Key Points
Lymphoma B cells trigger transcriptional reprogramming and deregulation of ECM organization in BM stromal cells.
Alteration of the tumor-supportive BM stromal niche in FL persists after treatment.
Visual Abstract
Bone marrow (BM) involvement is a common feature of germinal center–derived B-cell lymphomas and is associated with a poor prognosis. In particular, follicular lymphoma (FL) infiltrates the BM in 70% of cases, and analysis of in vitro–expanded FL BM mesenchymal stromal cells (MSCs) has revealed an extensive alteration of BM stromal cell phenotypic, transcriptomic, and functional profiles. However, the mechanisms underlying the direct interplay between lymphoma B cells and their permissive stromal niche in situ have not yet been identified. In this study, we identified a significant remodeling of extracellular matrix (ECM) composition and organization in the BM of patients with FL and in a murine model of lymphoma B-cell BM xenograft. In particular, murine leptin receptor (LepR+) MSCs were identified by single-cell RNA sequencing as engaged in a bidirectional cross talk with malignant B cells, triggering their specific and progressive reprogramming and commitment toward a phenotype resembling that of human ECM/transforming growth factor β (TGFβ) myofibroblastic cancer–associated fibroblasts (CAFs) and FL-CAFs. Kinetic analysis of FL BM samples showed that ECM and TGFβ deregulation persisted after treatment, suggesting it may contribute to disease persistence and relapse. Overall, this work sheds new light on the kinetics and mechanisms of BM stromal niche reshaping in B-cell lymphomas.
Introduction
Follicular lymphoma (FL) and diffuse large B-cell lymphoma (DLBCL) are the 2 most common B-cell lymphomas.1-3 FL is an indolent disease, characterized by the accumulation of germinal center (GC)-derived B cells with a disseminated pattern, infiltrating the bone marrow (BM) in 50% to 70% of cases at diagnosis.3 Compared with lymph node (LN) FL B cells, BM FL B cells remain organized as nodular follicle-like aggregates but exhibit a lower cytological grade, a reduced proliferation, a loss of CD10 expression, and a specific transcriptomic profile reflecting reduced metabolic activity.4-6 Furthermore, BM is a niche for long-lived lymphoma precursor cells, as highlighted by the synchronous development of a clonally related FLs in donor and recipient of allogeneic BM transplantation.7 DLBCL is a group of heterogeneous GC/post-GC aggressive lymphomas, with a BM involvement in 10% to 25% of patients.8 FL and GC-derived DLBCL (GCB-DLBCL), in contrast to non-GC–derived B-cell lymphomas, share a preferential paratrabecular niche in invaded BM.9 FL transforms into DLBCL, usually of GCB type, at an annual rate of 3%.10,11 BM involvement is an adverse prognostic factor in both FL and DLBCL and is included in the lymphoma risk-stratification scores.8,12-14 The BM microenvironment provides a supportive niche for tumor B cells throughout lymphoma development, from pretumoral stage to overt lymphoma, relapse, and/or transformation,15 but the composition and function of BM lymphoma-supportive niches and how they acquire their protumoral activity remain elusive.
FL is a neoplasia highly dependent on a complex and dynamic tumor microenvironment (TME), including reprogrammed CD4+ T cells, myeloid cells, and lymphoid stromal cells (LSCs), that are closely related to the normal GC niche but exhibit specific phenotypic, transcriptomic, and functional features.3,16 FL-infiltrating LSCs are extensively remodeled in situ within FL LNs, showing deregulated production of chemokines and extracellular matrix (ECM) components, thus forming an organized network of heterogeneous cancer-associated fibroblasts (CAFs).17 Similarly, mesenchymal stromal cells (MSCs) obtained from FL BM display a specific transcriptomic profile associating an ectopic LSC-like signature with upregulation of CCL2 and interleukin-8 (IL-8).18,19 In accordance, FL BM-MSCs have both a greater capacity to directly support FL B-cell survival and to recruit protumoral monocytes and neutrophils compared with BM-MSCs obtained from healthy donors (HDs). Both CCL2 and IL-8 are inducible in vitro by coculture of HD BM-MSCs with malignant B cells, suggesting a direct role for FL B cells in the priming of BM FL-CAFs.18-20 Although LN FL-CAFs have been characterized by single-cell RNA sequencing (scRNAseq) approaches,21 data on BM FL-CAFs have only been obtained using long-term in vitro expanded cells. Furthermore, the ECM profile of BM FL-CAFs remains unexplored. In DLBCL, despite convincing data revealing LSC remodeling within invaded LNs, associated with alteration of chemokine and ECM profiles,22 BM DLBCL-CAFs have never been studied.
In this study, we used BM samples from patients with lymphoma and a model of lymphoma B-cell xenograft to decipher the progressive reprogramming of BM stromal cells in lymphoma-invaded BM. Through scRNAseq and immunohistofluorescence analyses of stromal heterogeneity, we identified Leptin receptor (LepR+) MSCs as the major cell compartment affected by tumor B-cell invasion and describe a deregulation of ECM organization directly driven by B-cell growth. The alteration of the CAF-derived ECM pattern within FL BM persisted after treatment, suggesting a role in disease persistence and relapse in this incurable disease. This work offers new insights into the kinetics of BM stromal niche remodeling in lymphoma and highlights the role of the B-cell/stromal cell cross talk in BM TME polarization.
Materials and methods
For details, see online supplemental Methods.
Human samples and cell lines
The research protocol was conducted under French legal guidelines with informed consent and was approved by the local ethics committee. BM plasmas were obtained from patients with FL collected at diagnosis and after 12 months of treatment (Tazemetostat in Newly Diagnosed Diffuse Large B Cell and Follicular Lymphoma Patients Treated by Chemotherapy trial, ClinicalTrials.gov identifier: NCT02889523; supplemental Table 1) and from age-matched patients undergoing cardiac surgery used as HDs. BM biopsies were obtained from patient diagnosis and reviewed by expert pathologists as FL or normal BM before analysis by immunohistofluorescence. The FL cell line DOHH2 was obtained from the DSMZ cell collection (Braunschweig, Germany) and the GCB-DLBCL cell line OCI-Ly-19 was a gift from Lou Staudt (National Cancer Institute, Bethesda, MD). Luciferase-expressing DOHH2 cells (Luc-DOHH2) were established after transduction with a lentivirus carrying the pHAGE PGK-GFP-IRES-LUC-W plasmid (research resource identifier: Addgene_46793).
