Key Points
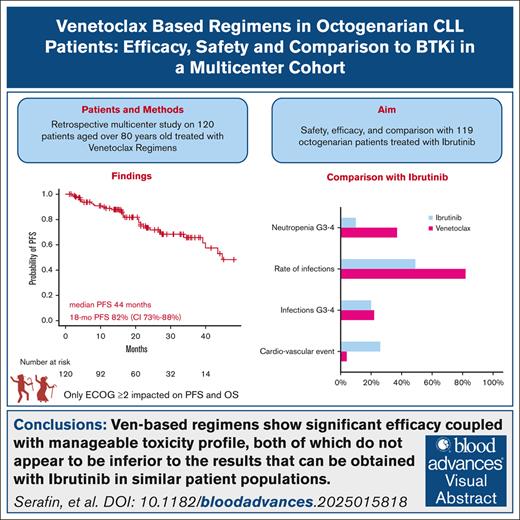
Ven is effective (91% ORR) and well tolerated in octogenarians, but 43% hd treatment holds and 32% prolonged ramp-up.
Ven causes more infections and neutropenia but fewer cardiovascular issues than BTKi, whereas serious infections (grade 3-4) are similar.
Visual Abstract
Octogenarians represent a significant fraction of patients with chronic lymphocytic leukemia (CLL) but, despite the prevalence of the disease in this age group, limited data are available on the safety and efficacy of novel drugs in this subgroup. We conducted a multicenter retrospective study enrolling 120 octogenarian patients who received venetoclax (Ven) regimens in any line. Regarding efficacy, we found Ven to perform similarly to what is reported in younger patients with CLL, with an overall response rate of 91%, a complete response rate of 44%, and median progression-free survival of 44 months. Concerning safety, we report a toxicity profile that is consistent with previous reports, with most high-grade adverse events being of hematologic or infectious nature, given that 37% and 22% of patients experienced neutropenia or infections of grade 3 or higher. As part of our study, we compared the safety and efficacy data we collected with those obtained in a comparable Bruton tyrosine kinase inhibitor (BTKi)-treated population. We found that these 2 treatments were comparable in terms of overall efficacy, barring a higher rate of complete responses with Ven; safety profiles were different among the 2 groups given that BTKi-treated patients had more cardiovascular toxicities (26% vs 4%) and Ven-treated subjects experienced more infectious events (82% vs 49%). Our data point out that Ven-based regimens are safe and effective in octogenarian patients with CLL despite their higher clinical complexity and comorbidity burden and should provide some basis for the design of prospective studies to further evaluate the optimal treatment regimen in this patient population.
Introduction
Given an incidence curve peaking in the seventh to the eighth decade of age,1-3 the indolent course of the disease,4,5 and the remarkable efficacy of current therapeutic regimens,6-9 a significant fraction of patients with chronic lymphocytic leukemia (CLL) is expected to be older than 80 years at the time they require treatment for the disease.
This specific patient population is usually under-represented in clinical trials, either by exclusion8,10 or by labeling comorbid individuals and subjects aged >65 to 70 years as unfit and the subsequent reporting of their outcomes as a whole.7,9,11,12 Moreover, even in the real-world setting, a specific description of outcomes of CLL treatment in this specific population is very limited, especially with novel targeted agents, even if some information on the use of ibrutinib (Ibr) has been recently reported.13-15 Considering that octogenarian patients come with specific clinical challenges, they often carry a high comorbidity burden with organ damage that can directly affect the safety and efficacy of the CLL-directed treatment.16 Although some effort has recently been made to address this unmet need within specifically designed clinical trials, such as the ongoing CLL-Frail study that is evaluating acalabrutinib monotherapy in this setting of patients,17 more evidence is required to allow clinicians to make informed choices in their day-to-day practice, especially within the realm of B-cell lymphoma 2 (BCL-2) inhibitors.
We conducted a retrospective multicenter clinical study enrolling octogenarian patients affected by CLL treated with venetoclax (Ven)-based therapeutic regimens, with the aim to report the efficacy and safety of these treatments in this patient population and to compare the outcomes with a similar patient group treated with Bruton tyrosine kinase inhibitors (BTKis).
Methods
Patients and procedures
This study enrolled patients affected by either treatment-naïve or relapsed/refractory (R/R) CLL older than 80 years at the time of treatment initiation with either a Ven-based regimen or a BTKi-based regimen. The indication for treatment initiation was established according to the International Workshop on Chronic Lymphocytic Leukemia guidelines.5,18 We included all patients for whom data were available, although informed consent was signed only by living patients.
Patients were retrospectively enrolled in 23 Italian centers and received treatment between 2014 and 2023, with BTKis being administered as continuous therapy and Ven being administered either as part of a fixed treatment regimen (Ven-rituximab [Ven-R] or Ven-obinutuzumab [Ven-G]) or as continuous therapy, with dosage and treatment schedules in keeping with previous publications and time-appropriate treatment guidelines.5,6,19-22
Outcomes and safety data for the population treated with first-line BTKis have already been reported elsewhere,13,14 although in the current work their follow-up data have been updated; on the contrary, we here include for the first time individuals treated with BTKis for R/R CLL from the same center.
