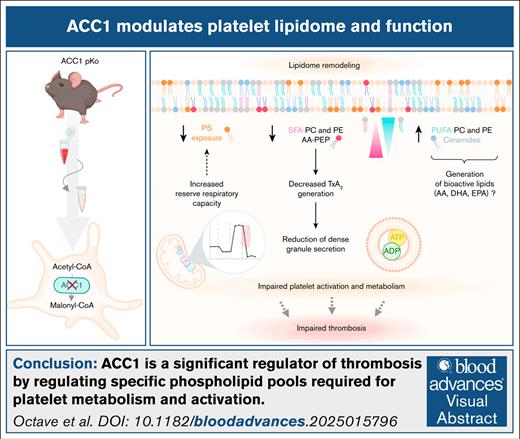
Key Points
Lipidomic analysis combined with a knockout mouse model identified ACC1 as a key regulator of the platelet lipidome.
Phospholipid pools regulated by ACC1 are essential for platelet metabolism and activation.
Visual Abstract
This study uncovers the pivotal role of acetyl-CoA carboxylase 1 (ACC1) in regulating platelet lipid composition, bioenergetics, activation, and thrombus formation, as demonstrated using a targeted glycoprotein Ibα (GPIbα)-Cre+/− mouse model. By comparing platelet-specific ACC1 knockout mice (GPIbα-Cre+/− × ACC1flx/flx) with both GPIbα-Cre+/− and ACC1flx/flx control groups, we showed that ACC1 deficiency profoundly reshaped the platelet phospholipidome. Specifically, ACC1 deletion led to decreased levels of arachidonic acid–containing phosphatidylethanolamine plasmalogens, thereby limiting thromboxane A2 synthesis, dense granule secretion, and platelet activation upon agonist stimulation. Bioenergetic analysis of ACC1-deficient platelets revealed reduced glycolytic activity, potentially worsening their activation defects. Notably, ACC1 deficiency also enhanced the mitochondrial reserve respiratory capacity, without altering basal respiration or adenosine triphosphate turnover. This increased reserve respiratory capacity correlated with reduced phosphatidylserine exposure, suggesting lower procoagulant activity. Importantly, we showed that ACC1 deficiency impaired thrombus formation without compromising hemostasis. Together, these findings identified ACC1 as a critical regulator of platelet function and highlighted its potential as a target for innovative antithrombotic therapies.
Introduction
Understanding the role of lipid composition in the platelet plasma membrane is crucial for unraveling hemostasis and thrombosis complexity. Lipids significantly influence platelet functionality, membrane fluidity, curvature, shape changes, and bioactive signaling molecule production.1-7 Several lipidomic studies have characterized lipid profiles of both resting and activated platelets,4,8-13 identifying glycerophospholipids, including phosphatidylcholine (PC), phosphatidylethanolamine (PE), phosphatidylserine (PS), and phosphatidylinositol, as the main lipid class.10 These glycerophospholipids differ in their head groups as well as length and saturation of their 2 fatty acyl chains.1,6,14 Watanabe et al reported that the main saturated fatty acids in the platelet plasma membrane are palmitic acid (16:0) and stearic acid (18:0), with oleic acid (18:1) and arachidonic acid (AA, 20:4) being the predominant unsaturated fatty acids.15 Arachidonic acid is particularly abundant in phosphatidylethanolamine plasmalogen (PEP), a glycerophospholipid subclass characterized by a vinyl-ether bond at the sn-1 position of the glycerol backbone.1 Upon platelet activation, these arachidonylated plasmalogens are selectively hydrolyzed by the phospholipase A2 (PLA2), releasing AA and generating lysophospholipid species.16-18 Subsequent AA oxidation via the cyclooxygenase and lipoxygenase pathways produces key lipid mediators, such as thromboxane A2 (TxA2) and other eicosanoids, which are crucial for platelet activation and bioenergetics.4,6,9,19,20
Platelets can synthesize lipids and fatty acyls de novo.21,22 Acetyl-coenzyme A (CoA) carboxylase (ACC) has emerged as a key enzyme in this process, catalyzing malonyl-CoA production, the rate-limiting substrate for de novo lipogenesis (DNL).23 Among the 2 isoforms (ACC1 and ACC2), ACC1 is the predominant isoform in platelets.19,24,25 Although DNL is typically considered a moderate contributor to intracellular lipid stores, a modulation of DNL can significantly alter membrane lipid saturation and the properties of various cell types, including endothelial26 and cancer cells.27,28 We have previously demonstrated that systemic activated protein kinase-ACC signaling inhibition and the resulting sustained ACC activation modifies AA-containing PEP levels in platelets, thereby influencing their reactivity to collagen and promoting thrombosis through increased TXA2 generation.19 Recent studies have also linked ACC to demarcation membrane formation in megakaryocytes (MKs), which is crucial for platelet biogenesis.29 However, whether platelets derived from ACC-deficient MKs exhibit altered lipidome composition, reduced activation, or functional impairments remains unknown.
In this study, we investigated the impact of ACC1 deletion on platelet lipid composition and function. Using glycoprotein Ibα (GPIbα)-Cre+/− mice for platelet-specific genetic ACC1 deletion, we demonstrated that ACC1 is essential for regulating the PEP lipid class and the overall levels of saturated and long polyunsaturated fatty acyl chains in platelet glycerophospholipids. These regulatory mechanisms directly affected platelet functionality, resulting in reduced thrombosis in mice. Our findings suggest that targeting DNL or ACC1 could be a promising strategy to prevent thrombotic disorders.
Methods
Additional materials and methods are outlined in the supplemental Data.
Mice
Platelet-specific ACC1 knockout mice were generated by crossing ACC1flx/flx females (The Jackson Laboratory) with GPIbα-Cre+/− males,30 yielding ACC1flx/flx × GPIbα-Cre+/− mice and ACC1flx/flx control mice. GPIbα-Cre+/− animals were used as additional controls. All mice were on a C57BL/6 background, males, aged 8 to 16 weeks, and maintained on standard chow diet.
Lipidomics
Platelets (2 × 108) were suspended in modified Tyrode buffer with 0.35% free fatty acid bovine serum albumin. Dry platelet pellets were obtained by centrifugation at 11 700g for 15 seconds, then stored at −80°C before lipid extraction. Lipid extraction and subsequent lipidomic analysis were conducted as previously described.1
Lipidomic data statistical analysis
Lipid species concentrations were analyzed using R.4.2.1 according to the following bioinformatics pipeline. The lipid species concentration matrix and associated metadata were imported into a SummarizedExperiment object (version 1.28.0), and lipid species with >80% missing values were excluded. Normalization was performed via the mass spectrometry total useful signal method, followed by log2 transformation. Linear models for each lipid species were built using limma (version 3.54.2), yielding fold changes and P values, which were false discovery rate (FDR)-adjusted (Benjamini-Hochberg, FDR ≤ 0.05). Visualizations were produced using ggplot2 (version 3.4.2), ggradar (version 0.2), and forestplot (version 3.1.1).
Statistical analysis
Statistical parameters including sample size, mean, standard error of the mean, and statistical significance are specified in the figure legends. P values ≤ .05 were considered statistically significant. Statistical analyses were performed using GraphPad Prism 9.1.2 (GraphPad Software, San Diego, CA).
Animal experiments followed Animal Research: Reporting of In Vivo Experiments guidelines and were approved by local ethics committees (Comité d’éthique facultaire pour l’expérimentation animale; 2016/UCL/MD/027 and 2021/UCL/MD/012).