Xenograft models
Mice were maintained in specific pathogen–free conditions in the Rennes animal facility. Seven- to 10-week-old male Rag2−/−γc−/− mice received transplantation with either 0.5 × 106 DOHH2, OCI-Ly-19, or Luc-DOHH2 cells by intrafemoral route or were injected with phosphate-buffered saline (PBS; sham mice). Two days before intrafemoral grafts, mice underwent a myeloablation using 2 intraperitoneal injections of hydroxyurea (H8627, Sigma Aldrich; 1 mg/g) separated by 8 hours. DOHH2 infiltration in the blood, spleen, and femurs (injected and contralateral) was studied by flow cytometry (supplemental Table 2) on a CytoFLEX (Beckman Coulter) at different time points as the percentage of human CD20+ cells compared with murine CD45+ cells. When indicated, tumor dissemination of Luc-DOHH2 was monitored using a PhotonIMAGER Optima system (Biospace Lab). BM plasmas were collected for soluble factor quantification and injected BM femurs were collected for immunofluorescence imaging.
Transcriptomic study of murine endothelial and stromal BM cells and DOHH2 cells
Mouse femurs of steady-state (control), PBS-injected (sham), and DOHH2-injected (grafted) mice were harvested at day 19 and day 40. Cells were sorted using a FACSAria II (BD Biosciences) as Cd45/Ter119−Cd31−Cd51+Cd200+ stromal cells, Cd45/Ter119−Cd31+Sca1+ endothelial cells, and human CD20+ human lymphoma B cells. Sorted DOHH2 were analyzed by bulk RNA sequencing. Sorted stromal and endothelial cells were merged at a 1:1 ratio before encapsulation into emulsion droplets using Chromium Controller (10× Genomics).
Results
Characterization of the ECM niche in the BM from patients with FL
FL LN stromal cells exhibit a specific ECM-related gene signature, including upregulation of numerous factors involved in ECM composition, stiffness, mechanical and biological properties, or turnover.17,21 We therefore explore the ECM profile of the BM stroma of patients with BM-invaded FL at diagnosis, by quantifying ECM components in the BM plasma and on BM biopsies. In addition to SPARC, a matricellular protein previously reported in LNs and BM infiltrated by GC-derived lymphomas,9 we identified fibroblast activation protein (FAP), Lumican, MMP-2, and tissue inhibitor of metalloproteinase 1 (TIMP1) (all previously found upregulated in FL LN CAF scRNAseq data21) as increased in the BM plasma of patients with FL at diagnosis compared with age-matched HDs (Figure 1A; supplemental Figure 1A). In addition, the network of collagen VI, a marker strongly expressed by LN fibroblastic reticular cells (FRCs),23 was variably amplified and reorganized in FL BM compared with HD BM, notably within or around B-cell aggregates (Figure 1B; supplemental Figure 1B). Finally, LOX, a collagen crosslinking factor overexpressed in CAFs from solid cancers and in LN FL-CAFs,21 was found upregulated in FL BM compared with HD BM with a gradient from paired box 5 (PAX5)-low noninvaded to PAX5-medium poorly invaded areas and, finally, PAX5-high malignant B-cell nodular aggregates (Figure 1C-E; supplemental Figure 1B-C). These results suggest a common ECM remodeling pattern in FL-CAFs from the LNs and BM and argue for a direct impact of tumor B cells on BM stroma reprogramming.
Characterization of the ECM niche in the BM from patients with FL. (A) Soluble matrix protein levels measured in the BM plasma from patients with FL (n = 11) and HDs (n = 12) using Luminex assay. Statistical significance was determined using the Mann-Whitney nonparametric U test. ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. (B) Immunofluorescence on FL BM sections for PAX5 (cyan) and collagen VI (ColVI; green). Nuclei were counterstained with DAPI (4′,6-diamidino-2-phenylindole). Scale bar, 200 μm. Boxes indicate 2 PAX5hi areas magnified in right panels for ColVI and PAX5 staining (scale bars, 50 μm). (C) Immunofluorescence on FL BM sections for PAX5 (cyan) and LOX (red). Nuclei were counterstained with DAPI. Scale bar, 500 μm. Boxes indicate areas magnified in right panels in which scale bars represent 50 μm. Each box illustrates a category of PAX5 expression area: (1) PAX5hi area, (2) PAX5med area, and (3) PAX5lo area. (D) Quantification of LOX signal intensity in HD (n = 5) vs patients with FL (n = 12). Each dot corresponds to the quantification of the whole slide for 1 sample (HD or FL). Statistical significance was determined using the Mann-Whitney nonparametric U test. ∗P < .05. (E) Quantification of LOX signal intensity in the 3 categories of PAX5 frequency areas in patients with FL (n = 12). Each symbol represents a different patient with FL. The green dotted line represents LOX intensity level in HDs. Statistical significance was determined using the Friedman test with Wilcoxon matched-pairs comparisons. ∗∗∗P < .001. hi, high; lo, low; med, medium; MFI, mean fluorescence intensity.
Characterization of the ECM niche in the BM from patients with FL. (A) Soluble matrix protein levels measured in the BM plasma from patients with FL (n = 11) and HDs (n = 12) using Luminex assay. Statistical significance was determined using the Mann-Whitney nonparametric U test. ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. (B) Immunofluorescence on FL BM sections for PAX5 (cyan) and collagen VI (ColVI; green). Nuclei were counterstained with DAPI (4′,6-diamidino-2-phenylindole). Scale bar, 200 μm. Boxes indicate 2 PAX5hi areas magnified in right panels for ColVI and PAX5 staining (scale bars, 50 μm). (C) Immunofluorescence on FL BM sections for PAX5 (cyan) and LOX (red). Nuclei were counterstained with DAPI. Scale bar, 500 μm. Boxes indicate areas magnified in right panels in which scale bars represent 50 μm. Each box illustrates a category of PAX5 expression area: (1) PAX5hi area, (2) PAX5med area, and (3) PAX5lo area. (D) Quantification of LOX signal intensity in HD (n = 5) vs patients with FL (n = 12). Each dot corresponds to the quantification of the whole slide for 1 sample (HD or FL). Statistical significance was determined using the Mann-Whitney nonparametric U test. ∗P < .05. (E) Quantification of LOX signal intensity in the 3 categories of PAX5 frequency areas in patients with FL (n = 12). Each symbol represents a different patient with FL. The green dotted line represents LOX intensity level in HDs. Statistical significance was determined using the Friedman test with Wilcoxon matched-pairs comparisons. ∗∗∗P < .001. hi, high; lo, low; med, medium; MFI, mean fluorescence intensity.
