–B-cell maturation antigen CAR-T therapy. We observed a significant decrease in bacterial diversity after ide-cel infusion, along with significant differences in the bacterial composition linked to therapy response and toxicities. Specifically, we found significant enrichment of Flavonifractor plautii, Bacteroides thetaiotaomicron, Blautia fecis, and Dysosmobacter species in ide-cel responders. A notable finding was the link of major microbiome disruption, defined as the presence of dominant specific taxa (>35% prevalence), and increased facultative pathobionts, like Enterococcus, with ide-cel toxicities, especially cytokine release syndrome (CRS). Patients with genus dominance in baseline samples had a higher incidence of grade 2 or higher CRS at 46.2% than those without genus dominance (11.1%; P = .043). In addition, network analysis and mass spectrometric assessment of stool metabolites revealed important associations and pathways, such as F plautii being linked to increased indole metabolites and pathways in responders. Our findings uncovered novel microbiome associations between ide-cel responses and toxicities that may be useful for developing modalities to improve CAR-T outcomes.
Introduction
In recent years, the human gut microbiome has been shown to play a role in modulating the efficacy of cancer immunotherapies in mouse models and cohorts of patients who underwent immune checkpoint inhibitor blockade1,2 and hematopoietic stem cell transplantation.3 The gut microbiome constitutes the primary source of foreign antigens encountered by the immune system, thereby significantly influencing immune responses. Consequently, when engineered adoptive cell therapies are administered to a patient, their efficacy and toxicity are likely modulated by the microbiome-driven immune environment. In a recent paper, vancomycin-induced microbiota changes enhanced tumor control and promoted the cross-presentation of tumor-associated antigens, highlighting, for the first time in a mouse model, the potential role of the microbiome in chimeric antigen receptor T-cell (CAR-T) therapy.4 Recently, our group and others also have expanded on the microbiome’s role in genetically engineered T cells expressing CARs in patients with lymphoma who received anti-CD19 CAR-T therapy.5-7 However, studies that explored the role of the microbiome in patients with multiple myeloma (MM) who received B-cell maturation antigen (BCMA)–directed CAR-T therapy have been limited with only 1 study reported in the literature.8
Anti-BCMA CAR-T therapy has revolutionized the management of patients with relapsed and refractory MM. The US Food and Drug Administration has granted approval for 2 CAR-T therapies for MM.9,10 Despite the promising initial responses observed with these CAR-T therapies, a significant number of patients experience relapse. In addition, the occurrence of CAR-T–related complications, such as cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS), further complicates the treatment landscape. Unfortunately, the lack of reliable biomarkers hinders the ability to predict both the treatment outcomes and potential toxicities associated with CAR-T therapy in MM. In this study, we investigated the alterations in the intestinal microbiome of patients with relapsed/refractory MM who underwent idecabtagene vicleucel (ide-cel) anti-BCMA CAR-T therapy and characterized the association of microbiome features with therapeutic response, risk of toxicity, and metabolite profiles.
Methods
Clinical data collection and regulatory compliance
Our study included 33 patients with MM who underwent ide-cel treatment at The University of Texas MD Anderson Cancer Center (MDACC; n = 20) and the Moffitt Cancer Center (n = 13) between September 2021 and July 2022. Ide-cel was the first CAR-T therapy that was approved for MM in 2021, nearly a year before ciltacabtagene autoleucel, which received approval in 2022. Consequently, we started collecting stool samples from patients who were treated with ide-cel earlier, leading to a larger sample set for this cohort than for the cohort treated with ciltacabtagene autoleucel. Therefore, we focused our metagenomic sequencing primarily on the ide-cel group to minimize heterogeneity in our analysis. In addition to the general epidemiologic patient characteristics, such as age and gender, we recorded indices of myeloma tumor burden at leukocyte apheresis and/or lymphodepletion, including serum immunoglobulin levels, monoclonal protein, beta-2-microglobulin, bone marrow biopsy plasma cell percentages before lympho-depleting chemotherapy, and Eastern Cooperative Oncology Group status. We collected information from patient records on the number of prior oncological therapies and whether or not they underwent bridging chemotherapy and/or radiotherapy. Serum C-reactive protein and ferritin levels were recorded at apheresis and lymphodepletion. The acquisition and examination of clinical data and patient biomaterials in this study received ethical approval through the Advarra Institutional Review Board under protocol Pro00029290 at the Moffitt Cancer Center, whereas at MDACC, approval was granted under Institutional Review Board protocol LAB04-717. Informed written consent was diligently obtained from all participants in the study, and no compensation was provided to the patients for their participation.
Fecal sample collection
At MDACC, patients sample collection was achieved using the OMNIgene GUT kit for both inpatient and outpatient sample collection, and the collected samples were frozen at −80°C before DNA extraction. At the Moffitt Cancer Center, samples were collected on fecal occult blood test cards and stored at −80°C, as previously described.11 At both participating centers, fecal specimens were systematically gathered from patients at various time intervals that encompassed periods before, during, and shortly after CAR-T infusion.
DNA extraction for microbiome analyses and microbiome sequencing
The stool samples from both the MDACC and Moffitt Cancer Center were collectively processed for DNA extraction at the Microbiome Core at MDACC. Our pipeline for DNA extraction and sequencing has been described previously in detail.5 Briefly, for the MDACC and the Moffitt Cancer Center samples, bacterial genomic DNA was extracted using the QIAamp Fast DNA stool mini kit (Qiagen) according to the manufacturer’s protocol with modification to include an intensive bead-beating lysis step. A 3.2 mm steel bead and zirconium beads were added to the DNA bead beating steps for intensive lysis. Sequencing libraries were prepared using the Illumina DNA preparation kit (Illumina, catalog no. 20060060) according to the manufacturer’s protocol. The library quality was determined using an Agilent high-sensitivity D1000 screen tape assay (Agilent; catalog no. 5067-5584), and libraries were pooled at an equal molar ratio. The final libraries were sequenced on an Illumina NovaSeq 6000 platform with a 2 × 150 bp end read protocol, producing ∼5 Gb per sample.
