Key Points
Sign and symptom scores represent distinct dimensions of PTS, relating differently to clinical profiles and outcomes.
Clinicians should recognize that combining these scores may obscure risk stratification and impede personalized treatment strategies.
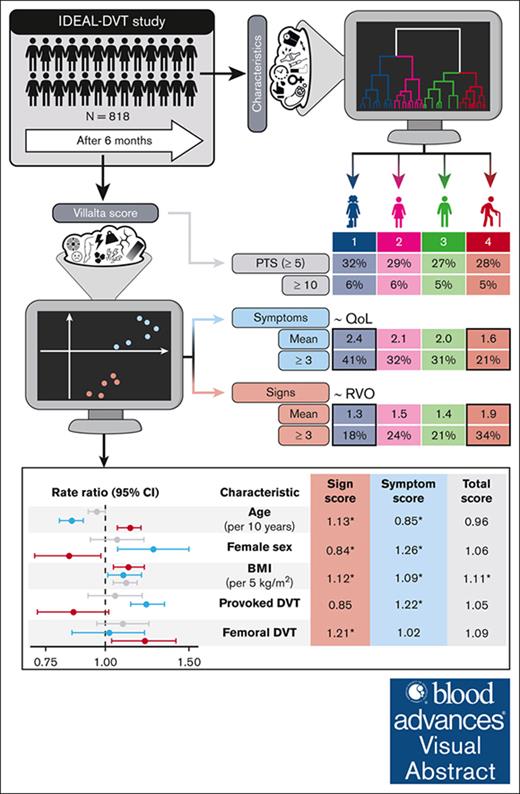
Visual Abstract
Postthrombotic syndrome (PTS) is a chronic condition that can develop after deep vein thrombosis (DVT) and is diagnosed using the Villalta scale. This study applied unsupervised machine learning to investigate the heterogeneity of PTS among patients and within the Villalta scale. In 818 patients from the IDEAL DVT study, clustering identified 4 clinical profiles: (1) younger patients with provoked DVT, (2) women with joint pain, (3) men with isolated popliteal DVT, and (4) older men with diabetes and femoral vein involvement. Clustering of Villalta items revealed a distinction between signs and symptoms. Sign scores increased with older age, male sex, higher body mass index (BMI), and DVT extent, whereas symptom scores increased with younger age, female sex, higher BMI, and provoked DVT. Residual venous obstruction was significantly associated with the sign score (odds ratio, 1.18 per point) but not the symptom score. Quality of life was related to the symptom score more than the sign score. At 6 months, sign and symptom scores differed significantly across profiles, especially between profile 1 and 4, because the former had most symptoms (41% vs 21% ≥ 3; P < .001), whereas the latter had most signs (18% vs 34% ≥ 3; P = .004]). After 2 years, symptoms decreased in profile 1 but increased in profile 4. Other profiles showed intermediate scores over time. These findings suggest that reappraising the PTS scoring system to distinguish its dimensions would enable more personalized risk prediction and prevention. This trial was registered at www.ClinicalTrials.gov as #NCT01429714.
Introduction
Postthrombotic syndrome (PTS) is a chronic venous disease that develops secondary to deep vein thrombosis (DVT).1 Although many patients develop persistent venous abnormalities after DVT, the objective presence of these without accompanying symptoms is insufficient to diagnose PTS.2 In absence of a stand-alone objective test, the diagnosis of PTS is based on characteristic signs and symptoms using the Villalta scale.3 This composite score comprises 11 items: 6 signs and 5 symptoms, each scored within a range from 0 to 3 points. In 2009, the Scientific and Standardization Subcommittee on the Control of Anticoagulation of the International Society on Thrombosis and Haemostasis reached consensus to standardize the definition of PTS as a Villalta score of ≥5 measured at least 3 months after DVT,4 although 6 months has been the earliest time point used in most recent trials.5-9 Using this consensus definition, up to 50% of patients are diagnosed with PTS within 2 years after DVT.10
Because signs and symptoms wax and wane over time, the consensus definition of PTS is prone to over-diagnose mild PTS (ie, score of 5-9),11 and stricter definitions have been proposed to improve associations of PTS with clinical outcomes such as quality of life (QoL).12 As research continues to debate the nuances of defining “true” PTS, there is a risk of overlooking that PTS is a heterogenous disease with varying clinical presentations.13 Although the Villalta scale is easily applicable, combining all items into a single score might obscure rather than enhance associations with outcomes of therapeutic interventions, because distinct aspects of PTS may require different therapeutic strategies due to the heterogeneity of underlying its pathogenic mechanisms. Accordingly, the risk factors included in validated prediction models have varied considerably,9,14-16 and the presence of residual venous obstruction (RVO), a major pathogenic mechanism, only moderately increases the risk of developing PTS.17
In this study, we aimed to assess the heterogeneity within the diagnosis of PTS by taking a closer look at different groups of patients with DVT as well as by reappraising the Villalta scale. This was done using unsupervised machine learning, specifically hierarchical cluster analysis, which allows for unbiased identification of homogenous groups within a heterogenous population.18 Using this potent method, we aimed to identify differences in patient profiles and dimensions of the Villalta scale associated with these profiles.
