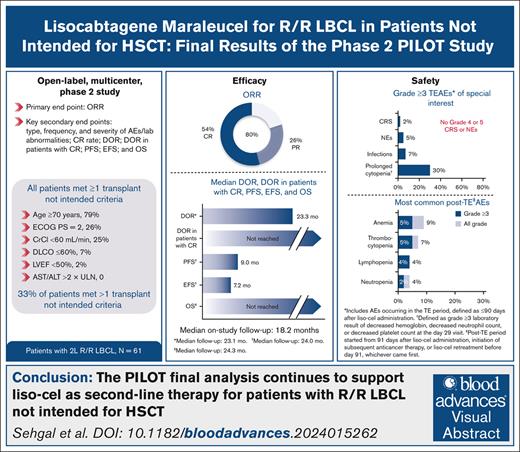
Key Points
In this final analysis of PILOT with median follow-up of 18.2 months, responses after liso-cel were durable and median OS was NR.
No new safety concerns were identified after longer follow-up, supporting second-line use of liso-cel for patients not intended for HSCT.
Visual Abstract
We report final analysis results from the PILOT study of lisocabtagene maraleucel (liso-cel) for patients with relapsed/refractory (R/R) large B-cell lymphoma (LBCL). Sixty-one adults with R/R LBCL who had received 1 previous line of therapy and met ≥1 hematopoietic stem cell transplantation (HSCT) not intended criterion. Overall response rate (primary end point) was 80%; 54% achieved complete response. After median on-study follow-up of 18.2 months, median duration of response was 23.3 months (95% confidence interval [CI], 6.2 to not reached [NR]). Median progression-free survival (PFS) was 9.0 months (95% CI, 4.2 to NR), median overall survival (OS) was NR (95% CI, 16.3 to NR), and 18-month PFS and OS rates were 43% (95% CI, 30-55) and 59% (95% CI, 45-70), respectively. In the treatment-emergent (TE) period (≤90 days after liso-cel administration), 79% had grade ≥3 adverse events (AEs), 38% had cytokine release syndrome (2% grade 3; no grade 4/5), 31% had neurological events (5% grade 3; no grade 4/5), and 7% had grade ≥3 infections. Of 57 patients in the post-TE period (≥91 days after liso-cel administration), 18% experienced grade ≥3 AEs; 1 patient had grade ≥3 infections. Thirty patients in the leukapheresis set (n = 74) died, mostly of disease progression (n = 24). In this population with high incidence of high-grade B-cell lymphoma, primary-refractory disease, advanced age, and comorbidities, liso-cel demonstrated durable efficacy and a favorable safety profile, consistent with previous reports. These results support liso-cel as second-line therapy for this underserved population of patients with R/R LBCL not intended for HSCT. This trial was registered at www.clinicaltrials.gov as #NCT03483103.
Introduction
For patients with relapsed or refractory (R/R) large B-cell lymphoma (LBCL) not intended to receive high-dose chemotherapy (HDCT)/hematopoietic stem cell transplantation (HSCT), therapeutic options have historically been limited and outcomes have been particularly poor.1,2 For patients with LBCL for whom transplant is an appropriate treatment, studies have shown a survival benefit with chimeric antigen receptor (CAR) T-cell therapy over HDCT and HSCT in the second-line setting, but few studies have specifically investigated CAR T-cell therapy for patients for whom transplant is not appropriate.3-7
The safety and efficacy of lisocabtagene maraleucel (liso-cel), an autologous, CD19-directed, 4-1BB CAR T-cell product, is well established across multiple disease states, including R/R LBCL, chronic lymphocytic leukemia, mantle cell lymphoma, and follicular lymphoma (FL).3,4,6,8-12 The open-label, phase 2 PILOT study (ClinicalTrials.gov identifier: NCT03483103) evaluated efficacy and safety of liso-cel in patients with R/R LBCL not intended for HSCT after 1 previous line of therapy.6 In the primary analysis of PILOT (data cutoff: 24 September 2021), the median on-study follow-up was 12.3 months.6 The primary end point was met, with an overall response rate (ORR) of 80% (95% confidence interval [CI], 68-89) and a complete response (CR) rate of 54% (95% CI, 41-67); the overall median duration of response (DOR) was 12.1 months (95% CI, 6.2 to not reached [NR]), and 21.7 months (95% CI, 12.1 to NR) in patients with a CR.6 Importantly, responses were observed in all prespecified subgroups, including those with high-risk features. The safety profile was consistent with previous reports.3,4,6,8-11,13 Here, we summarize the final analysis from the PILOT study after all patients completed or discontinued the study.
Methods
Study design and patients
The full methodology of the PILOT study was published in the primary analysis article.6 Briefly, adult patients were eligible if they had R/R (defined as less than a CR to first-line therapy) diffuse LBCL (DLBCL) de novo or transformed from FL; high-grade B-cell lymphoma with MYC and BCL2 and/or BCL6 rearrangements with DLBCL histology (double-hit or triple-hit lymphoma), or FL grade 3B; and positron emission tomography (PET)–positive disease. Patients must have received 1 previous line of therapy containing an anthracycline and a CD20-targeted agent. Patients must not have been intended for HSCT by the investigator and were required to meet ≥1 transplant-not-intended (TNI) criteria, which were based on institutional definitions across study sites in the United States and Europe (supplemental Methods).6
Procedures
Leukapheresis was performed on all eligible patients to obtain peripheral blood mononuclear cells to produce liso-cel. Optional bridging therapy for disease control was allowed before leukapheresis and/or while liso-cel was being manufactured, consisting of a salvage low-dose chemotherapy or noncurative standard-of-care chemotherapy regimen, but reconfirmation of study eligibility, including PET-positive disease, was required before lymphodepleting chemotherapy (LDC) could be administered. After availability of liso-cel and confirmation of infusion eligibility, LDC comprising IV fludarabine (30 mg/m2) and IV cyclophosphamide (300 mg/m2) daily for 3 days was administered. Liso-cel was infused 2 to 7 days after LDC for a total target dose of 100 × 106 CAR+ T cells. Liso-cel could be administered in either an inpatient or outpatient setting at the investigator’s discretion. After liso-cel infusion, patients entered posttreatment follow-up, and were monitored for 2 years for safety, cellular kinetics and biomarkers, disease status, health-related quality of life (HRQOL), and survival. After study completion, patients could enroll in a separate long-term follow-up study (ClinicalTrials.gov identifier: NCT03435796) assessing survival, adverse events (AEs), and viral vector safety for up to 15 years after liso-cel treatment.