All patients provided a written informed consent for study participation (4430/AO/18), which was approved by the local ethics board on 27 June 2019 and was conducted according to the principles of the Declaration of Helsinki and the Good Clinical Practice Guidelines.
Data
Data regarding patient and disease characteristics at therapy start (baseline), treatment-emergent adverse effects (TEAEs), and outcomes were collected.
The collected baseline data included age, gender, Rai and Binet stages of CLL, interphase FISH analyses for trisomy of chromosome 12 (12+), deletions of the long arm of chromosome 11 [del(11q)] and 13 [del(13q)] or the short arm of chromosome 17 [del(17p)], mutational status of the immunoglobulin heavy chain variable (IGHV), and TP53 genes. The assessment of IGHV status and TP53 abnormalities, including deletion and/or mutation of the gene, has been locally assessed before starting Ven in a certified European Research Initiative on CLL site as previously reported.23-25
Regarding baseline patient data, comorbidities were evaluated using the cumulative illness rating scale (CIRS), and a score higher than 6 was considered indicative of significant comorbidities.26 The performance status of each enrolled patient was recorded according to the Eastern Cooperative Oncology Group (ECOG) scale.27 For patients being treated for R/R CLL, data on previous treatment history were also collected.
Tumor lysis syndrome (TLS) risk was evaluated at baseline6,28 for Ven-treated patients, with TLS prevention and monitoring being performed according to local guidelines. Clinical and biochemical TLS were defined according to the Cairo-Bishop modification of the Hande-Garrow TLS classification system.29
TEAEs were recorded and classified following the Common Terminology Criteria for Adverse Events (version 5.0), and the occurrence of dose modification of either Ven or BTKis owing to toxicity was specified. The incidence of adverse events (AEs) was calculated considering the number of patients experiencing the specified TEAEs over the total number of patients.
The response to treatment and the occurrence of disease progression were defined in accordance with the time-appropriate International Workshop on Chronic Lymphocytic Leukemia guidelines (either the 2008 or the 2018 version). Overall survival (OS) was defined as the time from either Ven or BTKi initiation to death from any cause, whereas progression-free survival (PFS) was defined as the time from treatment initiation to disease refractoriness (the occurrence of which was evaluated after the first 3 months of treatment), disease relapse after obtaining at least a partial response, or death from any cause, whichever occurred first. Univariate and multivariate analyses for PFS and OS of the Overall BTKi + Ven cohorts are reported in the supplemental Table S1.
Statistical analysis
Continuous variables were described using median, minimum-maximum ranges, and interquartile ranges. Dichotomous variables were described using percentages, and univariate comparison between dichotomous variables was performed with the χ2 test.
Time-to-event analysis was performed by the construction of survival curves with the Kaplan-Meier method and by the comparison of such curves with the log-rank test. Hazard ratios (HRs) for comparisons were calculated using Cox test and are reported with the inclusion of their relative 95% confidence interval (CI). The cumulative incidence of the initiation of a new treatment was calculated using the method of Fine and Gray, with death being considered as a competing risk.
All analyses were performed using R version 4.4.2 for Windows.
Results
Study population
A total of 120 octogenarian patients with CLL were included in the study. The median age at the start of therapy was 81 years (range, 80-94 years), and the baseline characteristics of the patient population are presented in Table 1. Most patients had significant comorbidities, as indicated by a CIRS score greater than 6 in 65 patients (54%) and an ECOG Performance Status (ECOG) ≥2 in 23 (19%). Impaired renal function, defined by a creatinine clearance <60 mL/min, was documented in 53% of patients. Most individuals (n = 63, 53%) had an intermediate TLS risk, whereas 33 (28%) were considered to be at a high risk of TLS. High-risk genetic abnormalities, such as TP53 mutations and del(17p), were found in 21 (18%) and 25 patients (21%), respectively. The presence of either a TP53 mutation or a del(17p) was used to define the so-called high-risk disease, with 33 patients (28%) falling in this category. An unmutated IGHV gene was present in 67 cases (56%).