Microbiome sequencing and analysis of sequencing data
Microbiome sequencing and analysis were performed as previous described.5 Sequencing reads from each sample in a pool were de-multiplexed based on their associated barcode sequence. All downstream sequencing data analyses were performed centrally at MDACC. Taxonomic and functional profiling of the metagenomes were performed using MetaPhlAn v3.0 and HUMAnN3 v3.0.0. alpha.4.12
MS analysis for metabolome analysis
To determine the relative abundance of short-chain fatty acids (SCFAs) in human fecal samples, extracts were prepared and analyzed using ultrahigh-resolution mass spectrometry (MS). Approximately 200 to 300 mg of human fecal pellets were flash frozen in liquid nitrogen and homogenized using a Precellys tissue homogenizer. Metabolites were extracted using 1 mL ice-cold 0.1% ammonium hydroxide in 80/20 (volume-to-volume ratio) methanol/water. Extracts were centrifuged at 17 000g for 5 minutes at 4°C, and supernatants were transferred to clean tubes, followed by evaporation to dryness under nitrogen. Dried extracts were reconstituted in deionized water, and 5 μL was injected for analysis by ion chromatography–MS. Ion chromatography mobile phase A (weak) was water, and mobile phase B (MPB; strong) was 100 mM KOH in water. A Thermo Scientific Dionex ICS-5000+ system with a Thermo IonPac AS11 column (4 μm particle size, 250 × 2 mm) with the column compartment kept at 30°C was used. The autosampler tray was chilled to 4°C. The mobile phase flow rate was 360 μL/min, and the gradient elution program was 0 to 5 minutes at 1% MPB; 5 to 25 minutes from 1% to 35% MPB; 25 to 39 minutes from 35% to 99% MPB; 39 to 49 minutes at 99% MPB; and 49 to 50 minutes from 99% to 1% MPB. The total run time was 50 minutes. To assist the desolvation for better sensitivity, methanol was delivered by an external pump and combined with the eluent via a low dead-volume mixing tee. Data were acquired using a Thermo Orbitrap Fusion Tribrid mass spectrometer under electrospray ionization negative ionization mode at a resolution of 240 000. Raw data files were imported to Thermo Trace Finder and Compound Discoverer software for spectrum database analysis. A standard curve was generated at increasing concentrations of reagent buffers to calculate the relative concentrations of SCFAs in the stool samples. To clarify, the values generated by these methods were not absolute concentrations present in the gut, because the standards in the reagent were not in the real biologic matrix. We also conducted nontargeted analysis that measured anionic metabolites, including SCFAs, organic acids, sugar phosphate, and nucleotides. This platform can detect 150 to 180 metabolites in varying types of samples.
Bioinformatics and statistical analysis
To characterize the diversity of the bacteria present in each sample (alpha diversity), we computed the Inverse Simpson index on the rarified count data using the R package phyloseq.13 The Wilcoxon rank sum test was used to compare the alpha diversity between 2 groups, whereas the Kruskal-Wallis test was used to compare the alpha diversity across multiple groups, with Dunn post hoc test to establish pairwise differences. To characterize the changes in microbial abundance between clinically relevant time points in relation to ide-cel infusion, a linear mixed model analysis was performed using centered log ratio transformed microbiome abundances.14 For the beta diversity analysis, principle coordinates analysis plots were generated using Bray-Curtis distances. Stacked bar plots were used to visualize the microbiome composition of the stool samples. To evaluate the multivariate community-level differences between groups of outcomes, a permutational analysis of variance was performed using the adonis2 function within the R package vegan.15 On a granular level, to investigate which specific taxa were differentially abundant between patient groups, a differential abundance analysis was performed using the R package Microbiome Multivariable Association with Linear Models (MaAsLin2).16 MaAsLin2 relies on generalized linear models to identify differentially abundant taxa and can accommodate longitudinal study designs. For multiplicity correction, the false discovery rate (FDR)17 was calculated using the raw P values.
For the progression-free survival (PFS) analysis, the categorization of subjects into high or low groups was achieved by identifying the most suitable threshold for a continuous variable using the cutp function of the R package survMisc.18 Specifically, in this instance, the focus was on the distribution of normalized species abundance. Time to progression was defined as the time interval in days between the initial ide-cel infusion and disease progression. Survival curves were constructed using the Kaplan-Meier method, and a comparison of survival curves was performed using the log-rank test.
For analysis of the metabolomics data, a correlation heatmap was plotted to understand the level of correlation between the different metabolites. A test of correlation was also performed between the metabolites and the differentially abundant species found using MaAsLin2 for different outcomes of interest to detect indirect associations between the metabolites and outcomes potentially mediated through the microbiome. For multiplicity correction, the FDR was calculated using the raw P values.
Declaration of generative AI and AI-assisted technologies in the writing process
During the final editorial process of this work, the author(s) used ChatGPT 3.5 to reduce the length of the article. After using this tool, the author(s) reviewed and edited the content as needed and take(s) full responsibility for the content of the published article.
Results
Baseline patient characteristics
Our study included 117 stool samples that were collected at different time points relative to CAR-T infusion from 33 patients with myeloma who received ide-cel therapy at MDACC (n = 20) and the Moffitt Cancer Center (n = 13) between September 2021 and July 2022. The patient demographic and disease characteristics are listed in Table 1. The median age of the participants was 62.0 years (range, 56.0-67.0 years) with 10 females and 23 males. Patients received a median of 4 lines of systemic therapy before ide-cel. In all, 27.2% (9/33), 15.2% (5/33), and 15.2% (5/33) of patients achieved complete remission (CR), very good partial response (VGPR), or partial response (PR), respectively, to ide-cel therapy. With a median follow-up of 378 days (95% confidence interval, 96-274 days), the median PFS was 98 days (range, 42-215). CRS was observed in 79% (26/33) of patients, including 17 (51.5%) patients with grade 1, 7 (21.2%) with grade 2, and 2 (6.1%) with grade ≥3 CRS. ICANS was reported for 5 patients (15.2%) of whom 3 (9.1%) and 2 (6.1%) had grade 1 and grade ≥2 ICANS severity, respectively.