Assessments and end points
The primary end point was ORR, defined as the proportion of patients who had a best overall response of either CR or partial response. Secondary efficacy end points included CR rate, DOR (time from the initial response to the earliest of disease progression or death), DOR for patients whose best overall response was CR, progression-free survival (PFS; time from liso-cel infusion to the earliest of disease progression or death), event-free survival (EFS; time from liso-cel infusion to the earliest occurrence of death, progressive disease, or initiation of a new anticancer therapy), and overall survival (OS; time from liso-cel infusion to death). All efficacy end points except OS were assessed by an independent review committee (IRC) based on Lugano 2014 criteria for up to 2 years.14 Subgroup analyses were performed for the following variables: previous response to first-line therapy (refractory/CR <3 months vs CR ≥3 and ≤12 months vs CR >12 months), age-adjusted International Prognostic Index (aaIPI; ≥2 vs ≤1), Eastern Cooperative Oncology Group performance status (ECOG PS; 0/1 vs 2 at screening), age (<75 vs ≥75 years at screening), and bridging therapy (yes vs no).
Safety end points were frequency and severity of AEs, along with laboratory abnormalities. All AEs were recorded from the start of LDC to 90 days after liso-cel infusion or the end-of-study visit, whichever was earlier. Treatment-emergent AEs (TEAE) were defined as those that started during the TE period, which was defined as the time from liso-cel infusion up to and including 90 days after liso-cel administration. Any AEs that occurred after the initiation of another anticancer treatment or liso-cel retreatment were not considered TEAEs. AEs related to liso-cel and/or protocol-mandated procedures were also recorded during the post-TE period, which started at 91 days after liso-cel administration or initiation of subsequent anticancer therapy, whichever came first and continued through the end of study visit. Lee 2014 criteria15 were used to grade cytokine release syndrome (CRS), and the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4,0316 were used to grade all other AEs, including CRS symptoms and neurological events (NEs), which were defined as investigator-identified neurological AEs related to liso-cel. The criteria for serious AEs are provided in the supplemental Methods.
As a secondary end point, liso-cel persistence was assessed by quantitative polymerase chain reaction,17,18 and was defined as a transgene count greater than or equal to the lower limit of detection. Data obtained after retreatment with liso-cel or start of subsequent anticancer therapy were excluded from the determination of persistence. B-cell aplasia as measured by flow cytometry was an exploratory end point and was defined as <3% CD19+ B cells in peripheral blood lymphocytes; B-cell measurements taken after retreatment with liso-cel or subsequent anticancer treatment were excluded.
Statistical analysis
This final analysis was conducted after all patients completed or discontinued the study for any reason. No formal hypothesis testing was performed. Analysis sets are defined in the supplemental Methods. The ORR and CR rate were calculated with corresponding exact Clopper-Pearson CIs; the Kaplan-Meier method was used to estimate medians and 95% CIs for DOR, PFS, EFS, and OS; the Kaplan-Meier method was also used to estimate DOR, PFS, EFS, and OS at 12 months and 18 months. Median on-study follow-up, OS analysis, and safety data included all available long-term follow-up data (ie, data from patients who completed the PILOT study and enrolled in the separate long-term follow-up study). Statistical analyses were performed with SAS version 9.4 (SAS Institute, Cary, NC).
Results
Patient population
Between 26 July 2018 and the data cutoff date of 1 December 2022, 74 patients underwent leukapheresis, 61 of whom received liso-cel (supplemental Figure 1). Of note, 13 patients in the leukapheresis analysis set did not receive liso-cel for the following reasons: death (n = 5); no longer meeting eligibility criteria (n = 4); rapid clinical progression and admittance to hospice (n = 1); PET-negative disease after bridging therapy (n = 1); investigator decision (n = 1); and receipt of nonconforming product, defined as any product wherein one of the CD8 or CD4 cell components did not meet one of the requirements to be considered liso-cel, but could be considered appropriate for infusion (n = 1). Of 61 patients who received liso-cel, all met the criteria for inclusion in both the liso-cel–treated analysis set and the liso-cel–treated efficacy analysis set. A summary of previous treatments is provided in supplemental Table 1. Two patients received retreatment with liso-cel after achieving a CR and subsequently relapsing. Complete details about the population can be found in Sehgal et al and Table 1.6 Thirty-two patients (52%) received bridging therapy.6 As described in the primary article, all patients were required to meet ≥1 TNI criteria, and 20 patients (33%) met >1 TNI criteria (supplemental Table 2).6 Subsequent therapies that patients received if they progressed after receiving liso-cel on study are provided in supplemental Table 3. Median combined on-study follow-up from the PILOT study and the long-term follow-up study was 18.2 months (range, 1.2-47.0).
Patient demographics and baseline characteristics
| Characteristic . | Liso-cel–treated analysis set (n = 61) . |
|---|---|
| Age, y | |
| Median (range) | 74 (53-84) |
| <70, n (%) | 13 (21) |
| 70-74, n (%) | 20 (33) |
| ≥75, n (%) | 28 (46) |
| Male sex, n (%) | 37 (61) |
| Race, n (%) | |
| White | 54 (89) |
| Other | 3 (5) |
| Missing | 4 (7) |
| Histology, n (%) | |
| DLBCL NOS | 33 (54) |
| Transformed FL | 9 (15) |
| HGBCL | 18 (30) |
| FL3B | 1 (2) |
| Double or triple hit, n (%) | |