Baseline characteristics of our patient population treated with Ven-based regimens
| Characteristics . | N = 120 . |
|---|---|
| Age, median (range) | 81 (80-94) |
| ECOG, median (range) | 1 (0-3) |
| ECOG ≥2, n (%) | 23 (19) |
| CIRS >6, n (%) | 65 (54) |
| TP53 disruption, n (%) | 32 (28) |
| Del(17p), n (%) | 25 (21) |
| TP53 mutation, n (%) | 21 (18) |
| Missing data, n (%) | 20 (17) |
| IGHV unmutated, n (%) | 67 (56) |
| High-risk genetic disease | 33 (28) |
| TLS risk categories | |
| Low risk, n (%) | 24 (20) |
| Intermediate risk, n (%) | 63 (53) |
| High risk, n (%) | 33 (28) |
| CrCl <60 mL/min, n (%) | 63 (53) |
| LDH ULN, n (%) | 61 (51) |
| Rai stages | |
| 0-I, n (%) | 35 (29) |
| II, n (%) | 27 (23) |
| III, n (%) | 27 (23) |
| IV, n (%) | 31 (26) |
| Binet stages | |
| A, n (%) | 15 (13) |
| B, n (%) | 47 (39) |
| C, n (%) | 58 (48) |
| Type of Ven regimen | |
| Ven monotherapy, n (%) | 60 (50) |
| Ven-R, n (%) | 45 (38) |
| Ven-G, n (%) | 17 (14) |
| Treatment naïve, n (%) | 20 (17) |
| Relapsed patients, n (%) | 100 (83) |
| Previous lines, median (range) | 2 (0-6) |
| 1, n (%) | 35 (29) |
| 2, n (%) | 34 (28) |
| ≥3, n (%) | 32 (27) |
| Type of previous therapy | |
| BTKi, n (%) | 51 (43) |
| Chlorambucil ± anti-CD20, n (%) | 43 (36) |
| FC-R, BR, n (%) | 48 (40) |
| Idelalisib-R, n (%) | 12 (10) |
| Others (alloSCT, lenalidomide, rituximab, CHOP, RCVP), n (%) | 17 (14) |
| BTKi pre-exposure, n (%) | 51 (43) |
| Efficacy (best response) | |
| ORR (CR + CRi + PR), n (%) | 109 (91) |
| SD, n (%) | 9 (8) |
| PD, n (%) | 2 (2) |
| Richter transformation, n (%) | 7 (6) |
| Characteristics . | N = 120 . |
|---|---|
| Age, median (range) | 81 (80-94) |
| ECOG, median (range) | 1 (0-3) |
| ECOG ≥2, n (%) | 23 (19) |
| CIRS >6, n (%) | 65 (54) |
| TP53 disruption, n (%) | 32 (28) |
| Del(17p), n (%) | 25 (21) |
| TP53 mutation, n (%) | 21 (18) |
| Missing data, n (%) | 20 (17) |
| IGHV unmutated, n (%) | 67 (56) |
| High-risk genetic disease | 33 (28) |
| TLS risk categories | |
| Low risk, n (%) | 24 (20) |
| Intermediate risk, n (%) | 63 (53) |
| High risk, n (%) | 33 (28) |
| CrCl <60 mL/min, n (%) | 63 (53) |
| LDH ULN, n (%) | 61 (51) |
| Rai stages | |
| 0-I, n (%) | 35 (29) |
| II, n (%) | 27 (23) |
| III, n (%) | 27 (23) |
| IV, n (%) | 31 (26) |
| Binet stages | |
| A, n (%) | 15 (13) |
| B, n (%) | 47 (39) |
| C, n (%) | 58 (48) |
| Type of Ven regimen | |
| Ven monotherapy, n (%) | 60 (50) |
| Ven-R, n (%) | 45 (38) |
| Ven-G, n (%) | 17 (14) |
| Treatment naïve, n (%) | 20 (17) |
| Relapsed patients, n (%) | 100 (83) |
| Previous lines, median (range) | 2 (0-6) |
| 1, n (%) | 35 (29) |
| 2, n (%) | 34 (28) |
| ≥3, n (%) | 32 (27) |
| Type of previous therapy | |
| BTKi, n (%) | 51 (43) |
| Chlorambucil ± anti-CD20, n (%) | 43 (36) |
| FC-R, BR, n (%) | 48 (40) |
| Idelalisib-R, n (%) | 12 (10) |
| Others (alloSCT, lenalidomide, rituximab, CHOP, RCVP), n (%) | 17 (14) |
| BTKi pre-exposure, n (%) | 51 (43) |
| Efficacy (best response) | |
| ORR (CR + CRi + PR), n (%) | 109 (91) |
| SD, n (%) | 9 (8) |
| PD, n (%) | 2 (2) |
| Richter transformation, n (%) | 7 (6) |
alloSCT, allogenic bone marrow stem cell transplantation; BR, bendamustine-rituximab; CHOP, cyclophosphamide, doxorubicine, vincristine, and prednisolone; CrCl, creatinine clearance; CR, complete response; CRi, complete response with incomplete count recovery; FC-R, fludarabine, cyclophosphamide and rituximab; idelalisib-R, idelalisib, rituximab; LDH, lactate dehydrogenase; PD, progressive disease; PR, partial response; RCVP, rituximab, cyclophosphamide, vincristine and prednisolone; SD, stable disease; ULN, upper limit of normal.
Ven was given as monotherapy in 60 individuals (50%), whereas combination therapy with rituximab (Ven-R) and obinutuzumab was administered in 45 (38%) and 17 patients (14%), respectively. Most patients (83%) had a relapsed or refractory disease, with a median of 2 previous lines of therapy (range, 0-6). Previous therapies included BTKis in 51 patients (43%), fludarabine-cyclophosphamide-rituximab or bendamustine-rituximab in 48 (40%), chlorambucil-based regimens in 43 (36%), and idelalisib plus rituximab in 12 (12%). Twenty patients (17%) received Ven as first-line therapy, 17 (14%) in combination with obinutuzumab, and 3 (3%) as monotherapy.
Efficacy and survival evaluation
Overall, a response to a Ven-based therapeutic regimen was documented in 109 individuals, leading to an overall response rate (ORR) of 91%, with a complete remission (CR) rate of 44%. Stable disease was reported in 9 patients (7%), whereas 2 subjects (2%) had disease progression during Ven therapy. A partial response or better was obtained in 18 treatment-naïve patients (90%) and in 91 of the R/R subjects (91%).