Results
Platelet-specific ACC1 knockout mice displayed reduced platelet counts and increased αIIbβ3 expression
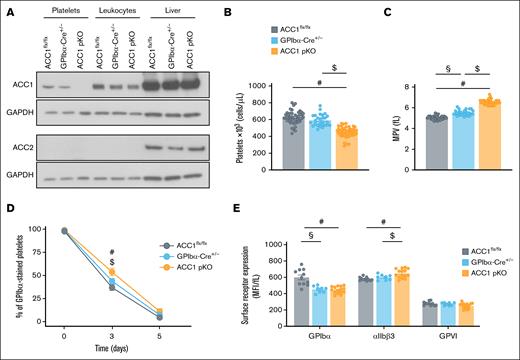
We developed a platelet-specific ACC1 knockout (pKO) mouse model and confirmed the absence of ACC1 expression in platelets, with no compensatory ACC2 upregulation (Figure 1A). ACC1 expression levels in leukocytes from ACC1 pKO mice were comparable with those from ACC1flx/flx and GPIbα-Cre+/− control mice, confirming the specificity of the ACC1 floxed gene deletion within the MK lineage by GPIbα-Cre–mediated recombination. Liver extracts were used as positive controls for detecting both ACC1 and ACC2. ACC1 deficiency resulted in a significant platelet count reduction compared with ACC1flx/flx and GPIbα-Cre+/− control mice, along with a platelet volume increase (Figure 1B-C). Minor changes were noted in other blood cell types, including erythrocytes, leukocytes, lymphocytes, and granulocytes, with hematocrit levels remaining similar across all 3 genotypes, suggesting that these effects were platelet-specific (supplemental Table 1). Flow cytometry analysis of platelets isolated from mice injected with Alexa 488–GPIbβ antibodies revealed a modest but significant reduction in platelet clearance in ACC1-deficient mice compared with both ACC1flx/flx and GPIbα-Cre+/− control groups 3 days after injection, indicating that the reduced platelet counts observed in ACC1 pKO mice is not because of increased platelet turnover (Figure 1D; supplemental Figure 1A). Given the role of CD36 in megakaryopoiesis,29,31,32 we assessed its expression in ACC1-deficient platelets as a readout for potential changes in megakaryocytes. CD36 levels were increased in ACC1 pKO platelets compared with both controls (supplemental Figure 1E), arguing against CD36 downregulation as a cause of the observed thrombocytopenia.
Characterization of ACC1flx/flx, GPIbα-Cre+/−, and ACC1 pKO mice. (A) Washed murine platelets or leukocytes were lysed and subjected to western blot analysis for ACC1 and ACC2 expression. Liver extract was used as positive control for ACC2. GAPDH served as loading control for platelet, leukocyte, and liver samples. (B-C) Platelet counts and MPV were determined by Cell-Dyn Emerald Hematology Analyzer (Abbott) in a single measurement conducted during the usage period of mice (8-16 weeks old). Data are expressed as mean ± standard error of the mean (SEM; n = ≥27). #P ≤ .0001 relative to ACC1flx/flx mice, $P ≤ .0001 relative to GPIbα-Cre+/− mice, and §P ≤ .0001 between ACC1flx/flx and GPIbα-Cre+/− mice. One-way analysis of variance (ANOVA) was used for data analysis. (D) Alexa 488–GPIbβ antibody was injected into ACC1flx/flx, GPIbα-Cre+/−, and ACC1 pKO mice to analyze platelet lifespan. Blood samples were collected on the day of the injection (day 0), as well as on days 3 and 5 after injection. Platelets were stained ex vivo with PE-conjugated anti-CD41 antibody, and the percentage of Alexa 488+ platelets among total CD41+ platelets was quantified by flow cytometry. Data are expressed as mean ± SEM (n ≥ 3). #P ≤ .05 relative to ACC1flx/flx mice, $P ≤ .01 relative to GPIbα-Cre+/− mice. Data were subjected to 2-way ANOVA analysis. (E) Platelet surface expression of αIIbβ3, GPIbα, and GPVI was measured by flow cytometry and normalized to MPV. Data are expressed as MFI adjusted to MPV (fL) ± SEM (n ≥ 8). #P ≤ .05 relative to ACC1flx/flx mice, $P ≤ .01 relative to GPIbα-Cre+/− mice, and §P ≤ .01 between ACC1flx/flx and GPIbα-Cre+/− mice. Data were subjected to 1-way ANOVA analysis. GAPDH, glyceraldehyde-3-phosphate dehydrogenase; GPVI, glycoprotein VI; MFI, median fluorescence intensity; MPV, mean platelet volume.
Characterization of ACC1flx/flx, GPIbα-Cre+/−, and ACC1 pKO mice. (A) Washed murine platelets or leukocytes were lysed and subjected to western blot analysis for ACC1 and ACC2 expression. Liver extract was used as positive control for ACC2. GAPDH served as loading control for platelet, leukocyte, and liver samples. (B-C) Platelet counts and MPV were determined by Cell-Dyn Emerald Hematology Analyzer (Abbott) in a single measurement conducted during the usage period of mice (8-16 weeks old). Data are expressed as mean ± standard error of the mean (SEM; n = ≥27). #P ≤ .0001 relative to ACC1flx/flx mice, $P ≤ .0001 relative to GPIbα-Cre+/− mice, and §P ≤ .0001 between ACC1flx/flx and GPIbα-Cre+/− mice. One-way analysis of variance (ANOVA) was used for data analysis. (D) Alexa 488–GPIbβ antibody was injected into ACC1flx/flx, GPIbα-Cre+/−, and ACC1 pKO mice to analyze platelet lifespan. Blood samples were collected on the day of the injection (day 0), as well as on days 3 and 5 after injection. Platelets were stained ex vivo with PE-conjugated anti-CD41 antibody, and the percentage of Alexa 488+ platelets among total CD41+ platelets was quantified by flow cytometry. Data are expressed as mean ± SEM (n ≥ 3). #P ≤ .05 relative to ACC1flx/flx mice, $P ≤ .01 relative to GPIbα-Cre+/− mice. Data were subjected to 2-way ANOVA analysis. (E) Platelet surface expression of αIIbβ3, GPIbα, and GPVI was measured by flow cytometry and normalized to MPV. Data are expressed as MFI adjusted to MPV (fL) ± SEM (n ≥ 8). #P ≤ .05 relative to ACC1flx/flx mice, $P ≤ .01 relative to GPIbα-Cre+/− mice, and §P ≤ .01 between ACC1flx/flx and GPIbα-Cre+/− mice. Data were subjected to 1-way ANOVA analysis. GAPDH, glyceraldehyde-3-phosphate dehydrogenase; GPVI, glycoprotein VI; MFI, median fluorescence intensity; MPV, mean platelet volume.
Further flow cytometry analysis of major platelet surface receptors, normalized to mean platelet volume, revealed a modest increase in αIIbβ3 expression in ACC1 pKO compared with ACC1flx/flx and GPIbα-Cre+/− platelets (Figure 1E). In contrast, the expression levels of other receptors, such as GPIbα or GPVI, were not elevated in ACC1-deficient platelets. Interestingly, GPIbα expression was even reduced in GPIbα-Cre+/− platelets, compared with both ACC1flx/flx and ACC1 pKO platelets (Figure 1E), despite a slightly increased platelet volume. This reduction in GPIbα expression has been previously documented in GPIbα-Cre+/− mice.30 Finally, western blot analysis of protease-activated receptor (PAR) 3, PAR4, and thromboxane A2 receptor receptors showed comparable expression levels across the 3 genotypes, with no consistent or statistically significant differences observed (supplemental Figure 1B-D).