Intrafemoral xenograft model reproduces lymphoma BM niche
To decipher the direct cross talk between lymphoma B cells and the BM stromal cell compartment, we developed an intrafemoral xenograft model in Rag−/−γc−/− mice using 2 GC-derived lymphoma B-cell lines: the GCB-DLBCL cell line, OCI-Ly-19; and the FL cell line, DOHH2. At 0.5 × 106 cells per mice, the engraftment rate was of 100% for both cell lines but with a median day of euthanasia of 25 for OCI-Ly-19–grafted mice and 42 for DOHH2-grafted mice (P < .001; supplemental Figure 2A). Although OCI-Ly-19 disseminated rapidly in all mouse tissues (data not shown), DOHH2 initially engrafted in the injected femur and expanded later, specifically in the contralateral femur, as shown by flow cytometry and bioluminescence imaging (Figure 2A). This slower tumor progression and preferential BM tropism support the use of DOHH2 intrafemoral xenograft as a model of B-cell lymphoma BM localization. To capture the kinetics of B-cell/stromal cell cross talk, we defined day 40 as the later evaluable time point (Gr.Late) and day 19 as the time point when BM involvement in the grafted femur was 10-fold lower (Gr.Early; supplemental Figure 2B).
Intrafemoral xenograft model reproduces lymphoma BM niche. (A) Tumor cell dissemination. Evolution over time of the proportion of viable human tumor cells (DAPI–hCD20+) compared with viable murine CD45+ cells in the blood (red), spleen (blue), and BM (grafted femur in green and contralateral femur in orange) of Rag−/−γc−/− mice that were transplanted with 0.5 × 106 DOHH2 cells by intrafemoral route (left). Luciferase imaging of representative mouse from 14 to 42 days after engraftment. Green star indicates grafted femur, and purple star indicates contralateral femur (right). (B) Experimental design of the B-cell and stromal cell transcriptomic characterization in Rag−/−γc−/− mice untreated, injected with PBS (sham), or grafted with DOHH2. (C) Using gene set enrichment analysis, enrichment for pathways upregulated in published data of primary FL B cells (FL) vs centrocytes24 were investigated in published data of DOHH2 cocultured with tonsil stromal cells and ECM in DT3D vs classical D2D25 and in DOHH2 recovered at the late time point (day 40) and/or at the early time point (day 19). Circle colors depict the NES and circle sizes the FDR (left). Spearman correlation plot of comparisons using NES values from previously selected pathways (right). ∗P < .05. CC, centrocytes; D, day; D2D, 2-dimensional DOHH2 culture; DT3D, 3-dimensional spheroids with tonsil stromal cells; FDR, false discovery rate; hCD20, human CD20; mCD45, murine CD45; max, maximum; min, minimum; NES, normalized enrichment score; q.val, q value.
Intrafemoral xenograft model reproduces lymphoma BM niche. (A) Tumor cell dissemination. Evolution over time of the proportion of viable human tumor cells (DAPI–hCD20+) compared with viable murine CD45+ cells in the blood (red), spleen (blue), and BM (grafted femur in green and contralateral femur in orange) of Rag−/−γc−/− mice that were transplanted with 0.5 × 106 DOHH2 cells by intrafemoral route (left). Luciferase imaging of representative mouse from 14 to 42 days after engraftment. Green star indicates grafted femur, and purple star indicates contralateral femur (right). (B) Experimental design of the B-cell and stromal cell transcriptomic characterization in Rag−/−γc−/− mice untreated, injected with PBS (sham), or grafted with DOHH2. (C) Using gene set enrichment analysis, enrichment for pathways upregulated in published data of primary FL B cells (FL) vs centrocytes24 were investigated in published data of DOHH2 cocultured with tonsil stromal cells and ECM in DT3D vs classical D2D25 and in DOHH2 recovered at the late time point (day 40) and/or at the early time point (day 19). Circle colors depict the NES and circle sizes the FDR (left). Spearman correlation plot of comparisons using NES values from previously selected pathways (right). ∗P < .05. CC, centrocytes; D, day; D2D, 2-dimensional DOHH2 culture; DT3D, 3-dimensional spheroids with tonsil stromal cells; FDR, false discovery rate; hCD20, human CD20; mCD45, murine CD45; max, maximum; min, minimum; NES, normalized enrichment score; q.val, q value.
Based on these data, we sorted viable Lin–CD31+SCA-1+ endothelial cells and Lin–CD51+CD200+ stromal cells (supplemental Figure 2C) before any manipulation (control mice), in PBS-grafted mice at day 40 (sham mice), and in DOHH2-grafted mice at day 19 (Gr.Early mice) and day 40 (Gr.Late mice), and analyzed them by scRNA sequencing (Figure 2B). In addition, we studied the gene expression profile of DOHH2 at day 19 and day 40 compared with DOHH2 before transplantation (day 0) by bulk RNA sequencing and determined differentially expressed genes (DEGs) between these 3 conditions (n = 3; adjusted P value <.05; supplemental Table 3). We applied gene set enrichment analysis to these gene lists and highlighted progressively upregulated pathways relevant to lymphoma biology, including tumor necrosis factor, interferon, transforming growth factor β (TGFβ), and hypoxia (Figure 2C). Similar reprogrammed pathways were found in published data sets of DOHH2 encapsulated in 3-dimensional spheroids in the presence of stromal cells and ECM, a model found to reproduce in vitro FL B cell/stromal cell cross talk,25 as well as in primary FL B cells compared with normal centrocytes.24 TGFβ1 has previously been reported as involved in ECM deregulation in FL LNs and overexpressed in FL B cells compared with normal centrocytes.17 We identified an upregulation of TGFβ1 in Gr.Late mice BM plasma compared with sham mice (supplemental Figure 2D). These data suggest that our in vivo xenograft model is relevant to FL biology and will be useful to capture B-cell/stromal cell interactions.
Reprogramming of LepR+ MSCs in lymphoma-invaded BM
Our scRNAseq data set corresponded to a total of 15 590 cells organized in 15 clusters after unbiased uniform manifold approximation and projection clustering (Figure 3A). Based on a previously published atlas in steady-state C57BL/6 mice,26 we identified 5 clusters of chondrocytes and fibroblasts (clusters 8, 12, 9, 5, and 14), 1 cluster of pericytes (cluster 11), 4 clusters of sinusoidal and arteriolar/arterial endothelial cells (clusters 2, 3, 10, and 7), 2 clusters of osteolineage cells (clusters 6 and 13), and 3 clusters of LepR+ MSCs (clusters 4, 0, and 1). We then assessed the repartition of the different experimental conditions within each cluster (Figure 3B). According to a χ2 test, the most significantly unbalanced clusters between control, sham, Gr.Early, and Gr.Late mice were the 3 LepR+ MSC clusters. Cluster 4 consisted almost exclusively of LepR+ MSCs from control mice, cluster 0 was strongly enriched for LepR+ MSCs from sham mice, and cluster 1 consisted mainly of LepR+ MSCs from grafted mice. Interestingly, the LepR-MSC signature obtained from steady-state C57BL/6 mice26 was less enriched in cluster 1 than in clusters 0 and 4, despite a high Lepr expression (supplemental Figure 3A-B), indicating that DOHH2-grafted LepR+ MSCs displayed a specific gene expression profile, distinct from that of LepR+ MSCs from immunocompetent mice with normal B-cell hematopoiesis. Based on these observations, we decided to focus our study on LepR+ MSCs.