Methods
Study participants
Pooled data were used from patients who participated in the IDEAL DVT study, a randomized, controlled, noninferiority trial that compared fixed-duration with tailored-duration elastic compression therapy (ECT) for the prevention of PTS.5 This study established noninferiority of tailored-duration ECT5 and also revealed significantly more PTS in patients who did not receive acute phase compression.19 A detailed description of the methods can be found in the published protocol.20 In brief, between March 2011 and July 2015, consecutive adult patients with objectively confirmed proximal acute DVT were randomized (1:1) to a tailored duration of at least 6 months or a fixed 2-year duration of ECT. Exclusion criteria included previous ipsilateral DVT, preexistent venous insufficiency (ie, Clinical Etiological Anatomical and Pathophysiological score of at least C3), severe peripheral artery disease, and active thrombolysis (ie, systemic or catheter directed). Patients were recruited in 12 centers in The Netherlands and 2 centers in Italy. There was a 2-year follow-up with visits to the outpatient clinic at 4 time points: 3 months, 6 months, 1 year, and 2 years after diagnosis. Ten of 12 centers in The Netherlands assessed the presence of RVO 1 week before planned cessation of anticoagulant therapy for a substudy,19 in which Italian centers did not participate. The study obtained ethical approval by the institutional review board of Maastricht University Medical Centre and was acknowledged by the medical ethical review boards of participating centers (NL32073. 068.10). All patients gave written informed consent. This trial was registered at www.ClinicalTrials.gov (identifier: NCT 01429714).
Villalta scale
This is a composite score comprising 11 items, namely 6 signs (ie, edema, induration, redness, venous ectasia, hyperpigmentation, and pain upon calf compression) and 5 symptoms (ie, pain, pruritus, cramps, paresthesia, and heaviness). Each item is scored on a 4-point scale: 0 points when there is no deviation from normal, 1 if the sign or symptom is mild, 2 if it is moderate, and 3 if it is severe. In addition, 15 points are attributed in case of venous ulceration. In the IDEAL DVT study, signs and symptoms of the Villalta scale were recorded at each follow-up visit. Signs were scored by assessors who were blinded to treatment allocation, using the published and validated visual guide.21 All patients were instructed not to reveal treatment allocation and not to wear ECT on the day of the follow-up visit. Assessors were unaware of patients’ symptoms because these were scored by patients themselves through an online questionnaire, which they were asked to fill out on the day before each follow-up visit. Changes in the score after 2 years (ie, ▵ relative to 6 months) were given to show development over time.
PTS
PTS was diagnosed based on a score of ≥5 (and moderate-to-severe PTS based on a score of ≥10) on the Villalta scale or presence of ipsilateral leg ulcer at least 6 months after DVT, as has been recommended by the International Society on Thrombosis and Haemostasis.4 The score was assessed at the 6-month, 1-year, and 2-year visit, as has been mentioned earlier. For the purpose of this analysis, we report the proportion of patients diagnosed at 6 months and the proportion of patients diagnosed after 2 years.
RVO
Presence of RVO was assessed by compressive ultrasound examination at 3 points, namely at the popliteal, femoral, and common femoral vein of the affected leg. This examination was performed by independent radiologists of the participating centers according to a prespecified protocol based on literature.22 Technical devices were dependent on preference and availability at the participating center. The patients were in supine position, and examined veins were imaged in the transverse plane. RVO was defined by the common definition of a vein diameter of at least 2 mm during full compression in at least 1 of the assessed vein segments.23 Radiologists were required to describe findings systematically based on a standard form provided by the study.
QoL metrics
Generic QoL was assessed using the Short Form 36 (SF-36), which is 36-item questionnaire that can be converted to a total score based on characteristics of the Dutch population.24 Venous disease–specific QoL was assessed using the venous insufficiency epidemiologic and economic studies QoL (VEINES-QoL) questionnaire, which is a 26-item questionnaire that converts to a total intrinsic score,25,26 and is the recommended score to use in the context of PTS.4 Both scores were expressed from 0 to 100 in this study, with higher scores representing better QoL.
Hierarchical cluster analysis
Cluster analysis is an unsupervised machine learning method that uses unlabeled data sets to identify clusters (ie, groups) based on the patterns of shared characteristics.18 A hierarchical approach allows for explorative studies without a prespecified number of clusters, which can be deduced from the results and are typically visualized using a dendrogram. In this study, we applied hierarchical cluster analysis to 2 levels of data: variables and patients. Variable clustering was used to identify groups of Villalta items, whereas patient clustering was used to create patient profiles. Importantly, although these profiles are formed based on known clinical characteristics, the patterns might result from underlying unmeasured variables. Differences between clusters are typically analyzed descriptively, because no individual characteristic explains the whole pattern. To validate the meaningfulness of these clusters, clinical outcomes should be used instead, because these were not used in the clustering algorithm itself.