| Yes | 20 (33) |
| No | 37 (61) |
| Missing | 4 (7) |
| R/R, n (%) | |
| Relapsed | 28 (46) |
| Relapsed ≤12 months | 13 (21) |
| Relapsed >12 months | 15 (25) |
| Refractory∗ | 33 (54) |
| Bone marrow involvement, n (%) | 7 (11) |
| LVEF of <50%, n (%) | 1 (2) |
| CrCl of <60 mL/min, n (%) | 15 (25) |
| AST/ALT of >2 × ULN, n (%) | 0 |
| Screening ECOG PS, n (%) | |
| 0/1/2 | 19 (31)/26 (43)/16 (26) |
| Screening aaIPI, n (%) | |
| 0-1/2-3 | 34 (56)/26 (43) |
| Missing | 1 (2) |
| Pre-LDC LDH of ≥500 U/L, n (%) | 11 (18) |
| Pre-LDC SPD per IRC of ≥50 cm2, n (%) | 10 (16) |
| Median baseline CRP (range), mg/L | 14 (0.5-617) |
| Screening HCT-CI score, n (%) | |
| 0-2/≥3 | 34 (56)/27 (44) |
| Best previous response to any previous therapies after diagnosis, n (%) | |
| CR | 28 (46) |
| PR | 15 (25) |
| SD | 5 (8) |
| PD | 13 (21) |
| Received bridging therapy,† n (%) | 32 (52) |
| Months from diagnosis to first liso-cel infusion, median (range) | 14 (2-183) |
| Characteristic . | Liso-cel–treated analysis set (n = 61) . |
|---|---|
| Age, y | |
| Median (range) | 74 (53-84) |
| <70, n (%) | 13 (21) |
| 70-74, n (%) | 20 (33) |
| ≥75, n (%) | 28 (46) |
| Male sex, n (%) | 37 (61) |
| Race, n (%) | |
| White | 54 (89) |
| Other | 3 (5) |
| Missing | 4 (7) |
| Histology, n (%) | |
| DLBCL NOS | 33 (54) |
| Transformed FL | 9 (15) |
| HGBCL | 18 (30) |
| FL3B | 1 (2) |
| Double or triple hit, n (%) | |
| Yes | 20 (33) |
| No | 37 (61) |
| Missing | 4 (7) |
| R/R, n (%) | |
| Relapsed | 28 (46) |
| Relapsed ≤12 months | 13 (21) |
| Relapsed >12 months | 15 (25) |
| Refractory∗ | 33 (54) |
| Bone marrow involvement, n (%) | 7 (11) |
| LVEF of <50%, n (%) | 1 (2) |
| CrCl of <60 mL/min, n (%) | 15 (25) |
| AST/ALT of >2 × ULN, n (%) | 0 |
| Screening ECOG PS, n (%) | |
| 0/1/2 | 19 (31)/26 (43)/16 (26) |
| Screening aaIPI, n (%) | |
| 0-1/2-3 | 34 (56)/26 (43) |
| Missing | 1 (2) |
| Pre-LDC LDH of ≥500 U/L, n (%) | 11 (18) |
| Pre-LDC SPD per IRC of ≥50 cm2, n (%) | 10 (16) |
| Median baseline CRP (range), mg/L | 14 (0.5-617) |
| Screening HCT-CI score, n (%) | |
| 0-2/≥3 | 34 (56)/27 (44) |
| Best previous response to any previous therapies after diagnosis, n (%) | |
| CR | 28 (46) |
| PR | 15 (25) |
| SD | 5 (8) |
| PD | 13 (21) |
| Received bridging therapy,† n (%) | 32 (52) |
| Months from diagnosis to first liso-cel infusion, median (range) | 14 (2-183) |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; CrCl, creatinine clearance; CRP, C-reactive protein; FL3B, FL grade 3B; HCT-CI, hematopoietic cell transplant–specific comorbidity index; HGBCL, high-grade B-cell lymphoma; LDH, lactate dehydrogenase; LVEF, left ventricular ejection fraction; NOS, not otherwise specified; PD, progressive disease; PR, partial response; SD, stable disease; SPD, sum of the product of perpendicular diameters; ULN, upper limit of normal.
Defined as less than a CR to first-line therapy.
Defined as any systemic treatment or radiotherapy provided to patients for disease control after consent and before LDC (1 patient received radiotherapy only).
Efficacy
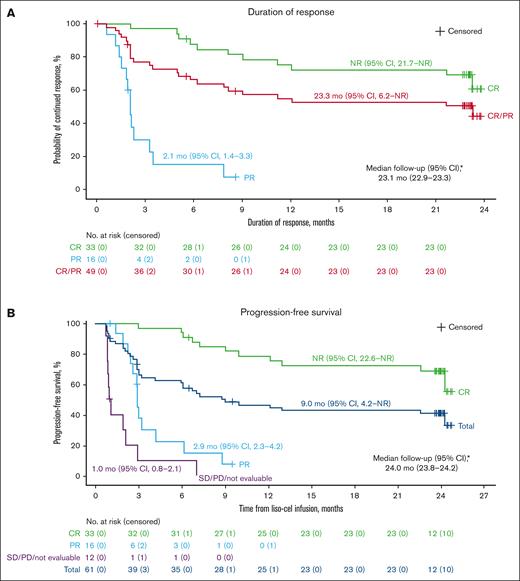
In the liso-cel–treated efficacy analysis set, the ORR was 80% (49/61 patients; 95% CI, 68-89), with 33 of 61 patients (54%; 95% CI, 41-67) achieving a CR (Table 2). Responses were durable, with median DOR of 23.3 months (95% CI, 6.2 to NR) and 18-month DOR rate of 53% (95% CI, 37-66; Figure 1A; Table 2). Median DOR for patients with a best response of CR was NR (95% CI, 21.7 to NR) and 18-month DOR rate was 72% (95% CI, 53-84; Figure 1A).
Summary of efficacy (liso-cel–treated efficacy analysis set)
| . | Liso-cel–treated efficacy analysis set (n = 61) . |
|---|---|
| ORR (CR + PR), n (%) [95% CI]∗ | 49 (80) [68-89] |
| CR rate, n (%) [95% CI]∗ | 33 (54) [41-67] |
| BOR, n (%) | |
| CR | 33 (54) |
| PR | 16 (26) |
| SD | 3 (5) |
| PD | 8 (13) |
| NE | 1 (2) |
| DOR | |
| Median (95% CI), mo† | 23.3 (6.2 to NR) |
| Continued response at 12 months, % (95% CI)† | 55 (40-68) |
| Continued response at 18 months, % (95% CI)† | 53 (37-66) |
| Median follow-up (95% CI), mo‡ | 23.1 (22.9-23.3) |
| PFS | |
| Median (95% CI), mo† | 9.0 (4.2 to NR) |
| 12-month PFS rate, % (95% CI)† | 47 (33-59) |
| 18-month PFS rate, % (95% CI)† | 43 (30-55) |
| Median follow-up (95% CI), mo‡ | 24.0 (23.8-24.2) |
| EFS | |
| Median (95% CI), mo† | 7.2 (3.2-24.3) |
| 12-month EFS rate, % (95% CI)† | 44 (31-56) |
| 18-month EFS rate, % (95% CI)† | 40 (28-52) |
| Median follow-up (95% CI), mo‡ | 24.0 (23.8-24.2) |
| OS | |
| Median (95% CI), mo† | NR (16.3 to NR) |
| 12-month OS rate, % (95% CI)† | 68 (55-78) |
| 18-month OS rate, % (95% CI)† | 59 (45-70) |
| Median follow-up (95% CI), mo‡ | 24.3 (24.0-24.8) |
| . | Liso-cel–treated efficacy analysis set (n = 61) . |
|---|---|
| ORR (CR + PR), n (%) [95% CI]∗ | 49 (80) [68-89] |
| CR rate, n (%) [95% CI]∗ | 33 (54) [41-67] |
| BOR, n (%) | |
| CR | 33 (54) |
| PR | 16 (26) |
| SD | 3 (5) |
| PD | 8 (13) |
| NE | 1 (2) |
| DOR | |
| Median (95% CI), mo† | 23.3 (6.2 to NR) |
| Continued response at 12 months, % (95% CI)† | 55 (40-68) |
| Continued response at 18 months, % (95% CI)† | 53 (37-66) |
| Median follow-up (95% CI), mo‡ | 23.1 (22.9-23.3) |
| PFS | |
| Median (95% CI), mo† | 9.0 (4.2 to NR) |
| 12-month PFS rate, % (95% CI)† | 47 (33-59) |
| 18-month PFS rate, % (95% CI)† | 43 (30-55) |
| Median follow-up (95% CI), mo‡ | 24.0 (23.8-24.2) |
| EFS | |
| Median (95% CI), mo† | 7.2 (3.2-24.3) |
| 12-month EFS rate, % (95% CI)† | 44 (31-56) |
| 18-month EFS rate, % (95% CI)† | 40 (28-52) |
| Median follow-up (95% CI), mo‡ | 24.0 (23.8-24.2) |
| OS | |
| Median (95% CI), mo† | NR (16.3 to NR) |
| 12-month OS rate, % (95% CI)† | 68 (55-78) |
| 18-month OS rate, % (95% CI)† | 59 (45-70) |
| Median follow-up (95% CI), mo‡ | 24.3 (24.0-24.8) |
BOR, best overall response; NE, not evaluable; PD, progressive disease.