Lymphoma B cells drive LepR+ MSC transcriptional reprogramming. (A) Uniform manifold approximation and projection (UMAP) plot of the scRNAseq data from endothelial and stromal cells of Rag−/−γc−/− mice untreated (Ctrl), injected with PBS (sham), or grafted with DOHH2 and euthanized at early (day 19) and late (day 40) time points (left). Heat map of the enrichment of mouse nonhematopoietic cell signature scores as previously defined by scRNA sequencing in steady-state C57BL/6 mice (right).26 (B) Stacked bar plot representing, for each cluster, the proportion of cells coming from the 4 conditions (control, sham, Gr.Early, and Gr.Late). The last bar of the plot represents the distribution of all cells between the different experimental conditions. The unbalance between experimental conditions in each cluster was evaluated using maximum residual values from a χ2 test. (C) UMAP plot of the subclustering of the 3 most unbalanced clusters (4, 0, and 1) after analysis of differential abundance using the differentially abundant sequencing (DA-seq) algorithm. Four LepR+ MSC subclusters were identified: MSC_Ctrl, MSC_Sh, MSC_Gr.Early, and MSC_Gr.Late. (D) Venn diagram of the differentially expressed genes between MSC_Gr.Late vs MSC_Gr.Early, MSC_Gr.Late vs MSC_Sh, and MSC_Gr.Early vs MSC_Sh (adjusted P value <.05; absolute value of log2 fold change of >.25). (E) Heat map of scores from human FL LN stromal cell subcluster signatures (reprinted from Abe et al21; upper part), human FL-LSC signature (reprinted from Mourcin et al17; middle part), and BM-MSC clusters obtained from HDs and patients with MM (reprinted from de Jong et al27; lower part), relative to the LepR+ MSC clusters identified by DA-seq.
Lymphoma B cells drive LepR+ MSC transcriptional reprogramming. (A) Uniform manifold approximation and projection (UMAP) plot of the scRNAseq data from endothelial and stromal cells of Rag−/−γc−/− mice untreated (Ctrl), injected with PBS (sham), or grafted with DOHH2 and euthanized at early (day 19) and late (day 40) time points (left). Heat map of the enrichment of mouse nonhematopoietic cell signature scores as previously defined by scRNA sequencing in steady-state C57BL/6 mice (right).26 (B) Stacked bar plot representing, for each cluster, the proportion of cells coming from the 4 conditions (control, sham, Gr.Early, and Gr.Late). The last bar of the plot represents the distribution of all cells between the different experimental conditions. The unbalance between experimental conditions in each cluster was evaluated using maximum residual values from a χ2 test. (C) UMAP plot of the subclustering of the 3 most unbalanced clusters (4, 0, and 1) after analysis of differential abundance using the differentially abundant sequencing (DA-seq) algorithm. Four LepR+ MSC subclusters were identified: MSC_Ctrl, MSC_Sh, MSC_Gr.Early, and MSC_Gr.Late. (D) Venn diagram of the differentially expressed genes between MSC_Gr.Late vs MSC_Gr.Early, MSC_Gr.Late vs MSC_Sh, and MSC_Gr.Early vs MSC_Sh (adjusted P value <.05; absolute value of log2 fold change of >.25). (E) Heat map of scores from human FL LN stromal cell subcluster signatures (reprinted from Abe et al21; upper part), human FL-LSC signature (reprinted from Mourcin et al17; middle part), and BM-MSC clusters obtained from HDs and patients with MM (reprinted from de Jong et al27; lower part), relative to the LepR+ MSC clusters identified by DA-seq.
To further characterize the condition-dependent heterogeneity of LepR+ MSCs and distinguish early from late time points, we used the differentially abundant sequencing multiscale approach, designed to delineate cell subpopulations with the most significant discrepancies between different biological states that are not captured by unsupervised clustering-based methods.28 We identified 4 clusters of differentially abundant LepR+ MSCs: 1 enriched for LepR+ MSCs from control mice (MSC_Ctrl), 1 enriched for LepR+ MSCs from sham mice (MSC_Sh), and 2 clusters enriched for LepR+ MSCs from Gr.Early or Gr.Late mice (named MSC_Gr.Early and MSC_Gr.Late; respectively; Figure 3C). Comparison of the DEGs (adjusted P value <.05; absolute value of log2 fold change of >0.25) between conditions revealed that the MSC_Gr.Early vs MSC_Sh signature was largely included within the MSC_Gr.Late vs MSC_Sh signature (147/182 genes [81%]), whereas 311 of 646 genes (48%) of the MSC_Gr.Late vs MSC_Sh signature overlapped with the MSC_Gr.Late vs MSC_Gr.Early signature (Figure 3D; supplemental Table 4). These data reveal that MSC_Gr.Early display an intermediate phenotypic state between MSC_Sh and MSC_Gr.Late.
Next, we assessed whether our murine MSC clusters were differentially enriched for signatures of human FL-CAFs. Both the whole FL-LSC signature, identified by comparing LSCs sorted from FL LNs vs reactive inflamed secondary lymphoid organs,17 and FL-LSC subset signatures, generated using a scRNAseq approach on stromal cells from FL LNs vs normal LNs,21 were enriched in MSC_Gr.Late compared with MSC_Gr.Early, MSC_Sh, and MSC_Ctrl (Figure 3E; supplemental Figure 3C). To explore a mature B-cell malignancy with primary BM involvement, we analyzed scRNAseq data of human BM stromal cells from patients with multiple myeloma (MM)27 (Figure 3E; supplemental Figure 3D). Although MSC_Gr.Late showed an enrichment for the MSC1 cluster signature, corresponding mainly to MM BM-MSCs, MSC_Ctrl were significantly enriched for the MSC4 cluster signature, enriched in HD BM-MSCs.