Statistical analysis
Patient clusters, hereafter referred to as profiles, were generated by hierarchical clustering with the Ward linkage method and the Gower distance using the R package “cluster” based on 10 categorical and 2 numeric baseline clinical characteristics (supplemental Table 1). The optimal number of clusters within a range of 2 to 10 was determined using the R package “NbClust.” Descriptive data were given as percentage for categorical variables and mean with standard deviation for continuous variables. Next, profiles were compared by their total Villalta score and proportion of (moderate-to-severe) PTS at 6 months, as well as the change in total Villalta score and proportion of (moderate-to-severe) PTS after 2 years. Comparisons were performed by t tests (or Mann-Whitney U tests) and χ2 tests (or Fisher exact tests), as appropriate, with multiple testing correction using a 5% false-discovery rate with the Benjamini-Hochberg method.
To investigate aspects of the Villalta scale, items were clustered hierarchically using the R package “ClustOfVar”; ipsilateral leg ulcer (n = 2) was excluded because of its rarity.5 These clusters were visualized using a loading plot, showing the influence of each item in a principal component analysis, as well as a using a correlation matrix with Pearson coefficients. To validate acquired scores as distinct from the total score, associations with clinical characteristics, RVO, and QoL metrics were assessed. Clinical characteristics were assessed in relation to the acquired scores by negative binomial regression, expressed as rate ratio with 95% confidence interval (CI) using the R package “MASS.” The relationship between acquired scores and RVO was assessed by multivariable logistic regression, expressed as an odds ratio with 95% CI. An optimal cutoff score for RVO was calculated using the Youden index. A sensitivity analysis of RVO for each vein segment (popliteal, femoral, and common femoral) was performed. Finally, the relationship between acquired scores and the QoL metrics was assessed by multivariable linear regression, expressed as regression coefficient with P value. Multivariable models were selected backward stepwise from clinical characteristics using the Akaike information criterion; scores were combined in 1 model. All analyses were primarily performed for 6-month scores, adjusted for acute phase compression. In addition, changes in scores after 2 years were analyzed, adjusted for treatment group and 6-month scores. Associations at 1- and 2-year follow-up are given in supplemental Table 2.
Finally, having established acquired scores, patient profiles were reassessed by comparing these scores descriptively between profiles, both as continuous variable and as proportion in the upper quartile (ie, ≥75th percentile). To perform cluster analyses, all missing values of clinical characteristics and Villalta items were imputed by random forest imputation using the R package “missForest.” Two-tailed P values < .05 were considered statistically significant for all statistical tests. Analyses were conducted in R statistical software, version 4.2.0.27
Results
Study participants
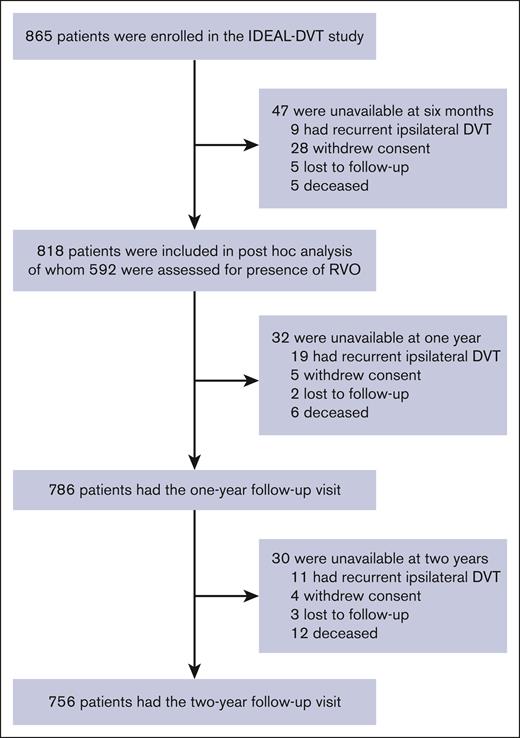
Of a total of 865 patients, 818 were included in this analysis after excluding patients who had died (n = 5), had recurrent ipsilateral DVT (n = 9), were lost to follow-up (n = 5), or had withdrawn consent (n = 28) within 6 months after the index DVT (Figure 1). Most patients were available for the 1-year (n = 786 [96.1%]) and 2-year (n = 756 [92.4%]) follow-up visits. Clinical characteristics are shown in Table 1; missing values were limited to 1.2% for previous contralateral DVT, and 0.2% for unprovoked DVT and the extent of DVT. Almost all patients (89.9%) received anticoagulation with a vitamin K antagonist, whereas a few patients received rivaroxaban (3.2%), low-molecular-weight heparins (4.0%), or an investigational anticoagulant drug (2.8%).