Two-sided 95% exact Clopper-Pearson CIs.
Kaplan-Meier method was used to obtain 2-sided 95% CIs.
Reverse Kaplan-Meier method was used to obtain the median follow-up and 95% CIs.
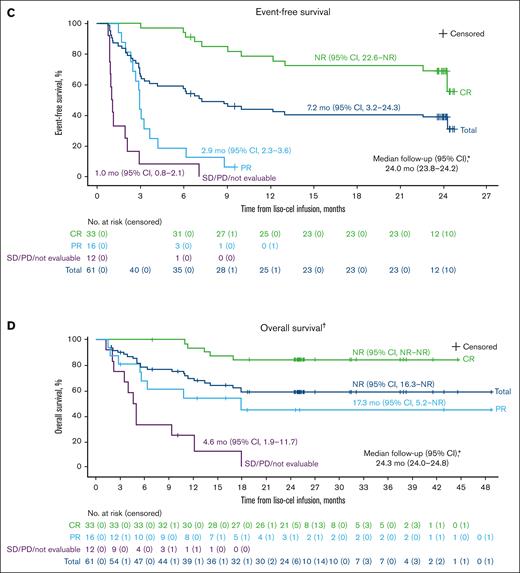
Kaplan-Meier curves for DOR, PFS, EFS, and OS in the liso-cel–treated efficacy analysis set. (A) Duration of response; (B) progression-free survival; (C) event-free survival; (D) overall survival. Data on Kaplan-Meier curves are expressed as median (95% CI). Crosses denote censored patients. ∗Reverse Kaplan-Meier method was used to obtain median follow-up and its 95% CIs. †OS data include data from the long-term follow-up study. PD, progressive disease; PR, partial response; SD, stable disease.
Kaplan-Meier curves for DOR, PFS, EFS, and OS in the liso-cel–treated efficacy analysis set. (A) Duration of response; (B) progression-free survival; (C) event-free survival; (D) overall survival. Data on Kaplan-Meier curves are expressed as median (95% CI). Crosses denote censored patients. ∗Reverse Kaplan-Meier method was used to obtain median follow-up and its 95% CIs. †OS data include data from the long-term follow-up study. PD, progressive disease; PR, partial response; SD, stable disease.
Median PFS was 9.0 months (95% CI, 4.2 to NR), with a median follow-up for PFS of 24.0 months (95% CI, 23.8-24.2; Figure 1B; Table 2). The 18-month PFS rate was 43% (95% CI, 30-55) in the overall population, and 72% (95% CI, 53-84) in those with a CR (Figure 1B; Table 2). Median EFS was 7.2 months (95% CI, 3.2-24.3), with a median follow-up for EFS of 24.0 months (95% CI, 23.8-24.2; Figure 1C; Table 2). The 18-month EFS rate was 40% (95% CI, 28-52) in the overall population and 72% (95% CI, 53-84) in those with a CR (Figure 1C; Table 2). The OS analysis included data from the PILOT study and the 17 patients who enrolled in the long-term follow-up study once they completed PILOT. Median OS was NR (95% CI, 16.3 to NR), with a median follow-up for OS of 24.3 months (95% CI, 24.0-24.8), and the 18-month OS rate was 59% (95% CI, 45-70; Figure 1D; Table 2).
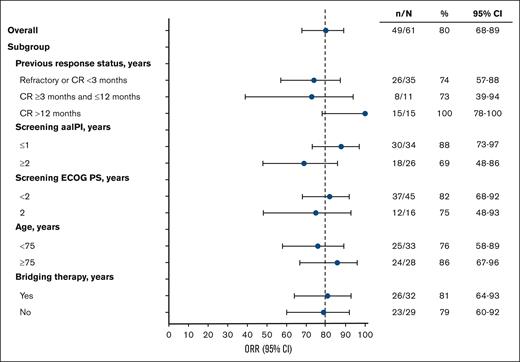
Efficacy end points were also assessed by predefined subgroups as follows: previous response status, screening aaIPI, screening ECOG PS, age, and bridging therapy in the liso-cel–treated efficacy analysis set. ORR was generally consistent across subgroups (Figure 2). DOR, PFS, and OS are reported for these subgroups in supplemental Table 4 and supplemental Figure 2 (DOR and PFS only). Based on response to most recent previous therapy, patients who achieved a best previous response of CR of >12 months demonstrated numerically improved survival compared with those who had a CR of <3 months and those who had a CR of ≥3 months and ≤12 months. Patients with aaIPI of ≤1 and patients who did not receive bridging therapy had numerically improved outcomes compared with patients with aaIPI of ≥2 and patients who received bridging therapy, respectively. Outcomes were similar for patients with ECOG PS of <2 vs 2 and those aged <75 years vs ≥75 years.
ORR by prespecified subgroup (liso-cel–treated efficacy analysis set). ORR and 2-sided 95% Clopper-Pearson CIs are displayed.
ORR by prespecified subgroup (liso-cel–treated efficacy analysis set). ORR and 2-sided 95% Clopper-Pearson CIs are displayed.
Efficacy results in the leukapheresis analysis set (intention-to-treat set) are presented in supplemental Table 5.