Characteristics of patients with MM who were treated with ide-cel CAR-T therapy
| Patient characteristics . | Total cohort (N = 33) . |
|---|---|
| Age, y | |
| Median (range) | 62 (56-67) |
| >65, n (%) | 10 (30.3) |
| Gender, n (%) | |
| Female | 10 (30.3) |
| Male | 23 (69.7) |
| Race, n (%) | |
| White | 24 (72.7) |
| Black | 7 (21.2) |
| Other | 2 (6.1) |
| Diagnosis, n (%) | |
| CR | 9 (27.2) |
| VGPR | 5 (15.2) |
| PR | 5 (15.2) |
| ECOG performance score, n (%) | |
| 0-1 | 24 (72.7) |
| ≥2 | 4 (12.1) |
| Unknown | 5 (15.1) |
| Extramedullary disease, n (%) | |
| No | 20 (60) |
| Yes | 13 (40) |
| Previous lines of therapy, n (%) | |
| 3-4 | 6 (18) |
| 5-6 | 10 (30) |
| >6 | 17 (52) |
| Autologous stem cell transplantation history (yes or no), n (%) | |
| Yes | 30 (90.91) |
| No | 3 (9.09) |
| Bridging therapy (yes or no), n (%) | |
| Yes | 26 (79) |
| No | 7 (21) |
| R-ISS staging at CAR-T infusion, n (%) | |
| I | 5 (15) |
| II | 16 (49) |
| III | 12 (36) |
| Patient characteristics . | Total cohort (N = 33) . |
|---|---|
| Age, y | |
| Median (range) | 62 (56-67) |
| >65, n (%) | 10 (30.3) |
| Gender, n (%) | |
| Female | 10 (30.3) |
| Male | 23 (69.7) |
| Race, n (%) | |
| White | 24 (72.7) |
| Black | 7 (21.2) |
| Other | 2 (6.1) |
| Diagnosis, n (%) | |
| CR | 9 (27.2) |
| VGPR | 5 (15.2) |
| PR | 5 (15.2) |
| ECOG performance score, n (%) | |
| 0-1 | 24 (72.7) |
| ≥2 | 4 (12.1) |
| Unknown | 5 (15.1) |
| Extramedullary disease, n (%) | |
| No | 20 (60) |
| Yes | 13 (40) |
| Previous lines of therapy, n (%) | |
| 3-4 | 6 (18) |
| 5-6 | 10 (30) |
| >6 | 17 (52) |
| Autologous stem cell transplantation history (yes or no), n (%) | |
| Yes | 30 (90.91) |
| No | 3 (9.09) |
| Bridging therapy (yes or no), n (%) | |
| Yes | 26 (79) |
| No | 7 (21) |
| R-ISS staging at CAR-T infusion, n (%) | |
| I | 5 (15) |
| II | 16 (49) |
| III | 12 (36) |
ECOG, Eastern Cooperative Oncology Group; R-ISS, Revised International Staging System.
Changes in the gut microbiome diversity during ide-cel therapy
To examine alterations in the gut microbiota during ide-cel therapy, we performed whole genome shotgun sequencing on fecal samples from patients at various time points relative to ide-cel infusion. A total of 117 stool samples were acquired from 33 patients, spanning dates from 49 days before to 91 days after ide-cel infusion (supplemental Figure 1). We categorized these samples into 5 clinically relevant time points in relation to ide-cel infusion, namely at apheresis (median, day −21, range: −49 to −21), before lymphodepletion (median, day −5; range, −10 to −3), at ide-cel infusion (median, day 0; range: −2 to +2), 1 week after ide-cel (median, day +7; range, +4 to +11), and ≥2 weeks after ide-cel (median, day +28; range, 14 to 91).
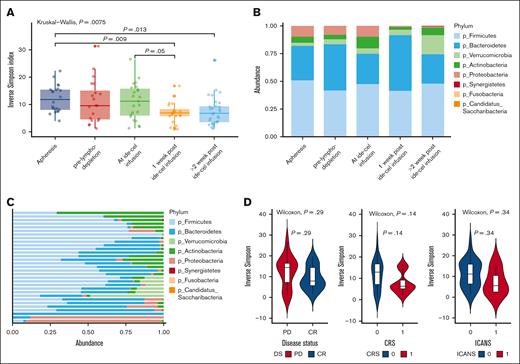
We first evaluated the diversity of the gut microbiota, as measured by the Inverse Simpson index,19 during ide-cel therapy. When compared with samples taken at the apheresis time point, a notable reduction in alpha diversity was observed following ide-cel infusion (Figure 1A). Furthermore, an investigation into the bacterial composition of the samples revealed the dominance of Firmicutes and Bacteroidetes at the phylum level at all time points (Figure 1B). Notably, a significant increase was observed in the relative abundance of Bacteroidetes in samples taken 1 week after ide-cel infusion when compared with those taken at the ide-cel infusion time point (Figure 1B; P = .0182).
Changes in the gut microbiome diversity during ide-cel therapy. (A) Bacterial diversity at different time points relative to CAR-T therapy, calculated using the Inverse Simpson index. This plot demonstrates the changes in bacterial diversity over time, highlighting how microbial communities evolve before, during, and after CAR-T therapy. (B) Relative abundance of different bacterial phyla in stool samples collected at various time points relative to ide-cel therapy. This bar plot shows the distribution of bacterial phyla, thereby enabling the comparison of microbial community composition at different stages of the treatment. (C) Phylum-level diversity of the baseline stool samples. This plot illustrates the diversity and abundance of bacterial phyla present in the baseline stool samples, providing a snapshot of the microbial community during initiation of therapy. (D) Box plots exploring the relationship between bacterial alpha diversity, measured at baseline, and the clinical outcomes of patients who underwent ide-cel therapy with the aim of identifying potential microbial predictors of treatment response and toxicities. PD, partial disease.