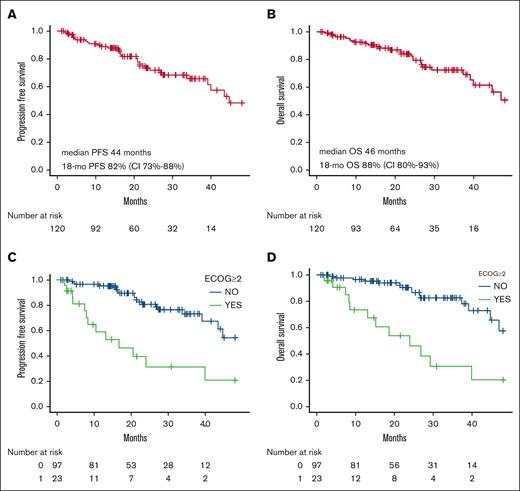
Across the entire cohort, after a median follow-up time of 21 months (range, 1-91 months), a 18-month PFS of 82% (95% CI, 73-88) and a 18-month OS of 88% (95% CI, 80-93) were documented (supplemental Table 3; Figure 1), with median survival times being 44 and 46 months, respectively. Overall, 20 patients (17%) experienced disease progression and 27 (23%) died. The most common cause of death was disease related, including CLL progression (7 patients) and transformation into Richter syndrome (5 patients). This was followed by infectious events in 8 patients, 4 of whom had a severe SARS-CoV-2 infection. Death causes were unspecified in 7 cases. Of those patients who progressed, 15 started a new therapy. In detail, 4 patients received Ibr, 1 pirtobrutinib, 2 rituximab-idelalisib, and 2 rituximab-miniCHOP for Richter transformation whereas the others were treated with chlorambucil or cyclophosphamide-based regimens. In our population, the start of a new treatment had a cumulative incidence of 12% (95% CI, 7.1-19) at 18 months, with all subjects receiving a further treatment line being R/R patients (supplemental Figure 1).
Survival outcomes and significant predictors. PFS and OS of the Ven-treated patients (A-B). PFS and OS of the significative variables (C-D). mo, months.
Survival outcomes and significant predictors. PFS and OS of the Ven-treated patients (A-B). PFS and OS of the significative variables (C-D). mo, months.
Factors influencing PFS and OS were evaluated through univariable and multivariable analyses. In the univariable analysis, an ECOG ≥2 (P<.0001) and pre-exposure to a BTKi (P = .008) were associated with a worse PFS, and the same factors significantly influenced OS (ECOG ≥2, P = .002; BTKi exposure, P = .017), as reported in supplemental Tables 1 and 2, Figure 1, and supplemental Figure 2. Notably, the line of therapy or the presence of a TP53 disruption did not influence outcomes in our univariable analysis.
When factors influencing patient survival were evaluated in a multivariable analysis, only ECOG ≥2 emerged as a significant predictor both for PFS (HR, 3.51; 95% CI, 1.57-7.85; P = .002) and OS (HR, 3.87; 95% CI, 1.64-9.12; P = .002; supplemental Table 2; Figure 1).
Temporary interruption of the drug for a period of 7 days or longer, definitive reduction in the dosage of the drug, and a ramp-up period of Ven longer than the scheduled 5 weeks did not have a statistically significant impact on PFS or OS in the evaluated population (supplemental Table 2).
Management issues and AEs
Among the enrolled individuals, a Ven ramp-up time longer than 5 weeks was reported in 38 cases (32%), and a total of 94 patients (78%) reached the full dose of the drug of 400 mg (Table 2). Temporary interruptions of the drug for at least 7 days occurred in 51 individuals (43%), whereas definitive interruption was documented in 59 individuals (49%), more often caused by disease progression (29%) or because of scheduled therapy interruption (34%); 49 patients (79%) completed the time-limited therapy whereas 62 patients were still on treatment at data cutoff.