Safety
TEAEs were consistent with those reported for the primary analysis.6 During the TE period (n = 61), 97% of patients had TEAEs of any grade and 79% of patients had grade ≥3 TEAEs of which the most common (occurring in >20% of patients) were neutropenia in 48%, leukopenia in 21%, and thrombocytopenia in 21% (Table 3). Ten patients received platelet transfusion, 16 patients red blood cell transfusion, and 6 patients IV immunoglobulin administration. Serious TEAEs were experienced by 33% of patients, most of which occurred only in 1 patient each (supplemental Table 6). Two patients died because of grade 5 TEAEs: 1 because of COVID-19 and 1 because of COVID-19 pneumonia. Incidences of CRS and NEs were also consistent with the primary analysis results,6 with 2% of patients experiencing grade 3 CRS and 5% experiencing grade 3 NEs, with no grade 4 or 5 CRS or NEs reported (Table 4). Steroid and tocilizumab use were previously reported in the primary analysis article.6 Grade ≥3 infections were reported in 7% of patients. Prolonged cytopenia at day 29 was observed in 30% of patients, and 72% of patients with prolonged cytopenia recovered by end of study.
AEs in the TE and post-TE periods
| TE period∗ . | ||
|---|---|---|
| TEAEs overview, n (%) . | Liso-cel–treated analysis set (n = 61) . | |
| Any grade . | Grade ≥3 . | |
| Any TEAE | 59 (97) | 48 (79) |
| Any serious TEAE | 20 (33) | 13 (21) |
| Liso-cel related | 48 (79) | 27 (44) |
| Most common any-grade TEAEs in ≥20% | ||
| Any grade | Grade ≥3 | |
| Neutropenia | 31 (51) | 29 (48) |
| Fatigue | 24 (39) | 0 |
| CRS | 23 (38) | 1 (2) |
| Anemia | 19 (31) | 7 (11) |
| Thrombocytopenia | 18 (30) | 13 (21) |
| Nausea | 15 (25) | 1 (2) |
| Leukopenia | 14 (23) | 13 (21) |
| Post-TE period† | ||
| AEs†overview, n (%) | Liso-cel–treated analysis set (n = 57) | |
| Any grade | Grade ≥3 | |
| Any AE | 29 (51) | 10 (18) |
| Any serious AE | 5 (9) | 3 (5) |
| Liso-cel related | 6 (11) | 3 (5) |
| Most common AEs†in ≥3% of patients | ||
| Any grade | Grade ≥3 | |
| Anemia | 5 (9) | 3 (5) |
| Thrombocytopenia | 4 (7) | 3 (5) |
| Lymphopenia | 2 (4) | 2 (4) |
| Neutropenia | 2 (4) | 1 (2) |
| TE period∗ . | ||
|---|---|---|
| TEAEs overview, n (%) . | Liso-cel–treated analysis set (n = 61) . | |
| Any grade . | Grade ≥3 . | |
| Any TEAE | 59 (97) | 48 (79) |
| Any serious TEAE | 20 (33) | 13 (21) |
| Liso-cel related | 48 (79) | 27 (44) |
| Most common any-grade TEAEs in ≥20% | ||
| Any grade | Grade ≥3 | |
| Neutropenia | 31 (51) | 29 (48) |
| Fatigue | 24 (39) | 0 |
| CRS | 23 (38) | 1 (2) |
| Anemia | 19 (31) | 7 (11) |
| Thrombocytopenia | 18 (30) | 13 (21) |
| Nausea | 15 (25) | 1 (2) |
| Leukopenia | 14 (23) | 13 (21) |
| Post-TE period† | ||
| AEs†overview, n (%) | Liso-cel–treated analysis set (n = 57) | |
| Any grade | Grade ≥3 | |
| Any AE | 29 (51) | 10 (18) |
| Any serious AE | 5 (9) | 3 (5) |
| Liso-cel related | 6 (11) | 3 (5) |
| Most common AEs†in ≥3% of patients | ||
| Any grade | Grade ≥3 | |
| Anemia | 5 (9) | 3 (5) |
| Thrombocytopenia | 4 (7) | 3 (5) |
| Lymphopenia | 2 (4) | 2 (4) |
| Neutropenia | 2 (4) | 1 (2) |
AEs were graded per the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.03, except for CRS, which was graded per Lee 2014 criteria.
TE period was defined as ≤90 days after liso-cel administration.
Post-TE period started from 91 days after liso-cel administration, initiation of subsequent anticancer therapy, or liso-cel retreatment before day 91, whichever came first.
AEs of special interest in the TE and post-TE periods (liso-cel–treated analysis set)
| AE, n (%) . | TE period (n = 61) . | Post-TE period (n = 57) . |
|---|---|---|
| CRS∗ | ||
| Any grade | 23 (38) | 0 |
| Grade 1/2 | 22 (36) | 0 |
| Grade 3 | 1 (2) | 0 |
| Grade 4/5 | 0 | 0 |
| NEs† | ||
| Any grade | 19 (31) | 0 |
| Grade 1/2 | 16 (26) | 0 |
| Grade 3 | 3 (5) | 0 |
| Grade 4/5 | 0 | 0 |
| Prolonged cytopenia at day 29‡ | 18 (30) | N/A |
| Grade ≥3 infections | 4 (7) | 1 (2)§ |
| Second primary malignancy | 0 | 2 (4)|| |
| AE, n (%) . | TE period (n = 61) . | Post-TE period (n = 57) . |
|---|---|---|
| CRS∗ | ||
| Any grade | 23 (38) | 0 |
| Grade 1/2 | 22 (36) | 0 |
| Grade 3 | 1 (2) | 0 |
| Grade 4/5 | 0 | 0 |
| NEs† | ||
| Any grade | 19 (31) | 0 |
| Grade 1/2 | 16 (26) | 0 |
| Grade 3 | 3 (5) | 0 |
| Grade 4/5 | 0 | 0 |
| Prolonged cytopenia at day 29‡ | 18 (30) | N/A |
| Grade ≥3 infections | 4 (7) | 1 (2)§ |
| Second primary malignancy | 0 | 2 (4)|| |
N/A, not available.
CRS was graded per Lee 2014 criteria.
NEs were defined as investigator-identified neurological AEs related to liso-cel and were graded per the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.03.
Prolonged cytopenia was defined as grade ≥3 laboratory result of decreased hemoglobin, decreased neutrophil count, or decreased platelet count at the day 29 visit.
Bacteremia and sepsis.
Squamous cell carcinoma of skin and malignant external ear neoplasm (n = 1) and myelodysplastic syndrome (n = 1).