Changes in the gut microbiome diversity during ide-cel therapy. (A) Bacterial diversity at different time points relative to CAR-T therapy, calculated using the Inverse Simpson index. This plot demonstrates the changes in bacterial diversity over time, highlighting how microbial communities evolve before, during, and after CAR-T therapy. (B) Relative abundance of different bacterial phyla in stool samples collected at various time points relative to ide-cel therapy. This bar plot shows the distribution of bacterial phyla, thereby enabling the comparison of microbial community composition at different stages of the treatment. (C) Phylum-level diversity of the baseline stool samples. This plot illustrates the diversity and abundance of bacterial phyla present in the baseline stool samples, providing a snapshot of the microbial community during initiation of therapy. (D) Box plots exploring the relationship between bacterial alpha diversity, measured at baseline, and the clinical outcomes of patients who underwent ide-cel therapy with the aim of identifying potential microbial predictors of treatment response and toxicities. PD, partial disease.
We next focused our analysis on baseline samples and examined whether there was an association between the composition of microbiota and ide-cel therapy responses and toxicities. To establish an individual’s baseline, we selected the sample closest to day 0 and following the criteria that this sample must fall within the timeframe of day −21 to day +2 relative to the ide-cel infusion (supplemental Figure 1). Of the 33 subjects, a baseline fecal sample was available for 31, and we identified 445 species-level taxa observed in at least 1 sample. The composition of these samples at the phylum and genus levels is depicted in Figure 1C and supplemental Figure 2, respectively. Most of these baseline samples exhibited a high prevalence of Firmicutes and Bacteroidetes, although there was considerable variability at the genus level.
We also analyzed the alpha diversity of the baseline samples (days −21 to +2 relative to ide-cel infusion) and found no significant association with ide-cel response (responders [CR, VGPR, or PR at day 90, n = 18] vs nonresponders [partial disease, stable disease, or minimal response at day 90, n = 13]; Figure 1D), CRS toxicity (grade 0-1 vs grade ≥2; Figure 1D), or ICANS (grade 0 vs grade ≥1; Figure 1D). Beta-diversity analysis of the gut microbiota in baseline samples also did not show any significant differences in CAR-T response (responders vs nonresponders; supplemental Figure 3A; supplemental Table 1), CRS toxicity (grade 0-1 vs grade ≥2; supplemental Figure 3B; supplemental Table 2), or ICANS (grade 0 vs grade ≥1; supplemental Figure 3C; supplemental Table 3), indicating a lack of association between the microbiome diversity indices and clinical outcomes and toxicities to ide-cel therapy.20
Association of the gut microbiome with ide-cel responses
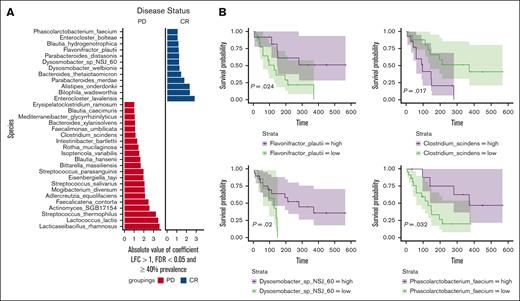
We next focused on differential abundance analysis to identify specific microbial features that differed at baseline between subjects who went on to be responders and nonresponders. We conducted a comparison between responders (CR, VGPR, or PR at day 90; n = 18) and nonresponders (partial disease, stable disease, or minimal response at day 90; n = 13) using MaAsLin2.21 Through this analysis, we identified a set of bacterial species that exhibited significant abundance differences (with a FDR <0.01; Figure 2A). Some of the species enriched in baseline samples from responder patients included Flavonifractor plautii, Bacteroides thetaiotaomicron, Bilophila wadsworthia, Alistipes onderdonkii, Parabacteroides, Dysosmobacter, and Enterocloster. We found that a higher relative abundance of microbial taxa within the class Bacteroidia, including the genera Bacteroides, Alistipes, and Parabacteroides, was associated with response. Conversely, a greater relative abundance of the Lachnospiraceae family, which includes genera such as Lachnoclostridium, Faecalimonas, and Enterocloster, and of the Streptococcaceae family, which includes the genera Lactococcus and Streptococcus, was observed in nonresponders (Figure 2A). In addition, to filter out spurious associations, the species were filtered to have a log-fold change (LFC) >1 and a prevalence of ≥40%. Although more features were initially discovered at a 25% prevalence level for the response analysis, we applied a 40% threshold to focus on clearer visualization and interpretation of the plots. Additional features that were identified at the 25% abundance threshold using MaAsLin2 analysis are presented in supplemental Figure 4. In addition, we also analyzed the abundance of these species individually as depicted in supplemental Figure 5.
Association of the gut microbiome with ide-cel responses. (A) Significant associations detected using MaAsLin2 for the disease status (DS) outcome. All hits were filtered for 40% prevalence, an LFC >1, and multiplicity corrected with an FDR <0.05. This plot highlights the taxa that were significantly associated with DS, indicating potential microbial markers of the condition. (B) Representative survival plots for differentially abundant species identified by MaAsLin2 showcasing the significant differences in survival probabilities associated with changes in DS. These plots illustrate how the presence and abundance of specific microbial species can influence survival outcomes, thereby emphasizing the clinical relevance of microbial composition in disease prognosis. PD, partial disease.
Association of the gut microbiome with ide-cel responses. (A) Significant associations detected using MaAsLin2 for the disease status (DS) outcome. All hits were filtered for 40% prevalence, an LFC >1, and multiplicity corrected with an FDR <0.05. This plot highlights the taxa that were significantly associated with DS, indicating potential microbial markers of the condition. (B) Representative survival plots for differentially abundant species identified by MaAsLin2 showcasing the significant differences in survival probabilities associated with changes in DS. These plots illustrate how the presence and abundance of specific microbial species can influence survival outcomes, thereby emphasizing the clinical relevance of microbial composition in disease prognosis. PD, partial disease.