Management issues and AEs during Ven regimens
| Management issues . | n (%) . | AEs All events, 343; grade ≥3 events, 111 . | n (%) . |
|---|---|---|---|
| Ramp-up >5 weeks | 38 (32) | Gastrointestinal, any grade | 31 (26) |
| Reached full dose of 400 mg | 94 (78) | Asthenia, any grade | 25 (21) |
| Interruption >7 days | 51 (43) | Anemia grade 1-2 | 29 (24) |
| Dose reduction | 43 (36) | Anemia grade 3-4 | 8 (7) |
| Definite interruption | 59 (49) | Neutropenia grade 1-2 | 51 (43) |
| Caused by toxicity | 19 (16) | Neutropenia grade 3-4 | 44 (37) |
| Caused by PD | 17 (14) | Thrombocytopenia grade 1-2 | 30 (25) |
| Caused by an infectious event | 11 (9) | Thrombocytopenia grade 3-4 | 16 (13) |
| Caused by end of therapy | 24 (20) | Febrile neutropenia | 12 (10) |
| Caused by death | 10 (8) | Infections grade 1-2 | 72 (60) |
| Deaths | 27 (23) | Infections grade 3-4 | 26 (22) |
| Deaths while on Ven | 10 (8) | SARS-CoV-2 infections | 46 (38) |
| Progressions | 20 (17) | Hospitalizations | 34 (28) |
| G-CSF administration owing to neutropenia | 66 (57) | TLS | 5 (4) |
| Management issues . | n (%) . | AEs All events, 343; grade ≥3 events, 111 . | n (%) . |
|---|---|---|---|
| Ramp-up >5 weeks | 38 (32) | Gastrointestinal, any grade | 31 (26) |
| Reached full dose of 400 mg | 94 (78) | Asthenia, any grade | 25 (21) |
| Interruption >7 days | 51 (43) | Anemia grade 1-2 | 29 (24) |
| Dose reduction | 43 (36) | Anemia grade 3-4 | 8 (7) |
| Definite interruption | 59 (49) | Neutropenia grade 1-2 | 51 (43) |
| Caused by toxicity | 19 (16) | Neutropenia grade 3-4 | 44 (37) |
| Caused by PD | 17 (14) | Thrombocytopenia grade 1-2 | 30 (25) |
| Caused by an infectious event | 11 (9) | Thrombocytopenia grade 3-4 | 16 (13) |
| Caused by end of therapy | 24 (20) | Febrile neutropenia | 12 (10) |
| Caused by death | 10 (8) | Infections grade 1-2 | 72 (60) |
| Deaths | 27 (23) | Infections grade 3-4 | 26 (22) |
| Deaths while on Ven | 10 (8) | SARS-CoV-2 infections | 46 (38) |
| Progressions | 20 (17) | Hospitalizations | 34 (28) |
| G-CSF administration owing to neutropenia | 66 (57) | TLS | 5 (4) |
PD, progressive disease; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
In 19 cases (16%), the therapy was discontinued owing to an AE, with 11 discontinuations (9%) being caused by infections; 20 patients (17%) experienced disease progression and 10 (8%) died while receiving Ven-based therapeutic regimens.
A total of 343 TEAEs were documented across the whole cohort, of which 111 were grade 3 or higher (Table 2). The most frequent grade 1-2 AEs were gastrointestinal symptoms (diarrhea, constipation, dyspepsia, 26%, n = 31), asthenia (21%, n = 25), and hematologic toxicity with rates of grade 1-2 neutropenia, thrombocytopenia, and anemia being 43% (n = 51), 25% (n = 30), and 24% (n = 29), respectively. Among AEs of grade 3 or higher, the most common were neutropenia (37%, n = 44), thrombocytopenia (13%, n = 16), and anemia (7%, n = 8). Infectious events were frequent, with 72 grade 1 to 2 (60%) and 26 grade 3 or higher infections (22%). We observed 15 bacterial pneumonias, 8 COVID-19 pneumonias, 8 upper airway infections, 7 herpes zoster reactivations, 6 urinary tract infections, and 3 cutaneous infections. Overall, 38% of patients contracted a SARS-CoV-2 infection of any grade. The remainder infectious events were not described regarding the site of infection and/or pathogenic agent. We recorded 34 hospitalizations (28%) that were considered to be associated with a TEAE. Granulocyte growth factor was administered to 66 patients (57%) owing to severe neutropenia. TLS was documented in 5 patients, of whom only 1 met the criteria for clinical TLS. Only 3 patients of the 17 receiving obinutuzumab developed a grade 1-2 infusion reaction.
In addition, during the follow-up, 7 cases (6%) developed Richter transformation, of whom 2 evolved while Ven was still being administered (Table 2).
Comparison with BTKi in octogenarians
Subsequently, we performed a comparison with a similar CLL population (named BTKi cohort in our analysis) treated within the same enrolling centers, of which data in the front line have already been published, with a shorter follow-up (Table 3). In brief, a total of 119 patients aged older than 80 years (range, 80-87 years) were treated with BTKi monotherapy between 2014 and 2021. Median follow-up was 25 months (range, 0-85), 69 patients (58%) had a CIRS score greater than 6, and 61 patients (53%) had an unmutated status of the IGHV gene. Compared with the Ven-treated patients, the BTKi cohort had notable differences in the median number of previous lines of therapy (0; range, 0-8) and the number of high-risk genetic lesions, with 47 TP53 mutations (40%) and 40 del(17p) (34%) being documented (P values of <.001 and .038 for the comparison with the Ven-treated cohort) (supplemental Table 3).