Clinical characteristics of the study population
| . | All patients (N = 818) . | Profile 1 (n = 204) . | Profile 2 (n = 209) . | Profile 3 (n = 235) . | Profile 4 (n = 170) . |
|---|---|---|---|---|---|
| Age, mean (±SD), y | 57.2 (±14.5) | 49.3 (±14.3) | 59.5 (±15.0)∗ | 57.9 (±12.4)∗,§ | 62.7 (±12.7)∗,‡ |
| Sex, % (n) | |||||
| Female | 58.3% (447) | 62.3% (127) | 88.5% (185) | 4.68% (11)∗,† | 10.6% (18)∗,† |
| Male | 41.7% (341) | 37.7% (77) | 11.5% (24) | 95.3% (224)∗,† | 89.4% (152)∗,† |
| BMI, mean (±SD) | 28.0 (±5.2) | 28.0 (±6.25) | 28.6 (±5.62)§ | 27.8 (±4.49) | 27.5 (±4.24)∗ |
| DVT extent, % (n) | |||||
| Common femoral vein | 21.8% (178) | 32.4% (66) | 14.4% (30) | 1.28% (3) | 46.5% (79) |
| Femoral vein | 27.8% (227) | 21.1% (43)§ | 21.1% (44)§ | 24.7% (58)§ | 48.2% (82) |
| Popliteal vein | 50.5% (413) | 46.6% (95) | 64.6% (135)∗,§ | 74.0% (174)∗,§ | 5.29% (9) |
| Affected side, % (n) | |||||
| Left | 54.2% (443) | 54.4% (111)§ | 51.2% (107)§ | 43.4% (102)§ | 72.4% (123) |
| Right | 45.4% (371) | 44.1% (90)§ | 48.8% (102)§ | 56.6% (133)§ | 27.1% (46) |
| Bilateral | 0.5% (4) | 1.5% (3) | 0.0% (0) | 0.0% (0) | 0.6% (1) |
| Unprovoked, % (n) | 69.3% (567) | 2.5% (5) | 88.0% (184)∗ | 94.0% (221)∗ | 92.4% (157)∗ |
| Previous contralateral DVT, % (n) | 9.8% (80) | 2.9% (6)‡ | 8.13% (17)‡ | 18.7% (44) | 7.7% (13)‡ |
| Chronic inflammatory disease, % (n) | 4.4% (36) | 4.4% (9) | 1.4% (3)‡ | 8.1% (19)† | 2.9% (5) |
| Diabetes, % (n) | 8.4% (69) | 3.9% (8)§ | 8.6% (18)‡,§ | 2.1% (5)†,§ | 22.4% (38) |
| Joint pain, % (n) | 11.6% (95) | 3.9% (8)†,‡ | 33.5% (70) | 0.4% (1) | 9.4% (16)†,‡ |
| Individualized ECT duration, % (n) | 49.9% (408) | 49.0% (100) | 50.7% (106) | 50.2% (118) | 49.4% (84) |
| Acute phase ECT, % (n) | 78.6% (643) | 72.5% (148)†,‡ | 83.3% (174)∗ | 82.1% (193)∗ | 75.3% (128) |
| . | All patients (N = 818) . | Profile 1 (n = 204) . | Profile 2 (n = 209) . | Profile 3 (n = 235) . | Profile 4 (n = 170) . |
|---|---|---|---|---|---|
| Age, mean (±SD), y | 57.2 (±14.5) | 49.3 (±14.3) | 59.5 (±15.0)∗ | 57.9 (±12.4)∗,§ | 62.7 (±12.7)∗,‡ |
| Sex, % (n) | |||||
| Female | 58.3% (447) | 62.3% (127) | 88.5% (185) | 4.68% (11)∗,† | 10.6% (18)∗,† |
| Male | 41.7% (341) | 37.7% (77) | 11.5% (24) | 95.3% (224)∗,† | 89.4% (152)∗,† |
| BMI, mean (±SD) | 28.0 (±5.2) | 28.0 (±6.25) | 28.6 (±5.62)§ | 27.8 (±4.49) | 27.5 (±4.24)∗ |
| DVT extent, % (n) | |||||
| Common femoral vein | 21.8% (178) | 32.4% (66) | 14.4% (30) | 1.28% (3) | 46.5% (79) |
| Femoral vein | 27.8% (227) | 21.1% (43)§ | 21.1% (44)§ | 24.7% (58)§ | 48.2% (82) |
| Popliteal vein | 50.5% (413) | 46.6% (95) | 64.6% (135)∗,§ | 74.0% (174)∗,§ | 5.29% (9) |
| Affected side, % (n) | |||||
| Left | 54.2% (443) | 54.4% (111)§ | 51.2% (107)§ | 43.4% (102)§ | 72.4% (123) |
| Right | 45.4% (371) | 44.1% (90)§ | 48.8% (102)§ | 56.6% (133)§ | 27.1% (46) |
| Bilateral | 0.5% (4) | 1.5% (3) | 0.0% (0) | 0.0% (0) | 0.6% (1) |
| Unprovoked, % (n) | 69.3% (567) | 2.5% (5) | 88.0% (184)∗ | 94.0% (221)∗ | 92.4% (157)∗ |
| Previous contralateral DVT, % (n) | 9.8% (80) | 2.9% (6)‡ | 8.13% (17)‡ | 18.7% (44) | 7.7% (13)‡ |
| Chronic inflammatory disease, % (n) | 4.4% (36) | 4.4% (9) | 1.4% (3)‡ | 8.1% (19)† | 2.9% (5) |
| Diabetes, % (n) | 8.4% (69) | 3.9% (8)§ | 8.6% (18)‡,§ | 2.1% (5)†,§ | 22.4% (38) |
| Joint pain, % (n) | 11.6% (95) | 3.9% (8)†,‡ | 33.5% (70) | 0.4% (1) | 9.4% (16)†,‡ |
| Individualized ECT duration, % (n) | 49.9% (408) | 49.0% (100) | 50.7% (106) | 50.2% (118) | 49.4% (84) |
| Acute phase ECT, % (n) | 78.6% (643) | 72.5% (148)†,‡ | 83.3% (174)∗ | 82.1% (193)∗ | 75.3% (128) |
Pairwise comparisons of profiles were corrected for multiple testing with a false-discovery rate of 5% (Benjamini-Hochberg method). Bold values indicate significant difference compared with all profiles.