We next assessed whether the differences in fecal sample collection methods used by the 2 centers impacted the identification of differential taxa. To evaluate the differences in global species composition, we performed a beta diversity analysis to examine variations in microbial community structure between the 2 sample collection methods. The permutational analysis of variance results were not significant with a P value of .403, indicating that there were no significant differences in beta diversity between the 2 centers (supplemental Figure 6). Consequently, we did not adjust for center as a covariate in the MaAsLin2 model when identifying differentially abundant taxa associated with disease status and toxicity variables. We further validated this by applying a center-adjusted MaAsLin2 model to identify differentially abundant features associated with disease status as shown in supplemental Figure 7A. Most differential features identified using the unadjusted model were retained as illustrated in supplemental Figure 7B.
Next, we conducted a Kaplan-Meier analysis by stratifying by the median baseline abundance of the selected differentially abundant species, which were filtered to have a prevalence of at least 40%, revealing significant associations with PFS for several species (Figure 2B). In particular, we found that patients with a high baseline abundance of F plautii, Dysosmobacter, and Phascolarctobacterium faecium exhibited a longer PFS than those with low abundance of these bacteria. In contrast, low abundance of Clostridium scindens was associated with longer PFS.
We subsequently performed a differential abundance analysis using MaAsLin2 on the longitudinal samples collected at various time points during ide-cel therapy from 33 patients. This analysis aimed to identify differential abundant species between responders and nonresponders across all time points. Notably, we observed enrichment of B thetaiotaomicron, Blautia faecis, and Dsysosmobacter species in samples from responder patients (supplemental Figure 8).
Association of the gut microbiome community dominance with ide-cel toxicities
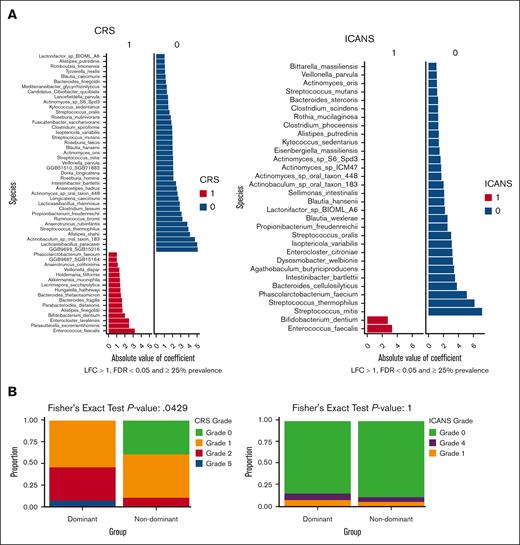
We also employed MaAsLin2 analysis on the baseline samples to identify species (filtered by an LFC >1 and a prevalence of ≥25%) that were differentially linked to CRS (grade 0-1 vs grade ≥2; Figure 3A) and ICANS (grade 0-1 vs grade ≥2; Figure 3B). We identified a number of species that exhibited differential abundance in individuals with lower or higher grades of CRS (supplemental Figure 9A) or ICANS (supplemental Figure 10A). The MaAsLin2 analysis conducted on baseline samples with species filtered by an LFC >1 and a prevalence of ≥ 40% is presented in supplemental Figure 9B for CRS and supplemental Figure 10B for ICANS. Similar to the previous publication that explored the role of the microbiome in CAR-T therapy for MM,8 we found that increased levels of facultative pathobionts, such as Enterococcus faecalis, corresponded with more severe cases of toxicities (Figure 3A-B).
Association of the gut microbiome community dominance with ide-cel toxicities. (A) Significant associations detected by MaAsLin2 for toxicity outcomes. CRS and ICANS were filtered to include only those with at least 25% prevalence, an LFC >1, and corrected for multiplicity with an FDR of <0.05. (B) Community dominance analysis illustrating the differences in the presence of dominant vs nondominant taxa in terms of toxicity outcomes.
Association of the gut microbiome community dominance with ide-cel toxicities. (A) Significant associations detected by MaAsLin2 for toxicity outcomes. CRS and ICANS were filtered to include only those with at least 25% prevalence, an LFC >1, and corrected for multiplicity with an FDR of <0.05. (B) Community dominance analysis illustrating the differences in the presence of dominant vs nondominant taxa in terms of toxicity outcomes.
In our cohort, we identified a striking observation concerning a patient whose baseline sample before ide-cel infusion exhibited substantial microbial disruption with the gut microbiota overwhelmingly dominated by a single genus of Bacteroidetes (abundance ∼95%). The patient was a 52-year-old female diagnosed with MM 4 years earlier with fluorescence in situ hybridization analysis showing translocation t(11;14) and 1q amplification. Before ide-cel therapy, she had received 6 lines of therapy, including an autologous stem cell transplant, and was penta-refractory. In the last 6 months, she experienced multiple hospital admissions for infections, including urinary tract infections and pneumonias with repeated exposure to broad-spectrum antibiotics, probably contributing to a dysbiotic microbiome. Before CAR-T therapy, bone marrow evaluations revealed a high tumor burden with 90% plasma cell neoplasm involvement. Following CAR-T infusion, the patient developed severe toxicity, reaching a maximum CRS grade of 5 and an ICANS grade of 4 within 3 days after ide-cel infusion. Her serum ferritin peaked at >97 000 ng/mL and C-reactive protein at 165 mg/L without other definitive criteria for hemophagocytic lymphohistiocytosis syndrome. Unfortunately, she succumbed to CRS on day 14 after infusion.