In the post-TE period (n = 57), no new safety signals were noted (Table 3; supplemental Table 7); 51% of patients had AEs of any grade and 18% of patients experienced grade ≥3 AEs, of which the most common were anemia and thrombocytopenia, each at 5%. Two patients received platelet transfusion, 5 patients red blood cell transfusion, and 1 patient IV immunoglobulin. Eight serious AEs occurred in 5 patients (supplemental Table 8). One patient died because of a grade 5 AE of sepsis (supplemental Table 9). As expected, no CRS events or NEs were reported in the post-TE period (Table 4). Overall, 2 patients had second primary malignancies, all occurring in the post-TE period and reported as serious AEs (1 squamous cell carcinoma of the skin [diagnosed on day 514, considered by the investigator to be unrelated to LDC and liso-cel] and malignant external ear neoplasm [diagnosed on day 681, considered by the investigator to be unrelated to LDC and liso-cel]; and 1 myelodysplastic syndrome [diagnosed on day 386, considered by the investigator to be related to LDC and unrelated to liso-cel]).
There were 30 (41%) deaths in the leukapheresis analysis set, of which most (n = 19) occurred in the post-TE period, with 4 additional deaths since the primary analysis6 (supplemental Table 9). Twenty-four deaths were due to disease progression, 2 were due to COVID-19, 1 was due to acute hypoxemic respiratory failure, 1 was due to grade 5 pneumonia, 1 was due to sepsis (as reported earlier), and 1 was due to an infected kidney stone. Both COVID-19 deaths occurred at the height of the pandemic, with one on day 43 and the other on day 63 after liso-cel infusion. In the case of sepsis, the patient had disease progression on day 29, received subsequent therapy, then developed grade 4 bacteremia, and died of sepsis on day 58.
Forty-one patients (67%) received liso-cel in the inpatient setting and 20 (33%) were monitored in the outpatient setting (supplemental Table 10). Of those monitored as outpatients, 9 patients were hospitalized after liso-cel infusion, of whom 7 patients were admitted because of AEs (3 because of grade 1 CRS, none due to NEs). Of 41 inpatients, 7 were admitted to the intensive care unit (ICU), including 4 for prophylaxis for CAR T-cell administration; and of 9 outpatients hospitalized, 1 was admitted to the ICU. The median length of hospital stay was 14 and 8 days for the 41 inpatients and 9 outpatients, respectively, and the median length of ICU stay was 7 days for the 7 inpatients and 3 days for the 1 outpatient.
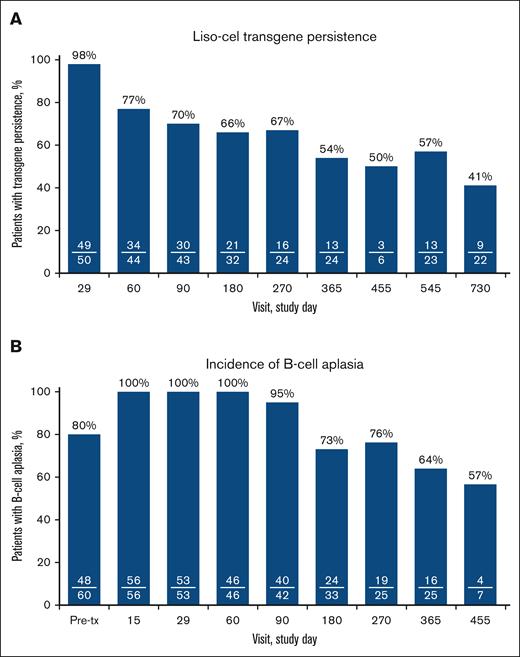
Cellular kinetic and pharmacodynamic data
At day 29, 49 of 50 patients (98%) demonstrated CAR T-cell persistence; at day 365, liso-cel transgene was detected in 13 of 24 patients (54%) with available samples; and, at day 730, liso-cel transgene was present in 9 of 22 patients (41%) with available samples (Figure 3A). B-cell aplasia increased initially after liso-cel infusion compared with pretreatment, then steadily decreased after 90 days. At day 15, 56 of 56 patients (100%) had <3% CD19+ B cells, at day 90 it was 40 of 42 patients (95%), which further decreased at day 365 to 16 of 25 patients (64%) and at day 455 to 4 of 7 patients (57%) (Figure 3B). Over the entire study, IV immunoglobulin was administered to 7 patients. An association between transgene persistence and the incidence of B-cell aplasia was observed beginning at 6 months after liso-cel infusion and was maintained through the last visit with B-cell aplasia data available (supplemental Figure 3).
Liso-cel transgene persistence∗ (A) (cellular kinetic set) and incidence of B-cell aplasia† (B) (liso-cel–treated efficacy analysis set). ∗Number of patients with persistence of liso-cel in the blood/number of patients with an available sample at the specific time point. Persistence is defined as a transgene count greater than, or equal to, the lower limit of detection. Data obtained after the initiation of retreatment with liso-cel or after start of a new anticancer therapy are not included in the determination of persistence. †Number of patients with B-cell aplasia/number of patients with an available sample at the specific time point. B-cell aplasia is defined as <3% CD19+ B cells in peripheral blood lymphocytes; B-cell measurements taken after the initiation of retreatment or after another anticancer treatment are excluded. tx, treatment.
Liso-cel transgene persistence∗ (A) (cellular kinetic set) and incidence of B-cell aplasia† (B) (liso-cel–treated efficacy analysis set). ∗Number of patients with persistence of liso-cel in the blood/number of patients with an available sample at the specific time point. Persistence is defined as a transgene count greater than, or equal to, the lower limit of detection. Data obtained after the initiation of retreatment with liso-cel or after start of a new anticancer therapy are not included in the determination of persistence. †Number of patients with B-cell aplasia/number of patients with an available sample at the specific time point. B-cell aplasia is defined as <3% CD19+ B cells in peripheral blood lymphocytes; B-cell measurements taken after the initiation of retreatment or after another anticancer treatment are excluded. tx, treatment.
Discussion
This final analysis of the PILOT study reflects one of the longest follow-up reports to date in a broad population of patients with R/R LBCL not intended for HSCT and treated with second-line CAR T-cell therapy. Efficacy results were consistent with the primary analysis, with an ORR of 80% and a CR rate of 54%, and no new safety signals were observed.6 In this population with historically low rates of survival and limited options for long-term disease control,1,2 liso-cel resulted in durable responses, with 18-month response rates of 53% in the overall population, and 72% in those with a best response of CR. Furthermore, median PFS, EFS, and OS were 9.0 months, 7.2 months, and NR, respectively, in the overall population, and all were NR in patients with CR. Efficacy outcomes were generally consistent across the predefined subgroups of clinical interest. The incidences of CRS and NEs were unchanged from the primary analysis, with only 2% and 5% of patients experiencing grade 3 CRS and NEs, respectively, with no grade 4 or 5 CRS or NEs. Additionally, although liso-cel persistence was observed in 41% of patients with samples available at day 730, incidences of grade ≥3 infections were low, and of the 30% of patients who experienced prolonged cytopenia, most recovered by end of study.