SD, standard deviation.
Indicates significant difference compared with profile 1.
Indicates significant difference compared with profile 2.
Indicates significant difference compared with profile 3.
Indicates significant difference compared with profile 4.
Patient profiles
Four profiles were identified based on patients’ clinical characteristics, as shown in Table 1. Overall, profile 1 (n = 204) consisted of relatively younger patients with provoked DVT; profile 2 (n = 209) was formed by women with joint pain and higher body mass index (BMI); profile 3 (n = 235) comprised men with isolated popliteal DVT, chronic inflammatory disease, and previous contralateral DVT; and profile 4 (n = 170) comprised older men with diabetes, left-sided DVT, and femoral vein involvement.
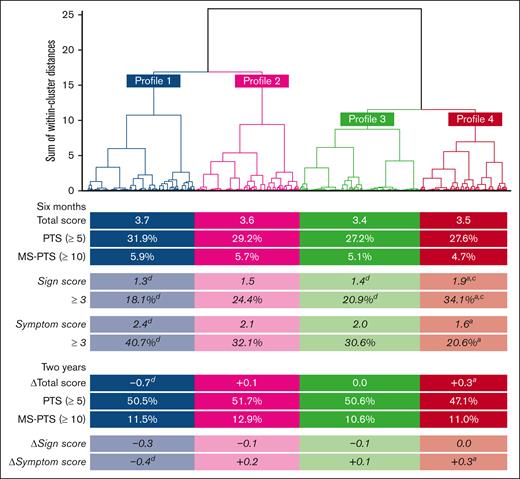
At 6 months, the total score and proportion of PTS and moderate-to-severe PTS were similar between profiles (Figure 2), although numerically slightly higher in the younger patients (profile 1). After 2 years, the total score had decreased substantially in the younger patients (profile 1), whereas the older men experienced a notable increase (profile 4); nevertheless, the proportion of PTS and moderate-to-severe PTS remained similar at 2 years, although PTS was numerically slightly lower in older men.
Comparing Villalta scores of patients’ profiles. Shows dendrogram of cluster analysis to obtain clinical profiles. Under each profile are total Villalta scores, proportions of MS-PTS, sign scores, and symptom scores at 6 months, as well as the score ▵s and cumulative proportion of MS-PTS after 2 years. Pairwise comparisons of profiles were corrected for multiple testing with a false-discovery rate of 5% (Benjamini-Hochberg method). a, b, c, dIndicates significant difference compared to profile 1, 2, 3, and 4, respectively. MS, moderate-to-severe.
Comparing Villalta scores of patients’ profiles. Shows dendrogram of cluster analysis to obtain clinical profiles. Under each profile are total Villalta scores, proportions of MS-PTS, sign scores, and symptom scores at 6 months, as well as the score ▵s and cumulative proportion of MS-PTS after 2 years. Pairwise comparisons of profiles were corrected for multiple testing with a false-discovery rate of 5% (Benjamini-Hochberg method). a, b, c, dIndicates significant difference compared to profile 1, 2, 3, and 4, respectively. MS, moderate-to-severe.
Villalta item clusters
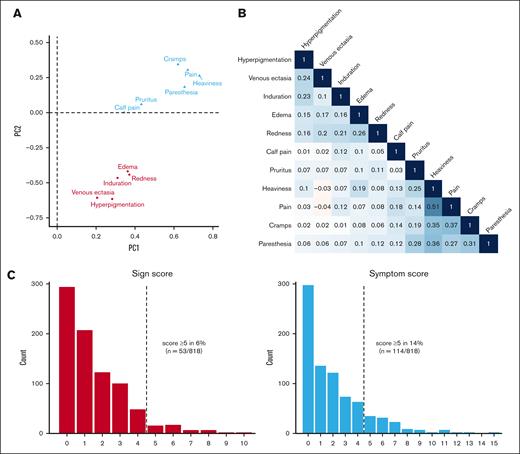
Two distinct clusters of items were identified: 1 with sign items and 1 with symptom items, including pain upon calf compression (Figure 3A). The strongest correlations between items were observed within the symptom cluster (Figure 3B), particularly between the items pain and heaviness (r = 0.51). Similar item clusters were found at the 1- and 2-year follow-up visits (supplemental Figure 1).
Clustering, correlation, and score distributions. Loadings plot of items colored by cluster (A); correlation matrix of items with Pearson coefficients (B); and bar plot of score distributions (C). Individual item scores at 6 months were missing in some patients, ranging from 1.3% to 4.9%, which were imputed. PC, principal component.
Clustering, correlation, and score distributions. Loadings plot of items colored by cluster (A); correlation matrix of items with Pearson coefficients (B); and bar plot of score distributions (C). Individual item scores at 6 months were missing in some patients, ranging from 1.3% to 4.9%, which were imputed. PC, principal component.