Stromal ECM profile is deeply modified in lymphoma-invaded BM
To further characterize lymphoma-induced alterations in BM stromal cells in situ, we explored the molecular pathways deregulated in grafted BM LepR+ MSCs. Gene set enrichment analysis highlighted a large set of ECM-related pathways as deregulated at both early and late stages, with a stronger upregulation in MSC_Gr.Late (Figure 4A). Focusing on the 100 most upregulated genes in MSC_Gr.Early and/or MSC_Gr.Late, we observed an enrichment for ECM-related genes, including Sparc, metalloproteinase-2 (Mmp2), Lox, Postn, Fn1, Acta2, or Bcn; cytoskeleton-related genes, including Actb, Tpm2, and Tpm4; collagens, including Col6a1, Col6a2, and Col6a3; and chemokines, including Cxcl9, Cxcl13, and Ccl2 (Figure 4B). These signatures were enriched in MSC_Gr.Late compared with MSC_Gr.Early. Immunohistofluorescence on BM sections of sham and Gr.Late mice femurs confirmed that B-cell infiltration was associated with increased expression of collagen VI, Sparc, and Lox in stromal cells (Figure 4C; supplemental Figure 4A). Similarly, we validated at the protein level the increase of CCL2 in BM plasma of day-40 grafted mice (supplemental Figure 4B). Conversely, genes related to cell signaling, including Jun, Heyl, Notch3, Isg15, or Igfbp3/5; and cholesterol metabolism, such as Ldlr, Fdsp, or Cyp51, were upregulated early in MSCs. We thus explored the metabolic states of LepR+ MSCs using Compass, a computational tool that models metabolic fluxes at the single-cell level.29 Compass highlighted major metabolic differences between MSC_Gr.Early/MSC_Gr.Late and MSC_Sh, with a generally increased metabolic activity in MSC_Gr.Early (supplemental Figure 4C; supplemental Table 5). In particular, the algorithm predicted that cholesterol metabolism and multiple amino acid metabolism reactions were more active in MSC_Gr.Early, whereas glycan metabolism reactions, regulating notably ECM organization and functions, were predominantly active in MSC_Gr.Late (Figure 4D).
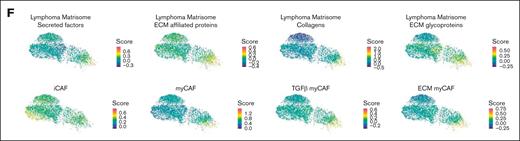
Stromal ECM profile is deeply modified in lymphoma-invaded BM. (A) Scatter plot of upregulated pathways in MSC_Gr.Late vs MSC_Sh (y-axis) and MSC_Gr.Early vs MSC_Sh (x-axis) as determined by gene set enrichment analysis (Hallmarks, Canonical pathways, and Gene Ontology databases). Pathways specific to each comparison are color coded: red for MSC_Gr.Late vs MSC_Sh, blue for MSC_Gr.Early vs MSC_Sh, and black for common pathways. ECM-related pathways are indicated by a star. (B) Heat map of the top 100 genes upregulated in at least 1 of the following comparisons: MSC_Gr.Late vs MSC_Gr.Early; MSC_Gr.Late vs MSC_Sh; and MSC_Early vs MSC_Sh. (C) Immunofluorescence on BM sections of sham (CA) or grafted (CB) mouse femurs for Sparc (red), collagen VI (ColVI; green), and human CD20 (hCD20; cyan; upper panel). Nuclei were counterstained with DAPI (blue); scale bars, 50 μm. Box (i) in Gr.Late femur indicates the area magnified below for Sparc, ColVI, and CD20 staining (scale bars, 50 μm). Immunofluorescence on BM sections of sham (CA) or grafted (CB) mouse femurs for Lox (red), and hCD20 (cyan; lower panel). Nuclei were counterstained with DAPI (blue); scale bars, 50 μm. (D) Compass-score differential activity of metabolic subsystems between MSC_Gr.Early vs MSC_Late (y-axis) and MSC_Gr.Early vs MSC_Sh (x-axis). Cohens d medians are calculated for each subsystem of Recon 2 pathways by taking each Cohens d value obtained for each reaction of this subsystem. Subsystems are categorized in global metabolic pathways and names of subsystems belonging to the “glycan metabolism” category are given in detail. (E) UMAP plot of non-endothelial stromal cells from human metastasis-free mesenteric LNs (MFLNs) and human FL LNs colored by sample origin (blue, MFLNs, and red, FL LNs; left). Visualization of the MSC_Gr. ECM and collagen signature scores derived from the 100 genes upregulated in MSC_Gr.Late and/or MSC_Gr.Early in MFLN vs FL stromal cells (right). (F) UMAP plot of scores from lymphoma matrisome signatures previously defined from DLBCL LNs30 and solid cancer CAF signatures previously defined from pancreatic cancers31 and from breast cancers32 were plotted on the LepR+ MSC clusters. iCAF, inflammatory CAF.
Stromal ECM profile is deeply modified in lymphoma-invaded BM. (A) Scatter plot of upregulated pathways in MSC_Gr.Late vs MSC_Sh (y-axis) and MSC_Gr.Early vs MSC_Sh (x-axis) as determined by gene set enrichment analysis (Hallmarks, Canonical pathways, and Gene Ontology databases). Pathways specific to each comparison are color coded: red for MSC_Gr.Late vs MSC_Sh, blue for MSC_Gr.Early vs MSC_Sh, and black for common pathways. ECM-related pathways are indicated by a star. (B) Heat map of the top 100 genes upregulated in at least 1 of the following comparisons: MSC_Gr.Late vs MSC_Gr.Early; MSC_Gr.Late vs MSC_Sh; and MSC_Early vs MSC_Sh. (C) Immunofluorescence on BM sections of sham (CA) or grafted (CB) mouse femurs for Sparc (red), collagen VI (ColVI; green), and human CD20 (hCD20; cyan; upper panel). Nuclei were counterstained with DAPI (blue); scale bars, 50 μm. Box (i) in Gr.Late femur indicates the area magnified below for Sparc, ColVI, and CD20 staining (scale bars, 50 μm). Immunofluorescence on BM sections of sham (CA) or grafted (CB) mouse femurs for Lox (red), and hCD20 (cyan; lower panel). Nuclei were counterstained with DAPI (blue); scale bars, 50 μm. (D) Compass-score differential activity of metabolic subsystems between MSC_Gr.Early vs MSC_Late (y-axis) and MSC_Gr.Early vs MSC_Sh (x-axis). Cohens d medians are calculated for each subsystem of Recon 2 pathways by taking each Cohens d value obtained for each reaction of this subsystem. Subsystems are categorized in global metabolic pathways and names of subsystems belonging to the “glycan metabolism” category are given in detail. (E) UMAP plot of non-endothelial stromal cells from human metastasis-free mesenteric LNs (MFLNs) and human FL LNs colored by sample origin (blue, MFLNs, and red, FL LNs; left). Visualization of the MSC_Gr. ECM and collagen signature scores derived from the 100 genes upregulated in MSC_Gr.Late and/or MSC_Gr.Early in MFLN vs FL stromal cells (right). (F) UMAP plot of scores from lymphoma matrisome signatures previously defined from DLBCL LNs30 and solid cancer CAF signatures previously defined from pancreatic cancers31 and from breast cancers32 were plotted on the LepR+ MSC clusters. iCAF, inflammatory CAF.