Lipidomic analysis revealed distinct changes in lipid profiles of ACC1 pKO platelets
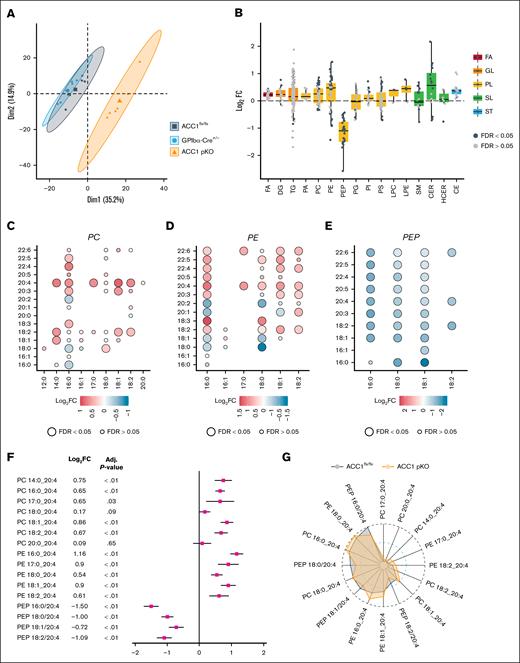
To investigate the impact of ACC1 deficiency on platelet lipid composition, we performed a lipidomic analysis on resting platelets from ACC1 pKO mice and compared the results with those of the 2 control groups (ACC1flx/flx and GPIbα-Cre+/−) using the Lipidyzer platform. Principal component analysis was conducted to distinguish the lipidome profiles of ACC1 pKO mice from those of the control groups. The principal component analysis revealed a clear separation between the lipid profiles of ACC1 pKO and control mice, with the 2 principal components accounting for 50.1% of the variance in the lipidome across the 3 genotypes (Figure 2A). The lipidomic analysis identified 446 lipid species across 16 lipid classes/subclasses, including fatty acids, diacylglycerol, triacylglycerol, phosphatidic acid, PC, PE, PEP, phosphatidylglycerol, phosphatidylinositol, PS, lysophosphatidyl-choline, lysophosphatidyl-ethanolamine, sphingomyelin, ceramide (CER), hydroxyceramide, and cholesterol ester when comparing ACC1 pKO with ACC1flx/flx mice. Notably, 168 lipid species (38% of the identified platelet lipid species) were significantly differentially expressed between these 2 groups (Figure 2B; supplemental Table 2). Similarly, when comparing ACC1 pKO and GPIbα-Cre+/− mice, 206 of 436 identified lipid species were differentially expressed (supplemental Table 2; supplemental Figure 2A). Class-wide analysis revealed that ACC1 deficiency led to a downregulation of PEP species, whereas CER, PC, and PE lipid classes were upregulated (Figure 2B; supplemental Figure 2A). To further explore changes in phospholipid species, we examined differences in fatty acyl chain length and unsaturation degree among groups. Consistent with previous studies,19,26,27 our analysis revealed that ACC1 deficiency was associated with a decrease in palmitic acid (16:0) across 3 phospholipid classes (PC, PE, and PEP). In contrast, levels of long polyunsaturated fatty acids (PUFAs) such as AA (20:4), eicosapentaenoic acid (20:5), and docosahexaenoic acid (22:6) were increased in PC and PE classes (Figure 2C-E; supplemental Figure 2B-D). Given that AA is a key precursor for the generation of signaling mediators in platelets, we specifically assessed the levels of AA-containing phospholipids (PC, PE, and PEP) across the 3 genotypes. Our findings showed that AA-containing PC or PE were upregulated, whereas AA-containing PEP levels were reduced in ACC1 pKO compared with both ACC1flx/flx and GPIbα-Cre+/− control platelets (Figure 2F; supplemental Figure 2E). A comparative analysis of the relative abundance of AA-containing phospholipids among groups revealed that the PEP 16:0/20:4 species was the most abundant within platelet phospholipid classes in control mice. In ACC1 pKO mice, the PEP 16:0/20:4 species is reduced, and PE 18:0/20:4 becomes the most abundant (Figure 2G; supplemental Figure 2F). Overall, these results underscored the pivotal role of ACC1 in the modulation of both saturated and polyunsaturated fatty acyl chain levels in platelet phospholipids. Notably, arachidonylated plasmalogen regulation may have significant implications for platelet responsiveness to agonists, potentially by affecting energy metabolism and/or TxA2 generation.
Lipidomic analysis revealed changes in lipid profiles of ACC1 pKO platelets. Lipid extraction was performed on washed murine platelets from 6 samples for 1 genotype, each sample consisting of platelets pooled from 2 or 3 mice of the same genotype. (A) Platelet lipidome overview of ACC1 pKO, and ACC1flx/flx, and GPIbα-Cre+/− controls by principle component analysis. The colors and symbols distinguish the 3 genotypes. The first and second dimensions with their associated percentage of explained variance are displayed on the x-axes and y-axes, respectively. (B-F) Lipidomic analysis comparing ACC1 pKO and ACC1flx/flx platelets. (B) Box and scatter plot representations of the modulation (log2FC) of 446 identified lipid species categorized into 5 (sub)classes, each represented by a distinct color (red, FA; orange, GL; yellow, PL; green, SL; and blue, ST). Lipid species are represented as black or gray dots based on their statistical significance (black dots representing a significant adjusted P value, FDR ≤ 0.05; whereas gray dots correspond to FDR ≥ 0.05). Lipids above the horizontal dotted line are upregulated, whereas lipids below are downregulated. Log FCs and FDR were calculated from the multivariate regression model. (C-E) Dot plots displaying the length and unsaturation levels of the 2 fatty acyl chains from PC (C), PE (D), and PEP (E). Color bars indicate the logFC from red (upregulation) to blue (downregulation). The adjusted P value is represented by the circle diameter (the largest circle corresponds to FDR ≤ 0.05). (F) Forest plot of AA-containing PC, PE, and PEP comparing ACC1flx/flx and ACC1 pKO mice. Data are presented as logFC (squares) relative to ACC1flx/flx platelets. (G) Radar plot showing the relative intensity of PC, PE, and PEP containing the 20:4 fatty acyl chains. The dark gray area corresponds to ACC1flx/flx mice, whereas the orange area corresponds to ACC1 pKO mice. CE, cholesterol ester; DG, diacylglycerol; FA, fatty acid; FC, fold change; GL, glycerolipid; HCER, hydroxyceramide; LPC, lysophosphatidylcholine; LPE, lysophosphatidylethanolamine; PG, phosphatidylglycerol; PI, phosphatidylinositol; PL, phospholipid; SL, sphingolipid; SM, sphingomyelin; ST, sterol; TG, triacylglycerol.
Lipidomic analysis revealed changes in lipid profiles of ACC1 pKO platelets. Lipid extraction was performed on washed murine platelets from 6 samples for 1 genotype, each sample consisting of platelets pooled from 2 or 3 mice of the same genotype. (A) Platelet lipidome overview of ACC1 pKO, and ACC1flx/flx, and GPIbα-Cre+/− controls by principle component analysis. The colors and symbols distinguish the 3 genotypes. The first and second dimensions with their associated percentage of explained variance are displayed on the x-axes and y-axes, respectively. (B-F) Lipidomic analysis comparing ACC1 pKO and ACC1flx/flx platelets. (B) Box and scatter plot representations of the modulation (log2FC) of 446 identified lipid species categorized into 5 (sub)classes, each represented by a distinct color (red, FA; orange, GL; yellow, PL; green, SL; and blue, ST). Lipid species are represented as black or gray dots based on their statistical significance (black dots representing a significant adjusted P value, FDR ≤ 0.05; whereas gray dots correspond to FDR ≥ 0.05). Lipids above the horizontal dotted line are upregulated, whereas lipids below are downregulated. Log FCs and FDR were calculated from the multivariate regression model. (C-E) Dot plots displaying the length and unsaturation levels of the 2 fatty acyl chains from PC (C), PE (D), and PEP (E). Color bars indicate the logFC from red (upregulation) to blue (downregulation). The adjusted P value is represented by the circle diameter (the largest circle corresponds to FDR ≤ 0.05). (F) Forest plot of AA-containing PC, PE, and PEP comparing ACC1flx/flx and ACC1 pKO mice. Data are presented as logFC (squares) relative to ACC1flx/flx platelets. (G) Radar plot showing the relative intensity of PC, PE, and PEP containing the 20:4 fatty acyl chains. The dark gray area corresponds to ACC1flx/flx mice, whereas the orange area corresponds to ACC1 pKO mice. CE, cholesterol ester; DG, diacylglycerol; FA, fatty acid; FC, fold change; GL, glycerolipid; HCER, hydroxyceramide; LPC, lysophosphatidylcholine; LPE, lysophosphatidylethanolamine; PG, phosphatidylglycerol; PI, phosphatidylinositol; PL, phospholipid; SL, sphingolipid; SM, sphingomyelin; ST, sterol; TG, triacylglycerol.