Baseline characteristics of the Ibr-treated cohort
| Characteristics . | N = 119 . |
|---|---|
| Age, median (range) | 80 (80-87) |
| CIRS >6, n (%) | 69 (58) |
| TP53 disruption, n (%) | 61 (51) |
| Del(17p), n (%) | 40 (34) |
| TP53 mutation, n (%) | 47 (40) |
| Missing data, n (%) | 6 (5) |
| IGHV unmutated, n (%) | 61 (51) |
| CrCl < 60 mL/min, n (%) | 79 (66) |
| Binet stages | |
| A, (%) | 11 (9) |
| B, (%) | 53 (45) |
| C, (%) | 55 (46) |
| Treatment naïve, n (%) | 79 (66) |
| Relapsed patients, n (%) | 40 (34) |
| Previous lines, median (range) | 0 (0-8) |
| 1, n (%) | 20 (17) |
| 2, n (%) | 10 (8) |
| 3+, n (%) | 10 (8) |
| Type of previous therapy | |
| Chlorambucil ± anti-CD20, n (%) | 24 (20) |
| FC-R, BR, n (%) | 26 (22) |
| Others (lenalidomide, rituximab, CHOP, RCVP, alemtuzumab, idelalisib-R), n (%) | 17 (14) |
| Efficacy (best response) | |
| ORR (CR + CRi + PR + PR-L), n (%) | 107 (90) |
| SD, n (%) | 12 (10) |
| PD, n (%) | 0 (0) |
| Richter transformation, n (%) | 7 (6) |
| Characteristics . | N = 119 . |
|---|---|
| Age, median (range) | 80 (80-87) |
| CIRS >6, n (%) | 69 (58) |
| TP53 disruption, n (%) | 61 (51) |
| Del(17p), n (%) | 40 (34) |
| TP53 mutation, n (%) | 47 (40) |
| Missing data, n (%) | 6 (5) |
| IGHV unmutated, n (%) | 61 (51) |
| CrCl < 60 mL/min, n (%) | 79 (66) |
| Binet stages | |
| A, (%) | 11 (9) |
| B, (%) | 53 (45) |
| C, (%) | 55 (46) |
| Treatment naïve, n (%) | 79 (66) |
| Relapsed patients, n (%) | 40 (34) |
| Previous lines, median (range) | 0 (0-8) |
| 1, n (%) | 20 (17) |
| 2, n (%) | 10 (8) |
| 3+, n (%) | 10 (8) |
| Type of previous therapy | |
| Chlorambucil ± anti-CD20, n (%) | 24 (20) |
| FC-R, BR, n (%) | 26 (22) |
| Others (lenalidomide, rituximab, CHOP, RCVP, alemtuzumab, idelalisib-R), n (%) | 17 (14) |
| Efficacy (best response) | |
| ORR (CR + CRi + PR + PR-L), n (%) | 107 (90) |
| SD, n (%) | 12 (10) |
| PD, n (%) | 0 (0) |
| Richter transformation, n (%) | 7 (6) |
BR, bendamustine-rituximab; CHOP, cyclophosphamide doxorubicine vincristine and prednisolone; CrCl, creatinine clearance; CRi, complete response with incomplete count recovery; FC-R, ludarabine, cyclophosphamide and rituximab; idelalisib-R, idelalisib, rituximab; PD, progressive disease; PR, partial response; PR-L, partial response with lymphocytosis; RCVP, rituximab, cyclophosphamide, vincristine and prednisolone; SD, stable disease.
Regarding efficacy, a comparable ORR (90%, n = 107; P = .809) was observed with the use of BTKis, but CR rate was notably lower (15%, n = 18, P < .001), with 18-month PFS and OS being 80% (95% CI, 71-86) and 90% (95% CI, 83-94), respectively (supplemental Table 3; supplemental Figures 2 and 3).
Regarding safety, permanent therapy discontinuation was documented in 51 cases (43%) owing to the use of BTKis, 18 cases (15%) owing to disease progression, and 17 cases (14%) owing to toxicity emerging as the dominant causes of discontinuation, whereas a lower incidence of interruption-leading infectious events (5%, n = 6) was reported (Table 4). Notably, 7 interrupted (6%) BTKi therapy owing to the diagnosis of a second primary malignancy. A total of 29 deaths (24%) were recorded during continuous BTKi therapy, of which 15 were caused by disease progression, 5 were caused by cardiovascular causes, 4 were caused by a second neoplasm, and 4 were caused by an infectious event.
Management issues and AEs that occurred in the BTKi cohort
| Management issues and AEs . | n (%) . |
|---|---|
| Hematologic toxicity grade 3-4 | 18 (15) |
| Anemia grade 3-4 | 6 (5) |
| Neutropenia grade 3-4 | 12 (10) |
| Infections grade 1-2 | 35 (29) |
| Infections grade 3-4 | 24 (20) |
| Cardiovascular events, any grade | 31 (26) |
| Cardiovascular events, grade 3-4 | 6 (5) |
| Bleeding grade 1-2 | 37 (31) |
| Bleeding grade 3-4 | 4 (3) |
| Second neoplasia | 12 (10) |
| Definite interruption | 51 (43) |
| Caused by toxicity | 17 (14) |
| Caused by PD | 18 (15) |
| Caused by an infectious event | 6 (5) |
| Caused by death | 7 (6) |
| Caused by second neoplasia | 7 (6) |
| Deaths | 29 (24) |
| Management issues and AEs . | n (%) . |
|---|---|
| Hematologic toxicity grade 3-4 | 18 (15) |
| Anemia grade 3-4 | 6 (5) |
| Neutropenia grade 3-4 | 12 (10) |
| Infections grade 1-2 | 35 (29) |
| Infections grade 3-4 | 24 (20) |
| Cardiovascular events, any grade | 31 (26) |
| Cardiovascular events, grade 3-4 | 6 (5) |
| Bleeding grade 1-2 | 37 (31) |
| Bleeding grade 3-4 | 4 (3) |
| Second neoplasia | 12 (10) |
| Definite interruption | 51 (43) |
| Caused by toxicity | 17 (14) |
| Caused by PD | 18 (15) |
| Caused by an infectious event | 6 (5) |
| Caused by death | 7 (6) |
| Caused by second neoplasia | 7 (6) |
| Deaths | 29 (24) |
PD, progressive disease.