Analysis of a human LN LSC scRNAseq atlas revealed a specific enrichment of collagen and ECM-related genes of the MSC_Gr signature (from Figure 4B) in LSCs, in particular in Podoplanin+CD21–COL6+ FRCs, from LNs of patients with FL compared with nonmalignant LNs (Figure 4E; supplemental Figure 4D-E). In addition, LepR+ MSCs from lymphoma-grafted mice, especially MSC_Gr.Late, were found significantly enriched for matrisome-secreted factor, ECM-affiliated protein, collagen, and ECM glycoprotein signatures previously defined from DLBCL LNs30 (Figure 4F; supplemental Figure 4F). Similarly, MSC_Gr.Late were significantly enriched for myofibroblastic CAFs (myCAFs) unlike inflammatory CAF signatures previously identified in solid cancers,31 and more precisely for TGFβ-secreting and ECM-organizing myCAF signatures,32 suggesting shared features of ECM deregulation in CAFs from lymphoma and solid cancers.
Taken together, these results show the polarization of BM LepR+ MSCs into an ECM-remodeling phenotype, mimicking lymphoma CAF reprogramming, upon direct contact with tumor B cells.
Study of the bidirectional B cell/stromal cell cross talk in lymphoma-invaded BM
To explore the interplay between tumor B cells and LepR+ MSCs, we performed a NicheNet interactome analysis using DOHH2 D40 as sender cells and MSC_Gr.Late as receivers, focusing on ligand-receptor interactions (Figure 5A-B) and ligand-targeted gene relationships (supplemental Figure 5A). In particular, expression of TGFβ1 and VEGF by DOHH2 was predicted to contribute to ECM remodeling in BM stromal cells, as previously reported for primary FL B-cell/FL LN LSC cross talk.17 Next, we inferred the gene regulatory network activity in LepR+ MSCs using the DecoupleR algorithm. Analysis of the top 50 most variable regulons predicted an increased activity of Smad3, the main transcription factor of the classical TGFβ pathway, along with several positive regulators of TGFβ signaling, including Pbx1, Tfe3, Atf2, and Srf, whereas the activity of Smad7 and Sox17, 2 inhibitors of TGFβ signaling, was reduced in MSC_Gr.Late (Figure 5C; supplemental Figure 5B). Finally, we used public scRNAseq data obtained from 20 purified primary FL B cells33 and estimated, using the CellPhoneDB repository,34 the number of bidirectional interactions between these malignant B cells and LepR+ MSCs, including our 4 clusters (MSC_Ctr, MSC_Sh, MSC_Gr.Early, and MSC_Gr.Late) and LepR+ MSCs from steady-state immunocompetent mice (Scadden_Lepr-MSC35; Figure 5D). A higher number of cell interactions were predicted between primary FL B cells and MSC_Gr.Late. When we highlighted the main pathways predicted to support signals from FL B cells to MSC_Gr.Late (active in at least 8 of 20 patients with FL), TGFβ1 emerged again as a major determinant.
Impact of malignant B cells on stromal cells in lymphoma-invaded BM. (A) Circos plot showing predicted interactions between ligands from DOHH2 cells and receptors from LepR+ MSC at the late stage of graft. D40 DOHH2 were defined as senders, and MSC_Gr.Late as receivers. (B) Heat map representation of ligand activity of D40 DOHH2 vs MSC_Gr.Late cells. Colors correspond to Pearson coefficient (quantifying ligand activities) and text to P values assessed by random permutation. (C) Heat map of the top 50 differentially expressed regulons detected in the 4 LepR+ MSC clusters using decoupleR algorithm36 and CollectTri database (left). Violin plots of Smad3 and Smad7 regulon activity for the LepR+ MSC clusters (right). (D) Heat map generated with CellPhoneDB repository, representing the number of significative bidirectional interactions between LepR+ clusters (MSC_Sh, MSC_Gr.Early, MSC_Gr.Late, and MSC_Ctrl) from this study and human primary FL B cells (left).33 Diagram of bidirectional interactions detected, by CellPhoneDB, between ligands from human FL B cells and receptors from MSC_Gr.Late, and observed in >8 of 20 patients with FL (right). Interactions also predicted in panel A are highlighted in red.
Impact of malignant B cells on stromal cells in lymphoma-invaded BM. (A) Circos plot showing predicted interactions between ligands from DOHH2 cells and receptors from LepR+ MSC at the late stage of graft. D40 DOHH2 were defined as senders, and MSC_Gr.Late as receivers. (B) Heat map representation of ligand activity of D40 DOHH2 vs MSC_Gr.Late cells. Colors correspond to Pearson coefficient (quantifying ligand activities) and text to P values assessed by random permutation. (C) Heat map of the top 50 differentially expressed regulons detected in the 4 LepR+ MSC clusters using decoupleR algorithm36 and CollectTri database (left). Violin plots of Smad3 and Smad7 regulon activity for the LepR+ MSC clusters (right). (D) Heat map generated with CellPhoneDB repository, representing the number of significative bidirectional interactions between LepR+ clusters (MSC_Sh, MSC_Gr.Early, MSC_Gr.Late, and MSC_Ctrl) from this study and human primary FL B cells (left).33 Diagram of bidirectional interactions detected, by CellPhoneDB, between ligands from human FL B cells and receptors from MSC_Gr.Late, and observed in >8 of 20 patients with FL (right). Interactions also predicted in panel A are highlighted in red.
To explore how BM LepR+ MSCs become more efficient to interact with and support lymphoma B cells, we applied the NicheNet computational method using MSC_Gr.Late as sender cells and DOHH2 at day 40 as receivers (Figure 6A-B). Several members of the TGFβ family pathway were found enriched at the stroma cell/B-cell interface, including Tgfb1 and Tgfb3, Bmp4, and Bmp5, consistent with the progressive enrichment for the TGFβ myCAF signature in MSC_Gr.Early and MSC_Gr.Late (Figure 4F). In addition, adhesion molecules, ECM remodeling factors, and collagens were strongly enriched in this interactome analysis, together with Cxcl12, Il7, and the Notch ligand, Jag1. Both CXCL12 and Jag1 have already been described as upregulated in human FL BM-MSCs compared with HD BM-MSCs.18,37 Interestingly, the CellPhoneDB analysis predicted a high number of shared pathways in the cross talk between primary human FL B cells and LepR+ MSCs (Figure 6C).