The PILOT study demonstrated long-term durability with no new safety signals in patients with LBCL who were not intended for transplant. These results complement those from the phase 3 TRANSFORM study, which demonstrated superiority of liso-cel over standard of care in patients with primary refractory or early relapsed (≤12 months) LBCL who were intended for HSCT.4 The AE profile observed in PILOT was consistent with TRANSFORM, which reported grade 3 CRS in 1% and grade 3 NEs in 4% of patients treated with liso-cel, with no grade 4 or 5 events. Together, the 2 studies demonstrate the benefit of liso-cel as a second-line treatment option for R/R LBCL regardless of intent for transplant.
Results were recently published from the open-label, phase 2 clinical trial (ALYCANTE) of the CD19-directed CAR T-cell therapy axicabtagene ciloleucel as second-line therapy for patients (n = 62) considered ineligible for HSCT.7 ALYCANTE enrolled patients with DLBCL, high-grade B-cell lymphoma, or FL grade 3B that was refractory, defined as a lack of CR to first-line therapy, or relapsed, defined as biopsy-proven disease relapse within 12 months of completion of first-line treatment. Patients were also required to meet ≥1 of the following 3 protocol-defined criteria for autologous stem cell transplantation (ASCT) ineligibility: age of ≥65 years, hematopoietic cell transplantation–specific comorbidity index score of ≥3, or previous ASCT (as first-line consolidation). ALYCANTE was conducted in France, and all patients were required to remain at the hospital for ≥10 days after infusion. After a median follow-up of 12 months, complete metabolic response at 3 months (primary end point) was reported for 71% of patients, and median PFS was 12 months. Grade 3/4 CRS was observed in 8% of patients, grade 3/4 immune effector cell–associated neurotoxicity syndrome in 15% of patients, and grade ≥3 prolonged cytopenia in 37% of patients. Although both the ALYCANTE and PILOT studies evaluated second-line CAR T-cell therapy for patients considered ineligible for HSCT, comparisons with PILOT should be made with caution. Compared with ALYCANTE, PILOT comprised a population of patients with older age (79% of patients aged ≥70 years); relapsed disease of >12 months (25%); and a high proportion with double- or triple-hit lymphoma (33%), hematopoietic cell transplantation–specific comorbidity index score of ≥3 (44%), and ECOG PS of 2 (26%). Furthermore, the primary efficacy analysis of ORR was assessed by an IRC in PILOT, compared with investigator-assessed complete metabolic response rate as the primary end point in ALYCANTE.
The treatment landscape for LBCL is changing rapidly. Bispecific antibodies are an emerging class of therapeutic agents that recruit and activate T cells at the tumor site, through either CD19 or CD20.19 Such agents, including blinatumomab, glofitamab, mosunetuzumab, odronextamab, and epcoritamab are being investigated in LBCL. However, these therapies have not yet demonstrated curative potential, and results are limited by short study follow-up periods.19 Additionally, the CD19-directed cytolytic monoclonal antibody tafasitamab, in combination with lenalidomide, is becoming a more commonly used treatment for patients with R/R DLBCL who are ineligible for ASCT.20 Although the phase 2 L-MIND study demonstrated ORR of 60% (CR rate, 43%) in this population, a real-world retrospective analysis in patients with R/R LBCL who received tafasitamab plus lenalidomide as standard-of-care therapy reported markedly lower efficacy outcomes (ORR, 31%; CR rate, 19%).20,21 This was most likely because of enrollment of patients with lower-risk disease and fewer comorbidities in the clinical study, which defined ineligibility for ASCT as age of >70 years, presence of organ dysfunction or other comorbidities that would preclude the use of HDCT or ASCT, failure to previous ASCT, no response to salvage therapy, or refusal or inability to receive ASCT.20,21
A distinctive feature of PILOT is that it allowed for, and demonstrated a successful approach to, outpatient monitoring of liso-cel, with fewer than half of patients receiving liso-cel as an outpatient admitted to the hospital. Because health care resource use is substantial in patients with R/R LBCL, with considerable need for hospital visits and high costs,22-24 outpatient liso-cel infusion and monitoring may have a positive economic impact.25 In addition, a recent analysis of patient-reported outcomes from the primary analysis of the PILOT study showed that liso-cel maintained or improved HRQOL with up to 545 days of follow-up.26 In patients enrolled in the PILOT study who completed the surveys (∼50%), baseline HRQOL, functional status, and symptom severity were slightly worse than the general population, which is expected in patients with R/R LBCL.26 After treatment with liso-cel and through day 545, significant and/or clinically meaningful improvement was reported for fatigue, pain, and appetite loss, as well as for different validated scales commonly used for measuring HRQOL in patients with lymphoma.26
One limitation of PILOT is that this study only enrolled patients in US sites, and ethnic/racial diversity of the study may not be fully representative of the prevalence of DLBCL by ethnic/racial breakdown. Another limitation is that half of patients who completed PILOT did not enroll in the long-term follow-up study, which may have affected the OS results. PILOT was designed to follow-up patients for 2 years after liso-cel treatment, after which patients could voluntarily enroll in a separate long-term follow-up study assessing safety and OS for up to 15 years. Additionally, IRC assessments were performed only in the initial 2-year PILOT study period, and DOR and PFS were censored at the completion of PILOT, which may have resulted in an underestimate of DOR and PFS per IRC, as evidenced by the high number of censoring events at 24 months on those Kaplan-Meier curves (Figure 1A-B).
Although the PILOT study followed a single-arm study design and did not include a comparator, a comparison between PILOT and a real-world external control cohort from a global noninterventional, retrospective, observational study showed that liso-cel resulted in greater efficacy compared with conventional second-line chemotherapy in patients with R/R LBCL who were not intended for HSCT.27 After using a trimmed stabilized inverse probability of treatment weighting method to balance baseline characteristics, liso-cel vs conventional chemotherapy resulted in higher ORR (79.6% vs 50.5%) and CR rate (53.1% vs 24.0%), and longer median DOR (12.1 vs 4.3 months), EFS (7.0 vs 2.8 months), PFS (7.0 vs 2.9 months), and OS (NR vs 12.6 months).27
Conclusions
Despite the high incidence of high-grade B-cell lymphoma, primary refractory disease, advanced age, and comorbidities in the PILOT population, responses were durable, with a median DOR of almost 2 years and an 18-month DOR rate of 53%. In addition, the longer follow-up did not result in any new cases of CRS or NEs, and the safety profile was consistent with previous liso-cel reports.3,4,6,8-12 Thus, the final analysis from the PILOT study continues to support liso-cel as second-line therapy for this underserved population of patients with R/R LBCL, including those with high-risk features, for whom HSCT is not intended.