Energy metabolism was modulated in ACC1 pKO platelets
Given that acyl-CoA, especially AA (20:4) and eicosanoids, serve as substrates for platelet mitochondrial β-oxidation,4,9 we investigated the impact of ACC1 deficiency on energy metabolism. We initially measured the basal oxygen consumption rate (OCR) and subsequently monitored changes after either thrombin injection (1 U/mL) or vehicle (Dulbecco's modified eagle medium) over a 30-minute period. Under nonstimulated conditions, OCR was comparable between control and ACC1 pKO platelets, with glucose as the metabolic substrate. Thrombin injection resulted in a similar OCR increase in ACC1-deficient and control platelets (Figure 3A-B; supplemental Figure 3A-B). To further evaluate oxidative phosphorylation, we injected oligomycin, an adenosine triphosphate (ATP) synthase inhibitor, which led to the anticipated OCR decrease. ATP-linked respiration did not differ significantly between ACC1 pKO and control platelets. Following this, FCCP (carbonyl cyanide-p-trifluoromethoxyphenylhydrazone), a mitochondrial uncoupler that disrupts membrane potential, was used to induce maximal respiration, which was found to be similar among all 3 genotypes under basal conditions (Figure 3A-B; supplemental Figure 3A-B). Upon thrombin stimulation, there was a decrease in maximal respiration and reserve capacity in ACC1flx/flx and GPIbα-Cre+/− platelets.4,33 Interestingly, ACC1 deficiency was associated with a significant increase in mitochondrial respiratory reserve after thrombin stimulation compared with ACC1flx/flx platelets, suggesting a greater availability of endogenous metabolic substrates to meet the increased energetic demand. The same trend was observed in comparison with GPIbα-Cre+/− platelets. Finally, the respiratory-chain complex I/III inhibitors rotenone/antimycin A reduced mitochondrial-induced OCR to a similar extent across all 3 genotypes, and proton leak remained unchanged among groups (Figure 3A-B; supplemental Figure 3A-B). In parallel, extracellular acidification rate (ECAR), which reflects proton release primarily due to lactate production and is commonly used as an indirect indicator of glycolytic activity, increased upon thrombin stimulation in all genotypes. Specifically, thrombin induced an increase in ECAR relative to resting conditions in both control and ACC1 pKO platelets. However, absolute ECAR values remained consistently lower in ACC1-deficient platelets, suggesting reduced glycolytic capacity (Figure 3C-D; supplemental Figure 3C-D). These findings suggest that ACC1 can be involved in the regulation of platelet energy metabolism, a feature that may affect their ability to respond effectively to thrombogenic stimuli.
ACC1 deficiency in platelets had an impact on both mitochondrial reserve capacity and glycolytic activity. OCR was measured in washed murine platelets from ACC1flx/flx or pKO mice. (A-B) OCR was assessed under basal conditions, after stimulation with 1 U/mL Thr, and after sequential treatment with 1 μM oligomycin (O), 0.4 μM carbonyl cyanide p-trifluoromethoxyphenylhydrazone (F), and a mix of 1 μM AtA/R. (A) Representative OCR profiles are shown as mean ± SEM (n = 4). (B) Quantification of mitochondrial respiratory parameters including basal (baseline OCR – R/AtA-sensitive OCR), Thr (Thr response − baseline OCR), ATP-linked (thrombin response − oligomycin sensitive OCR), maximal (FCCP [carbonyl cyanide-p-trifluoromethoxyphenylhydrazone]-sensitive – R/AtA-sensitive OCR), reserve capacity (FCCP-sensitive – Thr-responsive OCR), proton leak (oligomycin-sensitive – R/AtA-sensitive OCR), and nonmitochondrial (R/AtA-sensitive OCR). Results are expressed as mean ± SEM (n = 4). (C-D) ECAR, used as an indirect indicator of glycolytic activity, was measured in the same experimental conditions. (C) Representative ECAR profiles are shown as mean ± SEM (n = 4). (D) Quantification of basal and Thr-induced ECAR. Results are expressed as mean ± SEM (n = 4). ∗P ≤ .05 relative to unstimulated conditions. #P ≤ .05 relative to ACC1flx/flx mice. Statistical analysis was performed using 2-way ANOVA. Ata, antimycin A; DMEM, Dulbecco’s modified eagle medium; NS, nonstimulated; R, rotenone; Thr, thrombin.
ACC1 deficiency in platelets had an impact on both mitochondrial reserve capacity and glycolytic activity. OCR was measured in washed murine platelets from ACC1flx/flx or pKO mice. (A-B) OCR was assessed under basal conditions, after stimulation with 1 U/mL Thr, and after sequential treatment with 1 μM oligomycin (O), 0.4 μM carbonyl cyanide p-trifluoromethoxyphenylhydrazone (F), and a mix of 1 μM AtA/R. (A) Representative OCR profiles are shown as mean ± SEM (n = 4). (B) Quantification of mitochondrial respiratory parameters including basal (baseline OCR – R/AtA-sensitive OCR), Thr (Thr response − baseline OCR), ATP-linked (thrombin response − oligomycin sensitive OCR), maximal (FCCP [carbonyl cyanide-p-trifluoromethoxyphenylhydrazone]-sensitive – R/AtA-sensitive OCR), reserve capacity (FCCP-sensitive – Thr-responsive OCR), proton leak (oligomycin-sensitive – R/AtA-sensitive OCR), and nonmitochondrial (R/AtA-sensitive OCR). Results are expressed as mean ± SEM (n = 4). (C-D) ECAR, used as an indirect indicator of glycolytic activity, was measured in the same experimental conditions. (C) Representative ECAR profiles are shown as mean ± SEM (n = 4). (D) Quantification of basal and Thr-induced ECAR. Results are expressed as mean ± SEM (n = 4). ∗P ≤ .05 relative to unstimulated conditions. #P ≤ .05 relative to ACC1flx/flx mice. Statistical analysis was performed using 2-way ANOVA. Ata, antimycin A; DMEM, Dulbecco’s modified eagle medium; NS, nonstimulated; R, rotenone; Thr, thrombin.