Impact of stromal cells on malignant B cells in lymphoma-invaded BM. (A) Circos plot showing predicted interactions between ligands from LepR+ MSCs and receptors from DOHH2 recovered from the grafted mouse at day 40. MSC_Gr.Late were defined as senders, and DOHH2 D40 as receivers. (B) Heat map representation of ligand activity of MSC_Gr.Late vs DOHH2 D40 cells. Colors correspond to Pearson coefficient quantifying ligand activities and text to P values assessed by random permutation. (C) Diagram of significative bidirectional interactions detected, by CellPhoneDB, between ligands from MSC_Gr.Late and receptors from human primary FL B cells,33 and observed in >8 of 20 patients with FL. Interactions predicted in panel A are highlighted in red. (D) Quantification by Luminex of matrix proteins and cytokines in the BM plasma from patients with FL (n = 11; except for TGFβ1, n = 13) collected at diagnosis and after 12 months of treatment. Blue dots represent patients with FL and green dotted line represent HD median level. Statistical significance was determined using the Mann-Whitney nonparametric U test. ∗P < .05; ∗∗P < .01. ns, nonsignificant.
Impact of stromal cells on malignant B cells in lymphoma-invaded BM. (A) Circos plot showing predicted interactions between ligands from LepR+ MSCs and receptors from DOHH2 recovered from the grafted mouse at day 40. MSC_Gr.Late were defined as senders, and DOHH2 D40 as receivers. (B) Heat map representation of ligand activity of MSC_Gr.Late vs DOHH2 D40 cells. Colors correspond to Pearson coefficient quantifying ligand activities and text to P values assessed by random permutation. (C) Diagram of significative bidirectional interactions detected, by CellPhoneDB, between ligands from MSC_Gr.Late and receptors from human primary FL B cells,33 and observed in >8 of 20 patients with FL. Interactions predicted in panel A are highlighted in red. (D) Quantification by Luminex of matrix proteins and cytokines in the BM plasma from patients with FL (n = 11; except for TGFβ1, n = 13) collected at diagnosis and after 12 months of treatment. Blue dots represent patients with FL and green dotted line represent HD median level. Statistical significance was determined using the Mann-Whitney nonparametric U test. ∗P < .05; ∗∗P < .01. ns, nonsignificant.
Finally, we wondered whether the reprogramming of BM stromal cells into ECM-producing CAFs is reversible upon lymphoma cell clearance. We used BM plasma collected from patients with FL with high-tumor burden at diagnosis and 1 year after treatment, with a strong tumor mass reduction (supplemental Figure 6A). None of the soluble ECM factors tested, including SPARC, FAP, Lumican, MMP2, and TIMP1, showed a significant decrease after treatment in patients with FL, even in those who achieved near complete or complete disappearance of t(14;18)+ tumor B cells as shown using a very sensitive nested polymerase chain reaction assay (absolute frequency of <10−5; n = 6). Although significantly reduced, TGFβ1 level also remained higher in FL BM plasma than in HDs. Looking for an epigenetic regulation of BM stromal cell reprogramming in FL, we analyzed histone marks associated with DEGs in MSC_Gr.Late vs MSC_Sh. This study revealed an enrichment for the repressive mark H3k27me3 in downregulated genes, similar to that found when analyzing genes downregulated in FL-LSCs compared with normal LN-LSCs17 (supplemental Figure 6B).
Discussion
The role of stromal cells in lymphomagenesis is a subject of intense interest, in line with their capacity to directly support malignant B-cell survival and drug resistance and to impact the recruitment, activation, and polarization of the immune TME.38,39 Although lymphoma CAFs have been profiled by high-throughput molecular and phenotypic approaches in FL LNs,17,21,40 the heterogeneity and reprogramming of lymphoma stromal cells in the BM remain poorly understood. Yet, the BM has emerged as an important niche for lymphoma B cells, supporting early disease and relapse.38 Here, we provided additional clues on BM stromal cell reprogramming in B-cell lymphomas, with a specific focus on ECM reorganization and on the bidirectional cross talk between tumor B cells and BM stromal cells.
We first characterized ECM remodeling within FL-invaded BM, highlighting an upregulation of collagen VI, a marker associated with the ectopic LSC commitment of FL BM-MSCs,4,6 and LOX+ stromal networks. Additionally, BM plasmas from patients with FL showed increased amount of several soluble ECM components compared with HDs. Interestingly, modifications of ECM composition have also been described in FL and DLBCL LNs, suggesting the existence of common prosurvival tumor niches. In particular, SPARC, LOX, FAP, Lumican, MMP2, and TIMP1 are all upregulated at the transcriptomic level in LN FL-CAFs,17,21 whereas ECM displays both altered structure and increased stiffness in FL LNs compared with nonneoplastic LNs.41 Upregulation of stroma-derived ECM genes (CAF matreotype) is also described in DLBCL LNs,30 in which the FRC network is stretched and aberrantly remodeled.22 The phenotype of LepR+ MSCs in lymphoma-primed murine BM stromal cells showed partial overlap with human MM BM-MSCs, which similarly overexpress CCL2, COL6A3, and FN1.27 Indeed, in vitro–expanded BM-MSCs obtained from lymphoid neoplasia exhibit similarities in their transcriptomic profile, including an increased expression of multiple collagen genes.42 Tumor B cells in FL and GCB-DLBCL have a preferential paratrabecular localization in invaded BM but other mature B-cell lymphomas show different BM localization patterns, possibly reflecting interactions with different stromal cell subsets.43 How lymphomas with an intrasinusoidal infiltration pattern, such as splenic marginal zone lymphomas, have a specific BM stromal cell profile remains to be established. Overall, although the chemokine profiles of FL- vs DLBCL-CAFs are strongly different, our data suggest some communalities in the ECM remodeling profile across mature B-cell malignancies and tissues.