Single-taxon microbiome dominance, typically by facultative pathobionts, in a sample previously has been linked to microbiome injury.3 We therefore conducted an analysis to explore the relationship between taxonomic dominance and ide-cel toxicities. We defined a sample as having a taxonomic dominance if any genus in that sample had a prevalence of ≥35%. Among 31 baseline samples analyzed, dominance was observed in 13 samples within our cohort. Among these, 5 patients had a dominant species from the genus Bacteroides, whereas 1 patient had a dominant species from the genus Akkermansia. The incidence of high-grade CRS (grade 2 or higher) was significantly higher among patients with genus dominance in their baseline samples (46.2%) than among those without genus dominance (11.1%; P = .043; Figure 3B; supplemental Table 4). The clinical characteristics of patient cohorts with and without genus dominance are presented in supplemental Table 5. Among the patients with dominant species in their gut microbiota, each experienced a CRS event. Specifically, 7 patients had grade 1 CRS, 5 had grade 2, and 1 had grade 5 CRS. In contrast, among the 18 patients without species domination in their gut, 8 (44%) had no CRS event. Of those who had CRS events, 8 patients (44%) experienced grade 1 CRS, and only 2 patients (11%) had grade 2 CRS. No association was observed between the dominant taxonomic genus in the gut microbiome samples and ICANS (Figure 3B).
Association between gut microbial and stool metabolomics
We also conducted mass spectrometric assessments on stool samples collected on the day of ide-cel infusion (n = 16) to quantify essential metabolites, including SCFAs, indoles, and anionic metabolites. Subsequently, we conducted a correlation analysis between these metabolites and the bacterial species identified as differentially abundant (responders vs nonresponders) by MaAsLin2 analysis, focusing on various outcomes of interest to uncover potential indirect associations between metabolites and outcomes that are possibly mediated by these differentially abundant species. Figure 4A illustrates a correlation graph displaying the significant associations between microbial species linked to the responders to ide-cel (as in Figure 2A) and SCFAs. Our analysis revealed that genera, including Alistipes, Dysosmobacter, and Phascolactobacterium, that were more abundant in responders exhibited a significant correlation with elevated levels of SCFAs, such as propionic acid and valeric acid,22 in stool samples. Similarly, F plautii showed a notable correlation with increased levels of indoles in fecal samples (Figure 4B), suggesting that investigating the indole pathways in in vivo models is warranted to determine if specific indole derivatives enhance responses to CAR-T therapy. Additional correlation analyses between metabolites and bacterial species linked to toxicities (Figure 3A) are presented in supplemental Figures 11 and 12.
Association between gut microbial and stool metabolomics. (A) Significant correlations between taxa hits identified by MaAsLin2 for disease status outcome and indoles. (B) Significant correlations between taxa hits identified by MaAsLin2 for disease status outcome and SCFAs. (C) Representative significant associations between metagenomic (DNA) pathways and taxa hits identified for disease status outcome. PD, partial disease.
Association between gut microbial and stool metabolomics. (A) Significant correlations between taxa hits identified by MaAsLin2 for disease status outcome and indoles. (B) Significant correlations between taxa hits identified by MaAsLin2 for disease status outcome and SCFAs. (C) Representative significant associations between metagenomic (DNA) pathways and taxa hits identified for disease status outcome. PD, partial disease.
Pathways analysis
In addition to taxonomic associations, we also independently identified differentially abundant metagenomic functional pathways associated with responses through MaAsLin2 analysis. We detected 29 significant functional associations with metagenomic (DNA) pathways. MaAsLin2 considers a per-feature DNA covariate model in which per-feature normalized transcript abundance, along with other baseline covariates in the model, are regressed on per-feature normalized DNA abundances. In many of these pathways, functional perturbations were driven by shifts in their characteristic contributing taxa (Figure 4C). For example, the most significant DNA pathways enriched in patients with CR were characteristic of facultative anaerobes, such as F plautii and Escherichia coli. These included pathways such as carbohydrate degradation and fatty acid and lipid biosynthesis pathways among several others.
Discussion
In recent years, the gut microbiome has attracted considerable attention because of its associations with outcomes to anticancer therapies23 and their related toxicities, especially in the field of immune checkpoint blockade14,24,25 and allogeneic stem cell transplant therapy.3,16 Previous studies,6 including our own,5 have delved into gut microbiome associations in anti-CD19 CAR-T therapy for patients with lymphoma. To our knowledge, this is the first research that performed whole-genome shotgun sequencing on longitudinal stool samples collected at different time points from patients with MM who underwent standard-of-care, US Food and Drug Administration-approved ide-cel anti-BCMA CAR-T therapy. Our study offers novel microbiome findings and metabolome associations that warrant further mechanistic exploration for improving the clinical outcomes of ide-cel therapy. Key findings of our study include that the overall gut microbiome diversity was not linked to clinical responses; however, differential composition of bacterial species and pathways was associated with clinical outcomes and toxicities associated with ide-cel therapy. Another significant finding was that major microbiome injury was associated with certain ecologic taxa dominances, which were linked to higher grades of CRS and ICANS following ide-cel infusion. In addition, our network multiomics analysis identified differentially abundant species connected to specific gut metabolites and pathways, which affected the levels of immune-active metabolites and were associated with clinical outcomes.
In our literature review, we identified 1 study that performed gut microbiome analysis using 16S ribosomal RNA gene sequencing on stool samples from patients enrolled in an anti-BCMA CAR-T clinical trial.8 There are notable similarities and differences between that study and our own. Like the previous study, we observed a significant decline in gut bacterial diversity following CAR-T infusion. This observation aligns with findings from studies on anti-CD19 CAR-T therapy5,6 and allogeneic hematopoietic cell transplantation,3 which also reported a postinfusion decline in bacterial diversity. The reduction in bacterial diversity may be attributed to several factors, including the administration of antibiotics for febrile neutropenia or CRS complications, the effects of lymphodepleting chemotherapy, or reduced food intake caused by diminished appetite. However, in our study, the baseline bacterial diversity did not correlate with clinical responses or toxicities associated with CAR-T therapy. We did observe, however, a trend linking bacterial composition and diversity with CRS toxicity in patients who received ide-cel (supplemental Figure 3). In the other study,8 the authors similarly found no correlation between the baseline diversity at the time of infusion and clinical responses. However, they reported that patients with poor responses experienced a sharper decline in diversity after infusion than those with positive outcomes.