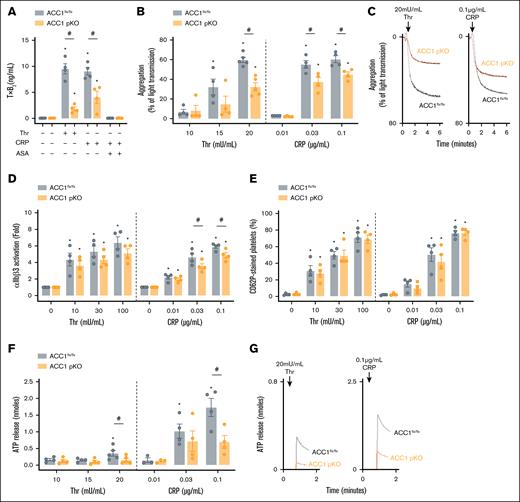
Reduced arachidonylated PEP content restricted TxA2 generation and dense granule release in ACC1 pKO platelets
Next, we evaluated the impact of ACC1 deficiency on TxA2 production by measuring TxB2 levels. Platelets lacking ACC1 showed a significant reduction in TxB2 generation after thrombin and collagen-related peptide (CRP) stimulation. Aspirin was used as a positive control for TxB2 generation inhibition (Figure 4A). Of note, the observed reduction in TxB2 production in ACC1 pKO platelets is unlikely to result from impaired PLA2 activation, as PLA2 activity remained comparable between ACC1 and control platelets in both resting and thrombin-stimulated conditions (supplemental Figure 4F). Because TxA2 acts as a secondary platelet agonist involved in αIIbβ3 integrin activation and dense granule secretion,34 we further examined platelet reactivity to thrombin and CRP. We first investigated αIIbβ3 inside-out signaling by analyzing platelet aggregation. ACC1 pKO platelets displayed reduced aggregation in response to both thrombin and CRP compared with ACC1flx/flx platelets (Figure 4B-C). This impaired aggregation correlated with decreased αIIbβ3 activation, as indicated by the reduced JON/A antibody binding compared with ACC1flx/flx platelets (Figure 4D). We then analyzed platelet granule secretion. After thrombin stimulation, surface expression of P-selectin, a marker for α-granule secretion, was similar between ACC1 pKO and ACC1flx/flx platelets. However, it tended to be reduced in ACC1 pKO platelets at low CRP concentrations (Figure 4E), suggesting that ACC1 deficiency may modestly impair α-granule secretion in response to this agonist. In contrast, ATP release, indicative of dense granule secretion, was significantly reduced in ACC1 pKO platelets compared with ACC1flx/flx platelets after thrombin or CRP stimulation (Figure 4F-G). Importantly, similar results in TxA2 generation, platelet aggregation, and dense granule release were observed when comparing platelets from ACC1 pKO mice with those from GPIbα-Cre+/− mice (supplemental Figure 4B-F). These findings highlight the pivotal role of ACC1 in regulating platelet reactivity through the synthesis of AA-containing PEP, used as a source for TxA2 production. To test whether impaired aggregation could be rescued by supplementing secondary mediators, we costimulated ACC1 pKO platelets with thrombin and exogenous adenosine diphosphate (ADP). However, this costimulation failed to restore aggregation to control levels, suggesting that the functional defect extends beyond impaired ADP release (supplemental Figure 4G-H).
ACC1 pKO platelets displayed reduced TxA2 generation, dense granule secretion, and aggregation upon agonist stimulation. (A-G) Washed murine platelets from ACC1 floxed or pKO mice were stimulated with various Thr or CRP concentrations. (A) Platelets were stimulated with 100 mU/mL Thr or 0.3 μg/mL CRP for 5 minutes, and TxB2 was measured in the supernatant using an enzyme-linked immunosorbent assay kit. Aspirin-treated platelets were included as a positive control for TxB2 generation inhibition in the CRP condition. Data are expressed as mean ± SEM (n = 4). ∗P ≤ .05 relative to unstimulated conditions. #P ≤ .05 relative to ACC1flx/flx mice. Data were analyzed via 2-way ANOVA. (B) Aggregation was analyzed by turbidimetry (Chrono-Log). Data are expressed as mean ± SEM (n ≥ 4). ∗P ≤ .05 relative to the first agonist concentration. #P ≤ .05 relative to ACC1flx/flx mice. Two-way ANOVA was used for analysis. (C) Aggregation profiles are shown. (D) Activated αIIbβ3 (JON/A) and (E) p-selectin (CD62P) exposure was detected by flow cytometry. Data are expressed as fold change ± SEM (n = 4) and mean ± SEM (n = 4) respectively. ∗P ≤ .05 relative to unstimulated conditions. #P ≤ .05 relative to ACC1flx/flx mice. Two-way ANOVA was used for analysis. (F) Dense granule secretion was assessed via addition of luciferase-luciferin reagent (Chrono-log). Data are expressed as mean ± SEM (n ≥ 3). ∗P ≤ .05 relative to the first agonist concentration. #P ≤ .05 relative to ACC1flx/flx mice. Two-way ANOVA was used for analysis. (G) Profiles are shown. ASA, aspirine; Thr, thrombine.
ACC1 pKO platelets displayed reduced TxA2 generation, dense granule secretion, and aggregation upon agonist stimulation. (A-G) Washed murine platelets from ACC1 floxed or pKO mice were stimulated with various Thr or CRP concentrations. (A) Platelets were stimulated with 100 mU/mL Thr or 0.3 μg/mL CRP for 5 minutes, and TxB2 was measured in the supernatant using an enzyme-linked immunosorbent assay kit. Aspirin-treated platelets were included as a positive control for TxB2 generation inhibition in the CRP condition. Data are expressed as mean ± SEM (n = 4). ∗P ≤ .05 relative to unstimulated conditions. #P ≤ .05 relative to ACC1flx/flx mice. Data were analyzed via 2-way ANOVA. (B) Aggregation was analyzed by turbidimetry (Chrono-Log). Data are expressed as mean ± SEM (n ≥ 4). ∗P ≤ .05 relative to the first agonist concentration. #P ≤ .05 relative to ACC1flx/flx mice. Two-way ANOVA was used for analysis. (C) Aggregation profiles are shown. (D) Activated αIIbβ3 (JON/A) and (E) p-selectin (CD62P) exposure was detected by flow cytometry. Data are expressed as fold change ± SEM (n = 4) and mean ± SEM (n = 4) respectively. ∗P ≤ .05 relative to unstimulated conditions. #P ≤ .05 relative to ACC1flx/flx mice. Two-way ANOVA was used for analysis. (F) Dense granule secretion was assessed via addition of luciferase-luciferin reagent (Chrono-log). Data are expressed as mean ± SEM (n ≥ 3). ∗P ≤ .05 relative to the first agonist concentration. #P ≤ .05 relative to ACC1flx/flx mice. Two-way ANOVA was used for analysis. (G) Profiles are shown. ASA, aspirine; Thr, thrombine.
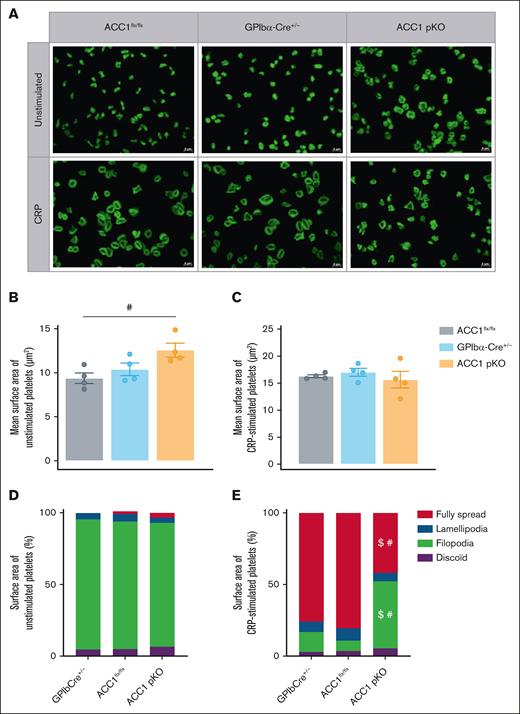
Interestingly, the absence of ACC1 also altered platelet morphology under both resting and stimulated conditions. At rest, ACC1-deficient platelets exhibited a significantly larger surface area when spread on fibrinogen (12.58 ± 0.7917 μm2) compared with ACC1flx/flx platelets (9.392 ± 0.5965 μm2) and GPIbα-Cre+/− platelets (10.39 ± 0.712 μm2; Figure 5A-C). However, upon CRP stimulation, ACC1-deficient platelets displayed a notably reduced proportion of fully spread platelets compared with controls. Instead, a higher number of filopodia-extending platelets was observed, indicating a failure to achieve full spreading under agonist stimulation (Figure 5A,D-E).