Sign and symptom scores
Based on the aforementioned clusters, Villalta scores were divided into 2 distinct scores, namely the “sign score” and “symptom score,” with a weak positive correlation between these scores (r = 0.19). The symptom score was observed to reach higher values more frequently, with scores of ≥5 occurring twice as often for the symptom score compared with the sign score (Figure 3C). This pattern persisted consistently at the 1-year and 2-year follow-up visits (supplemental Figure 2). Consequently, after 2 years, most patients diagnosed with PTS had a symptom score of ≥5 (41%) without a corresponding sign score of ≥5, whereas only a small proportion had a sign score of ≥5 (14%) without a corresponding symptom score of ≥5. The remaining patients either had scores of <5 (40%) or scores of ≥5 (5%) for both sign and symptom scores.
Relation to clinical characteristics
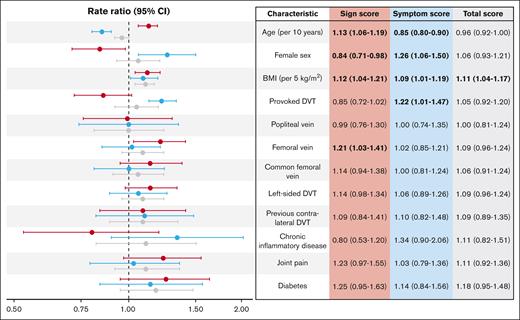
Characteristics associated with the sign score were older age, male sex, higher BMI, and femoral vein DVT, whereas associations with the symptom score were found for younger age, female sex, higher BMI, and provoked DVT (Figure 4). Consequently, BMI was the only characteristic associated with the total Villalta score. Similar associations were observed at the 1- and 2-year follow-up visits (supplemental Table 2). Changes in the sign score after 2-year follow-up were associated with older age (+0.15 point per 10 years; P < .001), provoked DVT (–0.26 point; P = .025), femoral vein DVT (+0.35 point; P = .001), and diabetes (+0.90 point; P < .001). None of the characteristics were associated with changes in the symptom score after the 2-year follow-up (supplemental Table 3).
Scores at 6 months in relation to clinical characteristics. Forest plot of rate ratios with 95% CI for clinical characteristics in relation to the sign (red), symptom (blue), and total (gray) scores, adjusted for acute phase compression. Vein segments were assessed separately. Rate ratios with P values < .05 are printed in bold to highlight statistically significant findings.
Scores at 6 months in relation to clinical characteristics. Forest plot of rate ratios with 95% CI for clinical characteristics in relation to the sign (red), symptom (blue), and total (gray) scores, adjusted for acute phase compression. Vein segments were assessed separately. Rate ratios with P values < .05 are printed in bold to highlight statistically significant findings.
Role of RVO
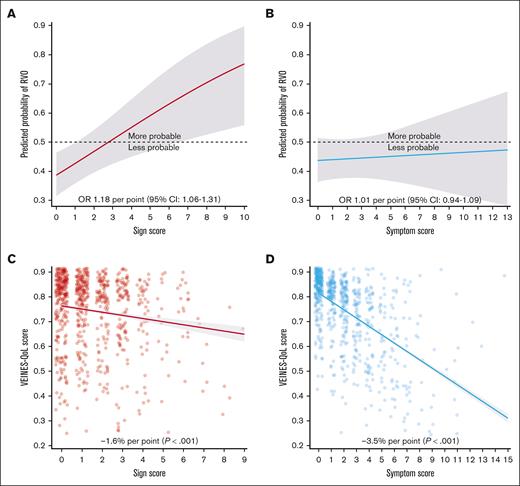
A total of 592 patients (72.4%) received compressive ultrasound at a mean of 5.3 (standard deviation, 1.9) months after DVT diagnosis. Their sign score was significantly associated with the presence of RVO (Figure 5A). A sensitivity analysis showed consistent effect estimates for RVO across all vein segments (supplemental Table 4). The optimal cutoff score was ≥3, showing higher sign scores were associated with increased odds of RVO (odds ratio, 2.01; 95% CI, 1.32-3.10) adjusted for sex, DVT extent, and acute phase compression. In contrast, there was no association between presence of RVO and the symptom score (Figure 5B). Similarly, RVO was associated with the sign score at the 1- and 2-year follow-up visits but not with changes in the scores over time (supplemental Table 5).
Scores at 6 months in relation to QoL and RVO. Partial effects plot of the sign score (A) and symptom score (B) in relation to RVO adjusted for sex, DVT extent, and acute phase compression. Scatterplot with regression line of the sign score (C) and symptom score (D) in relation to VEINES-QoL adjusted for age, sex, chronic inflammatory disease, joint pain, diabetes, and acute phase compression. Horizontal jitter (width = 0.3) was used for scatter plots to reveal overlapping dots. OR, odds ratio.
Scores at 6 months in relation to QoL and RVO. Partial effects plot of the sign score (A) and symptom score (B) in relation to RVO adjusted for sex, DVT extent, and acute phase compression. Scatterplot with regression line of the sign score (C) and symptom score (D) in relation to VEINES-QoL adjusted for age, sex, chronic inflammatory disease, joint pain, diabetes, and acute phase compression. Horizontal jitter (width = 0.3) was used for scatter plots to reveal overlapping dots. OR, odds ratio.