Another shared finding between the 2 studies was the expansion of certain pathobiont genera, particularly Enterococcus, which was associated with CAR-T–related toxicities. In the previous study, there was a significant expansion of Enterococcus in samples collected during episodes of CRS. Similarly, in our study, E faecalis was significantly linked to higher grades of CRS and ICANS. This post-therapy expansion of pathobionts may play a role in the pathophysiology of an exacerbated cytokine storm in CAR-T therapy. Despite these similarities, we did not identify overlapping genera or species associated with clinical responses between the 2 studies. In the previous study, certain genera, such as Prevotella, Collinsella, Bifidobacterium, and Sutterella, were linked to favorable responses. However, the taxa associated with CR in our study were entirely different. This discrepancy could be attributed to the smaller cohort sizes in both studies and the geographical differences between them. As we have shown in our previous work, geographic region can significantly influence the predictive capability of microbiome models.5
One of the interesting findings in our study was the strong association between profound microbiota injuries and higher grades of ide-cel–associated CRS. Patients whose baseline samples showed dominance of a single taxonomic genus were strongly associated with higher grades of CRS. Notably, 1 patient with more than 90% abundance of the Bacteroides genus in their baseline samples experienced grade 4 CRS and grade 5 ICANS and ultimately died as a consequence of toxicity. In addition, the finding that an increase in facultative pathobionts, such as E faecalis, in the damaged microbiome is significantly associated with CAR-T–related toxicities strongly indicates the potential of the microbiome to influence CRS associated with ide-cel therapy.
Our results also reveal novel associations of several key bacterial species that were abundant in responders to ide-cel therapy, which have previously been reported in the literature to impact immune responses significantly. Notably, F plautii stands out, because it is known to stimulate type I interferon signaling, thereby leading to enhanced T cell priming and improved efficacy of cancer immunotherapy in conjunction with checkpoint inhibitors.26 Our data also suggest that SCFAs are associated with ide-cel therapy responses,27 because beneficial bacteria in our cohort strongly associated with higher levels of SCFAs. SCFAs are known to influence key epigenetic and metabolic pathways, which are crucial for regulating T cell functions, such as differentiation, proliferation, apoptosis, and chemotaxis.28-30 These processes are vital for the proper activity of CAR-T cells, positioning SCFAs as important targets for enhancing CAR-T cell efficacy. Specifically, pentanoate has been shown to improve the effectiveness of CAR-T and adoptive T cell therapies in previous studies.22 Despite this, directly manipulating the in vivo SCFA levels in patients undergoing CAR-T therapy, either through probiotic supplementation or direct SCFA administration, may not produce the intended therapeutic benefits. This is because of the ability of SCFAs to activate regulatory immune cell subsets,31,32 which could suppress antitumor responses and negatively affect CAR-T cell outcomes. Our research, along with findings from other groups,33 supports the idea that supplementing CAR-T cells with SCFAs, such as valeric acid, during their production may enhance their metabolic activity, potentially leading to more potent CAR-T products. Considering the wide-ranging effects of SCFAs on both T cells and innate immunity, investigating their role in microbiome–T cell interactions presents a valuable opportunity to further optimize CAR-T cell therapies.
Through our MaAsLin2 analysis, we found that pathways related to amino acid metabolism, nucleotide biosynthesis, and cofactor and vitamin biosynthesis were significantly upregulated in ide-cel responders. Similar findings from another anti-BCMA CAR-T cohort8 highlighted the important role of amino acid biosynthesis pathway upregulation in favorable responses. The gut microbiome helps to metabolize amino acids from food and synthesize essential amino acids, thereby regulating the innate and adaptive immunity, which may be crucial for ide-cel therapy. We also found that tryptophan and its metabolites, such as tryptamine and indole-3-acetic acid, were significantly associated with responses in our cohort, indicating their important role.
In this study, to our knowledge, we report for the first time on the metagenomic sequencing of longitudinal samples from patients with MM who underwent standard-of-care ide-cel therapy. By analyzing these samples over time, we have uncovered novel associations between the microbiome and ide-cel response and toxicities. Further research into these associations using in vivo mouse models will be crucial to validate these findings and to elucidate the underlying mechanisms that link particular bacteria and metabolites to CAR-T responses and toxicities. Exploring these avenues could lead to the identification of specific microbiome modifications that enhance CAR-T cell efficacy or reduce toxicity. Such insights might pave the way for novel therapeutic strategies that involve manipulating the gut microbiome to improve patient outcomes in ide-cel therapy for MM.
Acknowledgments
This study was supported by the generous philanthropic contributions to The University of Texas MD Anderson Cancer Center Moon Shots Program and by the Platform for Innovative Microbiome and Translational Research at the Department of Genomic Medicine at The University of Texas MD Anderson Cancer Center. This work was supported, in part, by a Cancer Center Support Grant (P30CA016672) from the National Cancer Institute and the Microbiome Core Facility at the MD Anderson Cancer Center and by a Cancer Center Support Grant (P30CA076292) from the National Cancer Institute to the Moffitt Cancer Center and the Moffitt Flow Cytometry Core Facility. N.Y.S. was supported by grants from the Cancer Prevention and Research Institute of Texas (RP240326 and RP250131). R.R.J. was supported National Institutes of Health, National Heart, Lung, and Blood Institute grant R01HL124112. C.P. was partially supported by an Andrew Sabin Family Fellowship and CCSG P30CA016672 (Biostatistics Shared Resource Group). C.P. and S.S. were partially supported by National Heart, Lung, and Blood Institute grant R01HL158796. R.Z.O., from the Florence Maude Thomas Cancer Research, acknowledges support from the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation, the Riney Family Multiple Myeloma Research Fund, Moon Shot funding from MD Anderson Cancer Center, and the Paula and Rodger Riney Foundation.