Platelet-specific ACC1 deletion affects platelet spreading and actin cytoskeleton organization upon CRP stimulation. (A-E) Washed murine platelets were stimulated with or without CRP 0.3 μg/mL for 30 minutes. Platelets were stained with phalloidin-fluorescein isothiocyanate for 45 minutes at room temperature. (A) Panel of representative pictures. Scale bar, 5 μm. (B-C) Quantification of the mean surface area covered by (B) unstimulated or (C) CRP-stimulated platelets independently of the platelet morphology. Data are expressed as mean ± SEM (n = 4). #P ≤ .05 relative to ACC1flx/flx mice. Data underwent 1-way ANOVA. (D-E) Proportion of the surface area covered by (D) unstimulated or (E) CRP-stimulated platelets, being inactivated (discoid), extending filopodia, forming lamellipodia or fully spread after activation. Data are expressed as means ± SEM (n = 4). #P ≤ .05 relative to ACC1flx/flx mice. $P ≤ .05 relative to GPIb-Cre+/− mice. Data underwent 2-way ANOVA.
Platelet-specific ACC1 deletion affects platelet spreading and actin cytoskeleton organization upon CRP stimulation. (A-E) Washed murine platelets were stimulated with or without CRP 0.3 μg/mL for 30 minutes. Platelets were stained with phalloidin-fluorescein isothiocyanate for 45 minutes at room temperature. (A) Panel of representative pictures. Scale bar, 5 μm. (B-C) Quantification of the mean surface area covered by (B) unstimulated or (C) CRP-stimulated platelets independently of the platelet morphology. Data are expressed as mean ± SEM (n = 4). #P ≤ .05 relative to ACC1flx/flx mice. Data underwent 1-way ANOVA. (D-E) Proportion of the surface area covered by (D) unstimulated or (E) CRP-stimulated platelets, being inactivated (discoid), extending filopodia, forming lamellipodia or fully spread after activation. Data are expressed as means ± SEM (n = 4). #P ≤ .05 relative to ACC1flx/flx mice. $P ≤ .05 relative to GPIb-Cre+/− mice. Data underwent 2-way ANOVA.
Platelet-specific ACC1 deletion reduced thrombus formation on collagen-coated surfaces under flow conditions
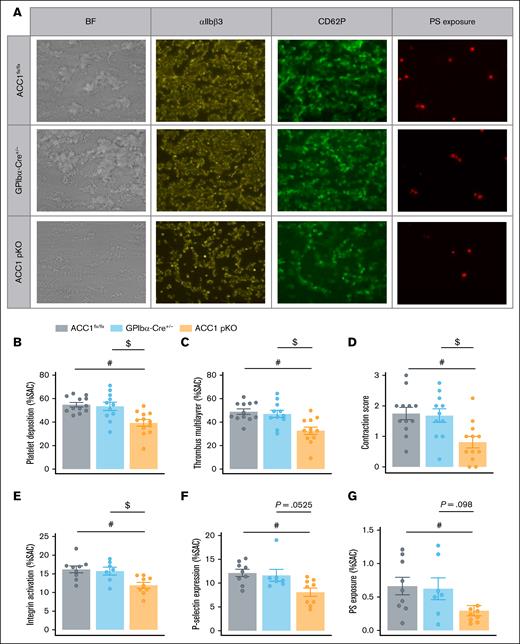
To evaluate platelet functions under dynamic conditions, we perfused anticoagulated blood over collagen 1– and collagen 3–coated surfaces at a shear rate of 1000/s and examined thrombus formation. Brightfield images were captured to assess platelet adhesion and thrombus buildup. Platelet adhesion was quantified by analyzing the surface area coverage, whereas thrombus formation was characterized by the surface area covered by multilayered thrombi and thrombus contraction score.35,36 Compared with ACC1flx/flx or GPIbα-Cre+/−, ACC1 pKO mice blood exhibited decreased platelet adhesion and multilayered thrombus formation, regardless of collagen-coated surface type (Figure 6A-D; supplemental Figure 5A-D). This reduction was likely because of the reduced TxA2 and ADP secretion, which are secondary agonists essential for platelet aggregation on collagen surfaces.19,37,38 Platelet activation in the flow chamber was simultaneously monitored using fluorescently labeled anti–P-selectin antibodies, JON/A antibodies, and annexin V to assess α-granule secretion, αIIbβ3 activation, and PS externalization (reflecting platelet procoagulant activity), respectively. Quantification showed significantly reduced activation levels of all markers in ACC1 pKO perfused blood on both collagen surfaces (Figure 6A,E-G; supplemental Figure 5A,E-G), confirming the loss-of-function phenotype of ACC1 pKO platelets even under flow conditions simulating thrombus formation in a blood vessel.
ACC1 deficiency in platelets impaired their activation and thrombus formation under flow conditions. (A-G) Whole blood from ACC1flx/flx, GPIbα-Cre+/−, and ACC1 pKO mice was perfused over collagen 1–coated surface (50 μg/mL) at a shear rate of 1000/s. (A) Representative images of multilayered platelet thrombi, and activated αIIbβ3, CD62P, and phosphatidylserine (PS) stainings. (B-D) Platelet deposition, thrombus formation and contraction score were assessed on BF images. (E) αIIbβ3 activation was evaluated using JON/A Ab, (F) P-selectin exposure was evaluated via staining with anti-CD62P antibody, and (G) PS exposure was determined using annexin V. The results are expressed as mean ± SEM (≥4 mice per group). #P ≤ .05 relative to ACC1flx/flx mice. $P ≤ .05 relative to GPIbα-Cre+/− mice. One-way ANOVA was used for analysis. BF, brightfield; JON/A Ab, JON/A antibody; SAC, surface area covered.
ACC1 deficiency in platelets impaired their activation and thrombus formation under flow conditions. (A-G) Whole blood from ACC1flx/flx, GPIbα-Cre+/−, and ACC1 pKO mice was perfused over collagen 1–coated surface (50 μg/mL) at a shear rate of 1000/s. (A) Representative images of multilayered platelet thrombi, and activated αIIbβ3, CD62P, and phosphatidylserine (PS) stainings. (B-D) Platelet deposition, thrombus formation and contraction score were assessed on BF images. (E) αIIbβ3 activation was evaluated using JON/A Ab, (F) P-selectin exposure was evaluated via staining with anti-CD62P antibody, and (G) PS exposure was determined using annexin V. The results are expressed as mean ± SEM (≥4 mice per group). #P ≤ .05 relative to ACC1flx/flx mice. $P ≤ .05 relative to GPIbα-Cre+/− mice. One-way ANOVA was used for analysis. BF, brightfield; JON/A Ab, JON/A antibody; SAC, surface area covered.
In vivo thrombus growth in ACC1 pKO mice was impaired, without effect on hemostasis
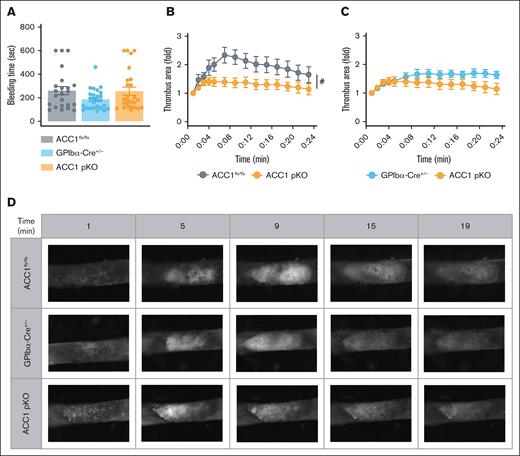
Finally, we investigated the role of platelet ACC1 in physiological hemostasis and thrombosis using in vivo mouse models. First, a bleeding time assay was performed, showing that the average bleeding time in ACC1 pKO mice was similar to that of ACC1flx/flx and GPIbα-Cre+/− animals (Figure 7A). Next, we studied the involvement of platelet-specific ACC1 in arterial thrombus formation using the well-established carotid artery thrombosis model induced by ferric chloride.39 Thrombus formation was monitored in real time by intravital fluorescence microscopy. In line with the impaired reactivity of ACC1-deficient platelets, thrombus growth was significantly reduced in ACC1 pKO mice compared with ACC1flx/flx mice (Figure 7B,D) and showed a moderate yet reproducible reduction when compared with GPIbα-Cre+/− controls (Figure 7C-D).