While comparing the toxicity profiles of the 2 regimens, we found that patients undergoing BTKi therapy had a lower rate of infections (49%, n = 59, vs 82%, n = 88; P = .001), but the proportion of grade ≥3 infections was similar (20%, n = 24, vs 22%, n = 26; P = .958) (supplemental Table 4). Among grade 3 or higher infections, 20 pneumonias and 8 septic events occurred during BTKi therapy.
When evaluating cardiovascular toxicity, we observed 31 events in the BTKi cohort (26% of patients), including 14 atrial fibrillation and 11 new-onset hypertension, of which 6 (5%) were grade 3-4; 37 patients (31%) had a grade 1-2 bleeding event whereas 4 (3%) had grade 3-4 bleeding. In contrast, only 5 cardiovascular events were recorded in the Ven cohort (3 myocardial infarctions and 2 atrial fibrillations), resulting in the temporary discontinuation of the drug. Twelve BTKi-treated (10%) and 4 Ven-treated patients (3%) developed a second neoplasm.
Discussion
Although the use of novel target therapies has been seldom explored in very old patients with CLL within specifically designed trials, such treatment options are commonly used in routine clinical practice, although data regarding their efficacy and safety in this specific subset of patients are lacking.
We herein report the results of an Italian multicenter retrospective study, exploring the use of Ven-based therapeutic regimens in the largest octogenarian cohort to date. In line with what is expected in this patient population, more than half of the enrolled subjects had a significant comorbidity burden, as indicated by a CIRS score >6 and ECOG of ≥2, and more than a quarter of the individuals had a high-risk disease, defined by the presence of either a TP53 mutation or a del(17p). Notably, the vast majority of the considered patients had R/R disease, and consequently, most of them received Ven-based therapies that are approved for prescription in the R/R setting, such as Ven-R (38%) or continuous Ven monotherapy (50%).
In our patient population, Ven-based therapeutic regimens showed remarkable efficacy, with an ORR >90% and a CR rate shy of 45%, yielding results that are comparable with what is reported in younger patients both within clinical trials and published real-world experiences30,31 and leading to notable survival results, with median OS and PFS almost reaching 4 years. Moreover, the data we obtained compare favorably with a recent report published by the German CLL study group (GCLLSG),32 where a pooled analysis of 7 GCLLSG trials was performed extrapolating patients aged 80 years or older from the trials’ safety populations (n = 152). In such analysis, the authors report on a population mostly treated with chlorambucil-based regimens, where, in comparison with what we observed with Ven treatment, lower rates of both overall and complete responses were achieved, albeit a slightly lower incidence of severe infectious complications was reported. A second, more recent, pooled analysis conducted by the GCLLSG extrapolates data from 6 clinical trials, focusing on the use of targeted agents as first-line treatment in patients with CLL aged 80 or older. In this publication, data on a small sample of subjects are reported (n = 33), and although the results of Ven-treated subjects are not reported separately, they represent most of the sample (82% Ven-G, 6% Ibr-Ven-G). The efficacy results detailed in this analysis are somewhat aligned with those described by our analysis, given that an ORR of 73% and a CR rate of 36% are disclosed, whereas the comparison of survival data with that of our own is made difficult by the rather different observation times, and the lack of reported information on AEs hinders further comparisons.33
Some additional information on the utilization of Ven-based regimens in elderly patients can also be obtained from a recent study coming from a joint United Kingdom-United States effort, 33 which reported on a cohort of 343 individuals treated with Ven-based regimens for R/R CLL within 66 centers. Notably, the elderly population in this study was defined as having an age of 75 years or older (n = 71), thus making comparisons with our results rather difficult, given that an age difference of 5 years can have marked effects on patient performance and fitness, especially in the higher age ranges. Nonetheless, the results reported by Eyre et al are in line with our data, given that they described an ORR >80% with a CR rate of 35% without any significant excess in toxicities in comparison with the younger patient cohort, with the sole exception of severe neutropenia that however did not translate into an increase of life-threatening infectious events.34 Interestingly, a higher rate of therapy discontinuation owing to AEs was reported in the older cohort (34%), suggesting lower tolerability of Ven in older patients. Such finding is slightly in contrast with what we observed within our population, given that the rate of discontinuation for AEs (infectious and noninfectious AEs combined) was ∼15%, with more than three-quarters of the enrolled subjects being able to reach the full dose of Ven. As reported in the English-American cohort, the most common AEs associated with Ven in our population were of hematologic or infectious nature and mostly mild in grading. While discussing the management of AEs, it is important to point out that, within our study, a significant fraction of patients required a therapy interruption longer than a week, and in some cases, a longer Ven ramp-up time was chosen by the treating physician to mitigate toxicities, but neither the former nor the latter dose modifications seemed to influence the overall efficacy of the chosen Ven regimen.