Authorship
Contribution: N.Y.S., L.R., A.R., D.M.W., M.B., M.G., S.K.T., H.C.L., M.D.J., F.L.L., M.L.D., M.M., S.C., R.A., and K.K.P. supervised patient recruitment and data and biospecimen collection; N.Y.S., M.D.J., R.R.J., D.K.H., and K.K.P. contributed to institutional review board filing and data safety; N.Y.S., M.D.J., D.K.H., and K.K.P. contributed to the study concept and design; C.-C.C. and the Platform for Innovative Microbiome and Translational Research core at the MD Anderson Cancer Center performed the DNA extractions from stool samples, microbiome sequencing, and metabolomic studies; S.S., N.Y.S., and C.P. designed and headed all computational and analytical aspects related to this study; N.Y.S. wrote the first draft of the manuscript; N.Y.S., D.K.H., C.P., and K.K.P. jointly supervised the work; and all authors contributed to the final version of the manuscript.
Conflict-of-interest disclosure: M.D.J. reports consultancy/advisory fees from Kite/Gilead, Novartis, Bristol Myers Squibb (BMS), and Myeloid Therapeutics, and research funding from Incyte and Kite/Gilead. S.S.N. reports research support from Kite/Gilead, BMS, Allogene, Precision Biosciences, Adicet Bio, Sana Biotechnology, and Cargo Therapeutics; serving as an advisory board member/consultant for Kite/Gilead, Merck, Sellas Life Sciences, Athenex, Allogene, Incyte, Adicet Bio, BMS, bluebird bio, Fosun Kite, Sana Biotechnology, Caribou, Astellas Pharma, MorphoSys, Janssen, Chimagen, ImmunoACT, Orna Therapeutics, Takeda, Synthekine, CARsgen, Appia Bio, and GlaxoSmithKline; having stock options from Longbow Immunotherapy, Inc; and having intellectual property related to cell therapy. C.R.F. reports serving as a consultant for AbbVie, Bayer, BeiGene, Celgene, Denovo Biopharma, Foresight Diagnostics, Genentech/Roche, Genmab, Gilead, Karyopharm, N-Power Medicine, Pharmacyclics/Janssen, Seagen, and Spectrum; having stock or stock options in Foresight Diagnostics and N-Power Medicine; and receiving research funding from 4D, AbbVie, Acerta, Adaptimmune, Allogene, Amgen, Bayer, BostonGene, Celgene, Cellectis EMD, Gilead, Genentech/Roche, Guardant, Iovance, Janssen Pharmaceutical, Kite, MorphoSys, Nektar, Novartis, Pfizer, Pharmacyclics, Sanofi, Takeda, TG Therapeutics, Xencor, Ziopharm, Burroughs Wellcome Fund, Eastern Cooperative Oncology Group, National Cancer Institute, V Foundation, and the Cancer Prevention and Research Institute of Texas: CPRIT Scholar in Cancer Research. R.Z.O. reports research funding unrelated to this work from Heidelberg Pharma AG, Asylia Therapeutics, and Biotheryx; serving on advisory boards for Amgen, Inc, BMS, Celgene, EcoR1 Capital LLC, Forma Therapeutics, Genzyme, GSK Biologicals, Ionis Pharmaceuticals, Inc, Janssen Biotech, Juno Therapeutics, Kite Pharma, Legend Biotech USA, Molecular Partners, Sanofi-Aventis, Servier, and Takeda Pharmaceuticals North America, Inc; and being a Founder of Asylia Therapeutics, Inc, with an equity interest. N.Y.S. reports research funding from Panbela Therapeutics. R.R.J. reports serving as a consultant or an advisory board member for Merck, Microbiome DX, Karius, MaaT Pharma, LisCure, Seres, Kaleido, and Prolacta, and having received patent license fees or stock options from Seres and Kaleido. K.K.P. reports research funding from and/or has served on advisory boards for AbbVie, Arcellx, AstraZeneca, BMS, Caribou Biosciences, Celgene, Genentech, Janssen, Kite, Merck, Oricel, Novartis, Legend Biotech, Pfizer, Regeneron, and Sanofi. N.Y.S., C.-C.C., S.S.N., and R.R.J. are inventors on patent applications through The University of Texas MD Anderson Cancer Center related to the results of the current study entitled “Serum Metabolomics Related to Chimeric Antigen Receptor (CAR) T-Cell Therapy” and “Gut Microbiome as a Predictive Biomarker of Outcomes for Chimeric Antigen Receptor T-Cell Therapy and its Modulation to Enhance Efficacy and Reduce Toxicity.” The remaining authors declare no competing financial interests.
Correspondence: Christine Peterson, Department of Biostatistics, The University of Texas MD Anderson Cancer Center, 1400 Holcombe Blvd, Unit 423, Houston, TX 77030; email: cbpeterson@mdanderson.org; Doris K. Hansen, Department of Blood and Marrow Transplant and Cellular Immunotherapy, Moffitt and Department of Oncologic Sciences, Tampa, FL 33612; email: dhansen@moffitt.org; Neeraj Y. Saini, Department of Stem Cell Transplantation & Cellular Therapy, The University of Texas MD Anderson Cancer Center, 1400 Holcombe Blvd, Unit 423, Houston, TX 77030; email: nsaini@mdanderson.org; and Krina K. Patel, Department of Lymphoma and Myeloma, The University of Texas MD Anderson Cancer Center, 1400 Holcombe Blvd, Unit 423, Houston, TX 77030; email: kpatel1@mdanderson.org.
References
Author notes
S.S. and L.R. are joint first authors.
C.P., D.K.H., N.Y.S., and K.K.P. contributed equally and are joint senior authors.
Data are available on request, as long as patient privacy is maintained, from the corresponding author, Neeraj Saini (nsaini@mdanderson.org). The underlying data presented in this study are available in the main manuscript and the supplemental Material. Any further needed information is available on request from the corresponding authors.
The full-text version of this article contains a data supplement.