Impact on QoL
SF-36 and VEINES-QoL scores at 6 months were available in 85.7% (n = 701) and 91.6% (n = 750) of patients, respectively. After adjusting for age, sex, chronic inflammatory disease, joint pain, diabetes, and acute phase compression, associations with the SF-36 were stronger for the symptom score (−1.9 per point; P < .001) than the sign score (−0.7 per point; P = .008). The VEINES-QoL also showed a more pronounced association with the symptom score (Figure 5C) than with the sign score (Figure 5D). At 1- and 2-year follow-up, symptom scores were similarly associated with QoL, whereas sign scores no longer associated with SF-36; score increases after 2 years associated with reductions in QoL (supplemental Table 5).
Revisiting profiles
When looking at the sign and symptom score, differences emerged between profiles (Figure 2). Older men (profile 4) had the highest sign score and lowest symptom score, whereas, inversely, younger patients (profile 1) had the lowest sign score and highest symptom score; these differences were statistically significant. Women with joint pain (profile 2) and men with isolated popliteal DVT (profile 3) had comparatively lower sign scores and intermediate symptom scores. Changes after 2 years revealed that sign scores remained overall similar, except in younger patients (profile 1) who had a notably decreased score. Similarly, the symptom score decreased significantly in younger patients (profile 1), whereas older men (profile 4) experienced an increase of the symptom score over time.
Discussion
Our aim in this study was to reveal the heterogeneity within PTS using unsupervised machine learning. This led us to uncover 4 distinct profiles of patients with DVT. Although these profiles did not differ notably in the proportion of PTS, our findings suggest that the total Villalta score obscured their differences. By untangling the Villalta score into the sign and symptom scores, we achieved a more meaningful differentiation of patients according to the manifestations of PTS. Next, we demonstrated the clinical and pathogenic relevance of these separate scores by showing how they relate differently to patient characteristics, the presence of RVO, and QoL metrics. Finally, through a longitudinal investigation with multiple time points, we showed that the sign and symptom scores might follow unique trajectories across patient profiles. We believe that these observations warrant a reappraisal of how PTS is defined in clinical studies and routine care.
Of the 4 identified profiles, young patients with provoked DVT (profile 1) experienced the highest symptom burden, with the affected leg showing the least signs. Over time, however, their symptoms were found to decrease substantially, possibly explained by physical recovery or psychological adaptation. In contrast, older men with diabetes and femoral vein involvement (profile 4) presented with the most signs, whereas reporting least symptoms initially but with a notable increase over time. The other profiles, women with joint pain (profile 2) and men with isolated popliteal DVT (profile 3), had comparatively few signs and intermediate symptoms, which seemed to be more stable over time. These findings suggest possible overdiagnosis of PTS in the young patients, because they experience spontaneous improvement over time, whereas PTS is defined as a permanent diagnosis. Conversely, there might be underdiagnosis of PTS in the older men, as sign scores tended to obtain lower values than symptom scores. This difference arises because of less overlap among items of the sign score, as indicated by lower within-score correlations. Consequently, most PTS is diagnosed based primarily on symptoms rather than signs. These observations of overdiagnosis and underdiagnosis are particularly relevant in routine care settings, in which the follow-up period for the onset of PTS is often <2 years.
Thus far, 4 studies have been published in which patient profiles were identified using clustering in patients with DVT or, more generally, venous thromboembolism.28-31 Although profiles vary between studies because of their different study samples and methodologies, they consistently observe young patients with provoked DVT (ie, profile 1) and older patients with cardiovascular comorbidities (ie, profile 4), as well as middle-aged profile(s) with few comorbidities. Of these studies, only 1 included PTS as an outcome,28 which similarly found no substantial differences in the proportion of PTS between profiles, except for a high risk of PTS in patients with previous venous thromboembolism; these patients were mostly absent in our study because of the exclusion of patients with previous ipsilateral DVT.