ACC1 pKO mice exhibited reduced arterial thrombosis while maintaining normal hemostasis. (A) Tail bleeding times of ACC1flx/flx, GPIb-Cre+/−, and ACC1 pKO mice in saline solution preheated at 37°C. Results are expressed as mean ± SEM (n ≥ 23). Data were analyzed via 1-way ANOVA. (B-D) Mice were subjected to thrombosis induced in vivo by FeCl3 application to the carotid artery (10% of FeCl3 during 5 minutes). Thrombus formation was visualized using intravital microscopy analyzing exogenous platelet accumulation stained with carboxyfluorescein succinimidyl ester. (B-C) Thrombus growth area quantification over time. Data are expressed as mean ± SEM (n ≥ 6). #P ≤.05 relative to ACC1flx/flx mice. Data were analyzed using unpaired t test comparing area under the curve. (D) Representative pictures of thrombus formation over time (1, 5, 9, 15, and 19 minutes) after FeCl3 injury.
ACC1 pKO mice exhibited reduced arterial thrombosis while maintaining normal hemostasis. (A) Tail bleeding times of ACC1flx/flx, GPIb-Cre+/−, and ACC1 pKO mice in saline solution preheated at 37°C. Results are expressed as mean ± SEM (n ≥ 23). Data were analyzed via 1-way ANOVA. (B-D) Mice were subjected to thrombosis induced in vivo by FeCl3 application to the carotid artery (10% of FeCl3 during 5 minutes). Thrombus formation was visualized using intravital microscopy analyzing exogenous platelet accumulation stained with carboxyfluorescein succinimidyl ester. (B-C) Thrombus growth area quantification over time. Data are expressed as mean ± SEM (n ≥ 6). #P ≤.05 relative to ACC1flx/flx mice. Data were analyzed using unpaired t test comparing area under the curve. (D) Representative pictures of thrombus formation over time (1, 5, 9, 15, and 19 minutes) after FeCl3 injury.
Discussion
Lipid metabolism in platelets has gained increasing attention in recent years, particularly regarding platelet activation and thrombotic diseases. Our previous research has demonstrated that systemic activation of ACC promotes platelet activation and thrombosis by extensively remodeling the platelet phospholipidome.19 However, selective ACC1 deletion effects on lipid composition and function of platelets had not yet been explored. Using the newly developed GPIbα-Cre+/− mouse model,30 we provided definitive evidence that ACC1 is a crucial regulator of the platelet phospholipidome, with substantial implications for thrombus growth, as shown in both in vivo and ex vivo studies. This not only corroborates previous findings but also clearly indicates that ACC1 and lipid metabolism are key modulators of platelet function.
The GPIbα-Cre+/− model enables the selective deletion of the floxed ACC1 gene within the MK lineage, avoiding the off-target effects associated with platelet factor 4-Cre. Platelet factor 4-Cre can exhibit leaky expression in nonmegakaryocytic and even nonhematopoietic cells, particularly under inflammatory conditions, and has been linked to aberrant chemokine expression, which may confound phenotypic analyses.30,40,41 GPIbα-Cre+/− mice display normal platelet function and hemostasis, with only minor changes in platelet volume and receptor expression.30 Interestingly, ACC1 deficiency further increases mean platelet volume, potentially explaining, at least in part, the increased αIIbβ3 surface expression observed in ACC1 pKO platelets compared with GPIbα-Cre+/− and ACC1flx/flx control mice.30,42,43 We also reported a significant platelet count reduction in ACC1 pKO mice, consistent with findings in humans and primates after pharmacological ACC inhibition.29 In these cases, reduced platelet counts have been linked to impaired DNL-dependent synthesis of saturated PC species in MKs, essential for the formation of the demarcation membrane during platelet biogenesis.29 Because we also noticed a reduction in PC 16:0/16:0 and PC 16:0/18:0 in ACC1 pKO platelets, we can speculate that similar changes occur in MKs, potentially contributing to the decreased platelet counts in ACC1 pKO mice. Although CD36 has also been implicated in megakaryopoiesis,29,31,32 its upregulation in ACC1-deficient platelets suggests it is unlikely to contribute to the platelet count reduction in this model.
Although the mild thrombocytopenia in ACC1 pKO mice (∼25% reduction relative to controls) could theoretically affect thrombus formation, previous studies have suggested otherwise. Bourne et al demonstrated that C57BL/6 mice maintain thrombus formation despite an ∼50% platelet reduction induced by GPIbα-depleting antibody treatment.44 This indicates that the decreased thrombus formation observed in ACC1 pKO mice likely results from altered platelet function rather than from reduced platelet count. Supporting this, ACC1 deficiency led to a significant decrease in TxA2 formation, resulting from the reduction in plasmalogens containing AA. This reduction was associated with impaired platelet activation, both in vitro after agonist stimulation and ex vivo in flow chamber studies. To assess whether defective aggregation in ACC1 pKO platelets could be rescued by a key secondary agonist, we costimulated thrombin-treated platelets with exogenous ADP. Aggregation remained impaired, indicating that deficient dense granule secretion is not solely responsible. Rather, changes in the phospholipidome may more broadly affect membrane organization, signaling, and metabolism. These alterations may increase membrane fluidity or expansion at baseline, thereby contributing to the larger surface area of ACC1-deficient platelets, while compromising the cytoskeletal remodeling required for full spreading upon activation.
Because exogenous AA is typically incorporated into phospholipids through fatty acid remodeling rather than de novo synthesis, further investigation is needed to elucidate ACC1-dependent mechanisms involved in the specific remodeling of the platelet phospholipidome. One hypothesis is that ACC1 deficiency could disrupt transacylation reactions required for the proper AA distribution across phospholipid pools, a process mediated by CoA-independent transacylase transferring AA from diacyl PC to vinyl-ether species such as PEP, which are key free AA reservoirs for eicosanoid synthesis.17,45 In support of this hypothesis, the reduction in PEP levels in ACC1 pKO platelets may reflect impaired ether lipid metabolism, which begins in peroxisomes. Although anucleate, platelets retain peroxisomal enzymes and express peroxisome proliferator-activated receptor α, a regulator of peroxisome-related lipid pathways.46-49 PE-plasmalogens support membrane architecture and oxidative homeostasis, and act as reservoirs for PUFAs such as AA (TxA2 precursor), and for PAF biosynthesis.50,51 Therefore, ACC1 deletion may limit lipid substrates required for TxA2 and PAF production, affecting platelet reactivity and inflammatory responses.
In addition to the reductions in saturated fatty acyl chains-containing PC and AA-containing PEP, we observed an increase in PUFAs within PC and PE classes. This pattern is consistent with findings in endothelial and cancer cells treated with ACC inhibitors, in which similar shifts in lipid composition have been linked to decreased membrane fluidity and integrity.26-28 PUFA influence on plasma membrane organization has also been demonstrated in platelets.1,52 Moreover, PUFAs participate in thromboinflammatory signaling pathways.53 Indeed, AA, docosahexaenoic acid, and eicosapentaenoic acid serve as precursors for inflammatory lipid mediators. Thus, the upregulation of PUFA-containing PC and PE in ACC1 pKO platelets is susceptible to influence thromboinflammatory responses, further highlighting the impact of ACC1 deficiency on platelet functions. Furthermore, ACC1 deficiency altered platelet sphingolipid content, primarily sphingomyelin and CER, which, together, account for ∼10% to 13% of the total dehydrated platelet mass.54 Increased CER levels, although not directly affecting hemostasis or thrombosis, have been associated with proinflammatory cytokine secretion and enhanced P-selectin–mediated platelet-leukocyte aggregation.55 Elevated CER levels in ACC1-deficient platelets might therefore contribute to a more inflammatory platelet profile, further supporting the role of ACC1 in modulating platelet-mediated inflammation.