Acknowledgments
Writing and editorial assistance were provided by Meredith Rogers of The Lockwood Group (Stamford, CT), funded by Bristol Myers Squibb. The authors thank Souha Fares and Muskan Mittal of Bristol Myers Squibb for assistance with performing some of the statistical analyses.
This study was funded by Juno Therapeutics, a Bristol-Myers Squibb Company and Bristol Myers Squibb.
Authorship
Contribution: A.S. contributed to the study conception or design and to data acquisition; D.H., P.A.R., N.G., M.H., G.C.H., J.E.G., P.M.R., N.D.W.-J., J.E., R.N., S.R.S., R.C., E.L., S.F., N.K.G., and L.I.G. contributed to data acquisition; V.A.C. and B.Y. contributed to data interpretation; Z.Y. contributed to data analysis; K.O. contributed to study conception or design, data analysis, and data interpretation; J.T. contributed to data analysis and data interpretation; and all authors contributed to the manuscript and approved it.
Conflict-of-interest disclosure: A.S. declares research funding from Kite/Gilead, Juno Therapeutics, a Bristol-Myers Squibb Company, Chimagen, and CytoAgents. D.H. declares speakers bureau fees from Bristol Myers Squibb. P.A.R. declares consulting fees from AbbVie, ADC Therapeutics, Bristol Myers Squibb, BeiGene, CVS Caremark, Roche/Genentech, Genmab, Intellia Therapeutics, Janssen, Kite/Gilead, Nektar Therapeutics, Novartis, Nurix Therapeutics, Pharmacyclics, and Sana Biotechnology; speakers bureau fees from Kite Pharma; and travel support from Nektar Therapeutics. N.G. declares consultancy fees from Seagen, TG Therapeutics, AstraZeneca, Pharmacyclics, Janssen, Bristol Myers Squibb, Gilead Sciences, Kite Pharma, BeiGene, Incyte, Lava Therapeutics, Roche/Genentech, Novartis, Loxo Oncology, AbbVie, Genmab, Adaptive Biotech, and ADC Therapeutics; research funding from TG Therapeutics, Roche/Genentech, Bristol Myers Squibb, Gilead Sciences, MorphoSys, AbbVie, and Pharmacyclics; speakers bureau fees from AstraZeneca, Janssen, Pharmacyclics, Kite Pharma, Bristol Myers Squibb, and Epizyme; and membership on a board of directors or advisory committee for Roche NHL Solutions Panel. M.H. declares research support from ADC Therapeutics, Astellas Pharma, Spectrum Pharmaceuticals, and Takeda Pharmaceutical; consultancy fees from AbbVie, ADC Therapeutics, Autolus, Bristol Myers Squibb, Caribou Biosciences, CRISPR Therapeutics, Genmab, Kite Pharma, and Omeros; and speakers bureau fees from ADC Therapeutics, AstraZeneca, BeiGene, CRISPR Therapeutics, DMC Inc, Genentech, Kite Pharma, and Myeloid Therapeutics. G.C.H. declares research funding from AstraZeneca and Incyte; speakers bureau participation for Missouri Oncology Society; travel support from Ono Pharmaceutical, Genmab, and Missouri Oncology Society; advisory board participation with AstraZeneca, Daiichi Sankyo, Genmab, Janssen, Ono Pharmaceutical, Rapa Therapeutics, and Sobi; and is an equity holder of AXIM Biotechnologies, AbbVie, Aimmune Therapeutics, Biogen, bluebird Bio, Cardinal Health, CareTrust REIT, Cellectis, Charlotte’s Web Holdings, Clovis Oncology, CVS Health, GW Pharmaceuticals, Insys Therapeutics, Merck, Micron Technology, Medical Properties Trust, Moderna, Mustang Bio, NeoGenomics Laboratories, OPKO Health, Viatris, and Zevra Therapeutics. P.M.R. declares a grant from Genentech; speakers bureau participation for Kite Pharma; and advisory board membership with Caribou Biosciences and Kite Pharma. N.D.W.-J. declares research funding from Astex Pharmaceuticals, Merck, and Genentech; and advisory board participation with BeiGene. J.E. declares speakers bureau participation for Bristol Myers Squibb, Genmab, and Kite Pharma. R.N. declares research funding from AbbVie, Bristol Myers Squibb, Genentech, Kite/Gilead, MorphoSys, and Pharmacyclics; and advisory board participation with AbbVie, ADC Therapeutics, AstraZeneca, BeiGene, Genentech, Kite/Gilead, Incyte, Janssen, Lava Therapeutics, Lilly, and Novartis. R.C. declares speakers bureau participation for AbbVie. S.F. declares speakers bureau participation for AbbVie, Bristol Myers Squibb, and Genmab. V.A.C., B.Y., Z.Y., K.O., and J.T. are current employees and equity holders of Bristol Myers Squibb. L.I.G. declares consultancy fees from Ono Pharmaceutical; advisory board participation for Bristol Myers Squibb and Kite Pharma; data and safety monitoring board participation for Janssen; is a cofounder of Zylem Biosciences; and reports patents for nanoparticles for cancer therapy (PCT/US2020/051549) and nanostructures for treating cancer and other conditions (PCT/US2013/027431). The remaining authors declare no competing financial interests.
J.E.G. is retired from the Earle A. Chiles Research Institute, Portland, OR.
Correspondence: Leo I. Gordon, Department of Medicine, Division of Hematology/Oncology, Northwestern University Feinberg School of Medicine, and the Robert H. Lurie Comprehensive Cancer Center of Northwestern University, 676 North Saint Clair St, Suite 850, Chicago, IL 60611; email: l-gordon@northwestern.edu.
References
Author notes
Presented, in part, at the 65th annual meeting and exposition of the American Society of Hematology, San Diego, CA, 9 to 12 December 2023; and the 2024 tandem meetings on Transplantation and Cellular Therapy of the American Society for Transplantation and Cellular Therapy and the Center for International Blood and Marrow Transplant Research, San Antonio, TX, 21 - 25 February 2024.
Bristol Myers Squibb policy on data sharing may be found at https://www.bms.com/researchers-and-partners/independent-research/data-sharing-request-process.html.
The full-text version of this article contains a data supplement.