Comparing the results observed within our Ven-treated cohort with the updated and expanded BTKi-treated octogenarian patient group,14 no significant differences in efficacy were found, in terms of both response rates and survival times. Although these results need to be interpreted with caution owing to the indirect nature of the comparison and the higher proportion of high-risk genetic lesions in the BTKi cohort, they still reinforce the point that Ven-based regimens are effective in this patient population. Furthermore, although the Ven-treated cohort exhibited a higher rate of all-grade infections, grade 3 to 4 infectious events had a similar incidence between the 2 groups. In contrast, the 2 groups significantly differed in terms of AEs that can be somewhat considered specific to either treatment regimen, with Ven-treated patients experiencing significantly more hematologic toxicity whereas cardiovascular toxicity being almost restricted to BTKi-treated patients.
Of note, some of the management issues that were described (eg, temporary interruption owing to hematologic toxicity, longer ramp-up phases) and the actions taken for their mitigation add on a layer of complexity in the evaluation of outcomes, toxicity, and the comparison with BTKi treatment, given that they often imply a higher medical burden for the involved patients owing to the increased number of blood tests and hospital visits, which are already typically higher in subjects treated with Ven-based strategies than in those treated with BTKi. However, owing to the design of the study and its main focus, we are unable to provide further information on the matter. Nonetheless, clinicians should not shun away from Ven-based treatments in this subset of patients, because they are a valid alternative to BTKi and they give the (at the time of writing) only option of delivering a time-limited treatment course, which may matter to patients and caregivers, even in the >80-year age range.
The results presented earlier are hampered by several limitations. First and foremost, the retrospective nature of the study is accompanied by issues with data collection and biases in the selection of patients. Furthermore, although we discuss the largest published cohort of Ven-treated octogenarian patients, a population such as ours is intrinsically heterogeneous, owing to both the varying degree of the comorbidity burden and the different biological nuances of CLL, which may have led to poor representation of some patient subsets. In addition, it needs to be underlined that the population included in this study is to be considered relatively fit considering its age, given that approximately half of the patients had a CIRS >6 and only ∼20% had an ECOG ≥2, and the efficacy and safety profiles of the discussed regimens may not be applicable to more comorbid individuals.
Nevertheless, our study represents a key step toward the achievement of a better standard of care for elderly patients requiring treatment for CLL, providing real-world evidence for the use of Ven-based combinations in this patient population and improving on some previously published data on the use of BTKis. Because we expect that the use of these therapeutic agents will increase in the near future, we hope that more research will focus specifically on the treatment of elderly patients, to provide further information, hopefully of a prospective nature, on the optimal therapeutic strategies to be used in such individuals.
Acknowledgments
The authors thank all the patients enrolled in this study and their caregivers.
This study was supported by Associazione Italiana per la Ricerca sul Cancro IG-25024, Progetti di Rilevanza Nazionale PRIN PNRR (P2022PSMX4), STARS UNIPD 2023, and the Organizzazione di Volontariato Ricerca per Credere nella Vita.
Authorship
Contribution: A. Serafin, A. Cellini, and A.V. contributed to this study’s concept, design, and data collection and wrote the initial draft of the manuscript; all the remaining authors participated in data collection, edited and revised the manuscript, provided critical and intellectual content, and approved the revised manuscript.
Conflict-of-interest disclosure: P.G. received honoraria from AbbVie, AstraZeneca, BeiGene, Bristol Myers Squibb, Johnson & Johnson, Galapagos, Lilly/Loxo Oncology, Merck Sharp & Dohme, and Roche; and research support from AbbVie, AstraZeneca, Bristol Myers Squibb, Johnson & Johnson, and Lilly/Loxo Oncology. I.F. received research support from AbbVie, BeiGene, and Eli Lilly. M.V. received research support from AbbVie, AstraZeneca, BeiGene, and Johnson & Johnson. A. Sanna received research support from AstraZeneca and Johnson & Johnson. R. Marasca received research support from AbbVie, Johnson & Johnson, BeiGene, and AstraZeneca. A. Cuneo received research support from AbbVie, AstraZeneca, BeiGene, Janssen, and Lilly. M. Marchetti received research support from Novartis, Sanofi, AstraZeneca, Roche, Gilead, and GlaxoSmithKline. A.M.F. received research support from Janssen, BeiGene, AbbVie, and AstraZeneca. P.S. received research support from AbbVie, Johnson & Johnson, BeiGene, and AstraZeneca. C.V. received research support from AbbVie, AstraZeneca, and Janssen. The remaining authors declare no competing financial interests.
Correspondence: Livio Trentin, Hematology Unit, Department of Medicine, University of Padova, via Nicolò Giustiniani 2, 35128 Padova, Italy; email: livio.trentin@unipd.it; and Massimo Gentile, Hematology Unit, Department of Onco-Hematology, Azienda Ospedaliera Annunziata, Cosenza, and Department of Pharmacy, Health and Nutritional Science, University of Calabria, 87100 Rende, Italy; email: massimo.gentile@tiscali.it.
References
Author notes
A. Serafin and A. Cellini contributed equally to this work.
The data supporting the findings of this study are available on reasonable request from the corresponding authors, Livio Trentin (livio.trentin@unipd.it) and Massimo Gentile (massimo.gentile@tiscali.it).
The full-text version of this article contains a data supplement.