Beyond lipid signaling, glycolysis is increasingly recognized as critical for platelet activation. Targeting metabolic enzymes such as pyruvate dehydrogenase kinases and pyruvate kinase M2 inhibits platelet activation and thrombosis by reducing aerobic glycolysis.56-58 Our results suggest glycolytic impairment in ACC1-deficient platelets, both in basal and activated conditions, although the exact mechanism remains unclear. Interestingly, studies in yeast, liver cells, and cancer cells showed that ACC1 deficiency leads to protein hyperacetylation, including of histones, cytoskeletal, and metabolic proteins.56-58 In line with this, our previous work demonstrated that pharmacological ACC inhibition limits α-tubulin deacetylation upon thrombin stimulation, impairing platelet aggregation.22 Future research should explore whether ACC1 deficiency affects glycolytic enzyme acetylation in platelets, with possible impacts on enzymatic activity and energy metabolism.
Regarding mitochondrial bioenergetics, ACC1 pKO platelets displayed unchanged basal and ATP-linked mitochondrial respiration but enhanced respiratory reserve capacity upon activation. This coincided with reduced PS exposure and protection against arterial thrombosis without impairing hemostasis. Notably, a similar phenotype occurs in 14-3-3ζ-deficient platelets,59 suggesting a shared mechanism. Although the link between ACC1 and respiratory reserve remains unclear, the enhancement may result from (1) increased endogenous substrate availability due to altered lipid metabolism, which may fuel the respiratory chain more effectively; and/or (2) mitochondrial remodeling, involving specific phospholipids. It is well established that specific phospholipids are essential for mitochondrial function.60,61 For example, cardiolipin supports respiratory reserve in various cells by stabilizing the electron transport chain and optimizing electron flux under high-energy demands.62,63 Exploring a potential ACC1-cardiolipin interaction could offer insights into how ACC1 regulates platelet bioenergetics, procoagulant activity, and thrombosis. Finally, the enhanced respiratory capacity may also reflect a metabolic shift compensating for impaired lipogenesis. Platelets could rely more on glucose oxidation, CD36-mediated fatty acid uptake, or amino acid catabolism to sustain mitochondrial function, an area warranting future investigation through metabolic flux analysis.
In conclusion, our study highlights the critical role of ACC1 in platelet function through the regulation of specific phospholipid pools required for membrane architecture, activation, and energy metabolism. We demonstrate that ACC1 is a key regulator of thrombosis while maintaining baseline platelet reactivity, which is essential for hemostasis. These findings provide a conceptual advance in understanding the physiological relevance of platelet ACC1 and establish this metabolic enzyme as a promising target for antithrombotic therapies.
Acknowledgments
The authors thank Emmanuel Vandenhooft for his dedicated animal care.
This work was supported by grants from the Fonds National de la Recherche Scientifique et Médicale (FNRS, Brussels, Belgium; grant J.0150.18) and the Action de Recherche Concertée of the Wallonia-Brussels Federation, Brussels, Belgium (ARC 23/28-132). M.O. received support from the Fonds pour la formation à la Recherche dans l’Industrie et dans l’Agriculture (FRIA, Belgium) and a Bourse du Patrimoine (Université catholique de Louvain, Belgium). L.P. and E.d.C.d. were supported by fellowships from the FRIA and FNRS, respectively. S.H. is an FNRS senior research associate.
Authorship
Contribution: M.O. and L.P. performed the experiments, analyzed the data, and wrote and edited the manuscript; E.C.Y. wrote and edited the manuscript; A.M. performed the experiments and acquired and analyzed the data, especially for the arterial thrombosis studies; J.A. carried out the biostatistical and lipidomic data analysis; M.G. and B.G. performed the experiments and acquired and analyzed the data, especially for the lipidomic studies; A.G. and V.R. performed the experiments and acquired and analyzed the data, especially for flow cytometry analyses; M.K., C. Baaten, and J.W.M.H. performed the experiments and acquired and analyzed the data, especially for the flow chambers studies; Z.N. and Y.S. generously provided the GPIbα-Cre mice; D.B. and C. Bouzin provided experimental and conceptual advice on flow cytometry and immunohistochemistry experiments, respectively; L.B. secured funding, discussed results and their implications, and edited the manuscript; C. Beauloye conceived and designed the study, secured funding, discussed results and their implications, and edited the manuscript; S.H. conceived and designed the study, secured funding, discussed results and their implications, and wrote and edited the manuscript; and all authors read and approved the final manuscript before submission.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Sandrine Horman, Pôle de Recherche Cardiovasculaire, Institut de Recherche Expérimentale et Clinique, Université Catholique de Louvain, 55 Ave Hippocrate, 1200 Brussels, Belgium; email: sandrine.horman@uclouvain.be.
References
Author notes
M.O. and L.P. contributed equally to this study and are joint first authors.
The data set will be made available upon request through contact with the corresponding author, Sandrine Horman (sandrine.horman@uclouvain.be).
The full-text version of this article contains a data supplement.

![ACC1 deficiency in platelets had an impact on both mitochondrial reserve capacity and glycolytic activity. OCR was measured in washed murine platelets from ACC1flx/flx or pKO mice. (A-B) OCR was assessed under basal conditions, after stimulation with 1 U/mL Thr, and after sequential treatment with 1 μM oligomycin (O), 0.4 μM carbonyl cyanide p-trifluoromethoxyphenylhydrazone (F), and a mix of 1 μM AtA/R. (A) Representative OCR profiles are shown as mean ± SEM (n = 4). (B) Quantification of mitochondrial respiratory parameters including basal (baseline OCR – R/AtA-sensitive OCR), Thr (Thr response − baseline OCR), ATP-linked (thrombin response − oligomycin sensitive OCR), maximal (FCCP [carbonyl cyanide-p-trifluoromethoxyphenylhydrazone]-sensitive – R/AtA-sensitive OCR), reserve capacity (FCCP-sensitive – Thr-responsive OCR), proton leak (oligomycin-sensitive – R/AtA-sensitive OCR), and nonmitochondrial (R/AtA-sensitive OCR). Results are expressed as mean ± SEM (n = 4). (C-D) ECAR, used as an indirect indicator of glycolytic activity, was measured in the same experimental conditions. (C) Representative ECAR profiles are shown as mean ± SEM (n = 4). (D) Quantification of basal and Thr-induced ECAR. Results are expressed as mean ± SEM (n = 4). ∗P ≤ .05 relative to unstimulated conditions. #P ≤ .05 relative to ACC1flx/flx mice. Statistical analysis was performed using 2-way ANOVA. Ata, antimycin A; DMEM, Dulbecco’s modified eagle medium; NS, nonstimulated; R, rotenone; Thr, thrombin.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/9/18/10.1182_bloodadvances.2025015796/2/m_blooda_adv-2025-015796-gr3.jpeg?Expires=1765041826&Signature=SbJoHAhtmP1GVY5VP5Q9pv4eQfvxpuPx5dm1W6UthwCP5iFN1n3WrwG4STqIcmnNhtxHYMj~34Gal-UOxTg4fAEBa~tWl0hhl9~7yMWYnUPAgh0YX5m8NRhCu1idGB9YAL-CgU9OLseQTf0caSTXLq-mDV534vEXaI0D9cGaGhowcOs4J8stBQ338uVBgKYyVUhjJgfGlBoUtSDUN3wgeYOqX7pR4BMBEkI9p8LxqkS4bENP9wMhX01qJ3HfFOemRAI1K1UKvrPLLkAGxjhHMbAVlBHLzoY8i9i9Je8r2a3d2kgobV1WOfK-X5PUFaN99PzvfrCA1BN~ujFl7wzG-A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)