Besides previous ipsilateral DVT, the identified risk factors of PTS vary considerably between clinical studies32 and even in validated prediction models.9,14,15 Our findings provide an explanation for the variability in frequently reported risk factors, as we reveal distinct associations with the sign and symptom score, which were opposite for age and sex, canceling each other out for the total score, whereas increasing BMI was the only risk factor associated with the total score. Moreover, provoked DVT was associated with the symptom score, whereas the extent of DVT was associated with the sign score. Although low correlation between the sign and symptom score has previously been reported,33 our findings highlight the need for future prediction models to treat these 2 scores as distinct outcomes to ensure stable risk factors, because these may otherwise simply be dependent on the composition of the study sample. Moreover, recognizing these separate scores and identifying clinically relevant threshold values might help reduce the within-patient variability currently observed over time for a Villalta score of ≥5, although assessments at multiple time points may still be necessary.11
This distinction between the signs and symptoms of PTS appears to be shaped by distinct underlying pathogenic mechanisms, as evidenced by the sign score being strongly associated with the presence of RVO, whereas the symptom score was not. This prominent role of RVO may clarify why the presence of thrombus in the femoral vein had a larger impact on the sign score than the presence of thrombus in the common femoral vein, because the femoral vein, given its length, may be more susceptible to RVO. However, absence of data on the iliac vein segment complicates our comparisons with iliofemoral DVT as a risk factor in prediction models.9,14 Our analysis of RVO was based on the most commonly used definition; however, the observed associations might differ if alternative definitions were applied, such as varying cutoff values for compressed vein diameter or incorporating flow assessment.23 Interestingly, an increase in the sign score over time was not associated with RVO but was strongly related to diabetes. Diabetes is known to affect skin health through oxidative stress and inflammation,34,35 and has been identified as a predictor of chronic venous disease.36
It is uncertain what mechanisms might underly the symptom score, but there may be a role for popliteal reflux or venous filling index, which are both associated with PTS.17,33 Studies in the adolescent population have made interesting observations that could further inform our understanding of pathophysiology in younger adults. Skin changes appear to be less common in this population, whereas symptoms are attributed a larger role.37 Pain intensity, in particular, has a major impact on adolescents, and was weighted heavily in the novel scoring algorithm CAPTSure.38 In a proof-of-concept study, worse CAPTSure scores associated with elevated compensatory calf pump activity at rest and impaired microvascular blood flow of the leg muscles during exercise.39 Therefore, these parameters are of interest for study in relation to symptoms experienced by the younger adult population. It should also be acknowledged that symptom scoring may be influenced by various comorbidities, only a limited number of which were recorded in the current study (ie, joint pain and diabetes).
The importance of uncovering the mechanisms underlying the symptoms of PTS is highlighted by its large effect on QoL compared with the sign score. A prior qualitative study observed that patients consider their skin changes as less important, whereas symptoms such as heaviness and paresthesia caused them a lot of discomfort.40 Nevertheless, completed and ongoing trials for the prevention and treatment of PTS, such as thrombolysis and venous stenting, have continued to put an emphasis on RVO, based on the open vein hypothesis.41 Our findings suggest that focusing on factors beyond patency, such as preventing venous reflux, might be more effective in reducing the onset of symptoms after DVT, possibly by physical exercise,42 ECT,19,43 or catheter-directed thrombolysis44 in selected patients. An exception to this may be venous claudication, the presence of which was unfortunately not recorded in this study. Further research is needed to understand how patients differently experience the symptoms of PTS, and how this interacts with their QoL. Finally, it should be noted that, although the sign score did not greatly affect QoL during our 2-year follow-up, over the years, severe skin changes can result in venous ulceration, which, although rare, has a profound impact on QoL.45 This implies that high sign scores may have a more significant influence on long-term QoL.
Our study has 3 important limitations that need to be discussed. First, our findings have not yet been externally validated, although we do provide internal validation by looking at the reproducibility of findings over time and clinical validation by the associations with outcomes. Second, our study sample excluded patients with previous ipsilateral DVT, preexistent venous insufficiency, severe peripheral artery disease, or active thrombolysis, and few patients were treated with direct oral anticoagulants, so it is unclear to what extent our findings can be extrapolated to these populations. Third, some clinical characteristics previously associated with PTS (eg, baseline Villalta score, varicose veins, and smoking) were unavailable, although we do provide other clinical characteristics of interest, namely joint pain and chronic inflammatory disease.46
Despite standardization and research efforts, the field of PTS has made limited progress in the last 15 years. We believe our observations provide a novel perspective on PTS that could move the field forward. Our proposal would be to consider the sign and symptom score of the Villalta scale as 2 distinct dimensions of PTS. Because these scores seem to have their own risk factors and pathogenic mechanisms, their distinction in clinical research should lead to better prediction models and interventions. By thinking in terms of clinical profiles, we might provide patients with a more tailored treatment, directed specifically at reducing signs or symptoms. We hope our findings will instigate an academic debate to reappraise the dimensions of PTS.
Acknowledgments
A.F.J.I. was generously supported through a Harry Struijker-Boudier Award For Talented Academics Talented PhD Candidates grant from the Cardiovascular Research Institute Maastricht. The IDEAL DVT trial was funded by ZonMw (The Netherlands; grant number 171102007).
ZonMw is a government-funded organization that had no role in study design, data collection and analysis, or writing of the report.
Authorship
Contribution: A.J.t.C.-H. conceived and conducted the IDEAL DVT trial; A.F.J.I. and A.J.t.C.-H. conceptualized and drafted the post hoc analysis; A.F.J.I. performed the statistical analysis under the supervision of V.t.C. and P.S.W.; A.F.J.I. wrote the manuscript under the supervision of A.J.t.C.-H.; A.J.t.C.-H. gave final approval of the version to be published; and all other authors contributed equally to writing and reviewing of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Aaron F. J. Iding, Thrombosis Expertise Center, Heart and Vascular Center, Maastricht University Medical Center, P. Debyelaan 25, 6229 HX Maastricht, The Netherlands; email: a.iding@maastrichtuniversity.nl.
References
Author notes
Study group author.
Original data are available on request from the corresponding author, Aaron F. J. Iding (a.iding@maastrichtuniversity.nl).
The full-text version of this article contains a data supplement.