ECM remodeling may exert protumoral effects, favoring FL B-cell implantation within the BM and promoting the activity of chemokines and cytokines. Many growth factors and cytokines/chemokines, such as TGFβ or CXCL12, but also CCL2, CXCL9, and CXCL10, were all found upregulated in LepR+ murine MSCs and FL and MM BM stromal cells (Guilloton et al18; de Jong et al27; and data not shown), could be released upon ECM degradation and reshape.44-46 Moreover, integrin interactions with ECM molecules activate several intracellular pathways in tumor cells, related to actin cytoskeleton or cell cycle.47 In agreement, LN CAF-derived ECM components predict a bad prognosis in patients with FL treated by chemotherapy only.21 DOHH2 themselves overexpressed ECM-related factors after in situ xenograft and could contribute to the local ECM modifications. In agreement, culture of lymphoma B-cell lines in 3-dimensional spheroids increases their production of ECM components.25 At day 40, DOHH2 also upregulated ITGA4 and ITGB1, together forming the very late antigen-4 integrin that, upon binding to vascular cell adhesion molecule-1 (expressed in MSC_Gr.Late), was associated with B-cell lymphoma growth and resistance to the anti-CD20 antibody rituximab.48 ECM reshaping within FL BM and CAF-derived TGFβ production may further affect immune cell infiltration and tumor elimination. In FL LN, CD8 T cells are essentially retained at the follicle border in contact with activated FRCs.49 In DLBCL, enrichment of the LN TME for a stromal cell/TGFβ signature is associated with a worse outcome in patients treated by chimeric antigen receptor T cells,50 in association with a reduced chimeric antigen receptor T-cell motility.22 Conversely, some CAF-derived ECM-derived components, in particular basigin and decorin, both overexpressed in murine LepR+ MSCs, display uncharacterized antilymphoma effects and correlate with a favorable prognosis in patients with DLBCL treated by R-CHOP (rituximab, cyclophosphamide, doxorubicin, Oncovin [vincristine], and prednisone).30 These data highlight the need for a comprehensive characterization of ECM composition, organization, and functional impact within lymphoma niches, including the BM. They also suggest that targeting stroma cells or the ECM, for example through TGFβ targeting, may be considered not only in solid cancers but also in B-cell lymphomas.
We identified tumor B cells as direct inducers of BM stroma reprogramming in lymphoma. Production of tumor necrosis factor α and lymphotoxin α1β2 by FL and DLBCL B cells has previously been shown to trigger LN stromal cell activation and commitment of BM-MSCs into LSCs in vitro.18 Moreover, TGFβ is overexpressed in LN FL B cells and may contribute to the induction of a TGFβ/myCAF program in FL-CAFs.17 FL B-cell–derived extracellular vesicles can prime BM stromal cells independently of direct cell interaction, a process also dependent on TGFβ signaling.4 In vitro, short-term culture with DLBCL B cells increases LN FRC metabolic activity relative to conditioning by nonmalignant B cells.22 Similarly, we identified early metabolic modifications in murine LepR+ BM-MSCs after tumor B-cell xenograft. Metabolic profile is known to influence lineage-differentiation fate51 and immunological properties of MSCs.52 These data argue for a role of metabolic reprogramming in BM lymphoma CAFs that needs further investigation. Apart from tumor B cells, other BM cell types could contribute to the lymphoma-induced BM-MSC phenotype in our mouse model. Activated neutrophils are important local inducers of the protumoral inflammatory BM-MSC phenotype in MM35 and have been shown to stimulate BM-MSC commitment to FL-supporting LSCs in vitro.19 Some nonmalignant immune compartments are missing or dysfunctional in our immunocompromised mouse models and could play a role in local stromal activation and ECM reorganization.37 There is currently no model to explore such a complex lymphoma BM niche in vivo.
In our cohort, TGFβ and soluble ECM components did not normalize after treatment, even in patients without detectable residual disease. Even if we cannot perform in situ staining to confirm the persistence of the stromal cell remodeling because of the lack of available BM biopsies after treatment, these data suggest a persistence of a deregulated ECM-supportive BM niche. This result is reminiscent of the persistence of inflammatory BM-MSCs in patients with MM after effective induction therapy.35 A possible explanation could be the persistence of nonmalignant B-cell–derived stimuli, coming from the residual TME. Alternatively, stromal epigenetic reprogramming, similar to that supporting LSC differentiation from stromal precursors,53 could support the prolonged FL MSC phenotypic alteration, as suggested by the analysis of histone marks enrichment in genes regulated upon lymphoma-dependent MSC reprogramming. Consistently, FL BM-MSCs maintain overexpression of CCL2, IL-8, or CXCL12 after in vitro expansion.18,19,37 Whether BM FL-CAFs promote the survival of treatment-resistant FL B cells remains to be explored. A deeper characterization of this niche is therefore required to better understand and prevent FL relapse, and ultimately improve patient prognosis.
Acknowledgments
Meso scale diagnostics quantification of murine soluble factors in the bone marrow (BM) plasma was performed by the InnovaBIO platform (Centre Hospitalier Universitaire Caen, France). The authors thank the staff of the flow cytometry (CytomeTRI), animal housing (ARCHE), Microscopy-Rennes Imaging Center (MRic), Facility for Artificial Intelligence and Image Analysis, and Histo-Pathology Hight Precision (H2P2) core facilities (UAR BIOSIT, Rennes, France). Both MRic and H2P2 platforms are members of France-BioImaging Infrastructure, supported by the French National Research Agency (ANR-24-INBS-0005 FBI BIOGEN). The authors also thank the GenOuest bioinformatics core facility (https://www.genouest.org) for providing the computing infrastructure and the LYSARC for organizing the collection of the BM aspirates from patients with follicular lymphoma included in the Tazemetostat in Newly Diagnosed Diffuse Large B Cell and Follicular Lymphoma Patients Treated by Chemotherapy trial.
This work was supported by research grants from the Institut National Du Cancer (INCA AAP PNP19-009), and Ligue Nationale contre le Cancer (Equipe Labellisée). B.B. is supported by the University of Rennes and the Région Bretagne.
Authorship
Contribution: E.D. designed experiments; E.D., B.B., A.B., C.M., T.L., J.D., F.J., and S.R. performed experiments; E.D., B.B., F. Mourcin, D.R., and K.T. analyzed data; E.D. contributed to the writing; S.L. coordinated bioinformatic analyses; S.L., V.I., N.B., and J.S. performed bioinformatic analyses; C.L. and F.L.-G. provided bone marrow biopsies; F. Morschhauser conducted the EpiRCHOP clinical study and provided human biological samples; F. Mourcin and D.R. discussed results; K.T. designed and supervised research; and B.B. and K.T. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Karin Tarte, Faculté de Médecine, INSERM UMR1236, 2 Av du Pr Léon Bernard, 35043 Rennes, France; email: karin.tarte@univ-rennes.fr.
References
Author notes
E.D. and B.B. contributed equally to this study.
D.R. and F.M. contributed equally to this study.
Other materials and protocols are available on request from the corresponding author, Karin Tarte (karin.tarte@univ-rennes.fr).
The full-text version of this article contains a data supplement.