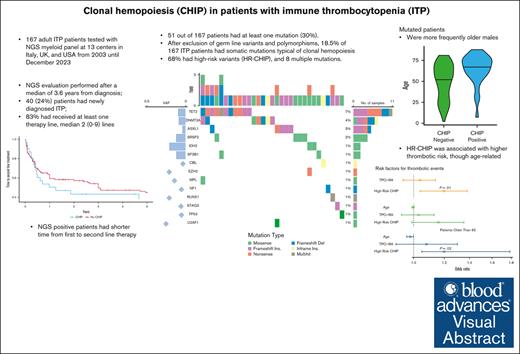
Key Points
Of 167 patients with ITP, 18.5% had somatic mutations typical of clonal hemopoiesis, 68% high-risk variants, and 8 multiple mutations.
Mutated patients were more frequently older males, with a shorter time from first- to second-line therapy, and had a higher thrombotic risk.
Visual Abstract
Diagnostic boundaries between immune thrombocytopenia (ITP) and other thrombocytopenic states, such as thrombocytopenic myelodysplastic syndromes, may be difficult to establish, and the detection of somatic mutations by next-generation sequencing (NGS) may be of aid. Here, we aimed at characterizing the prevalence and clinical significance of clonal hematopoiesis in ITP. In this multicentric retrospective observational study, we enrolled 167 adult patients with ITP, followed at 13 centers in Italy, United Kingdom, and the United States. Patients underwent NGS evaluation after a median of 3.6 years from ITP onset, and 83% had received at least 1 therapy line, for a median of 2 lines (range, 0-9); 51 of 167 patients (30%) had at least 1 mutation. After exclusion of germ line variants and polymorphisms, 31 of 167 (18.5%) were defined as having clonal hemopoiesis. Most commonly mutated genes were TET2, DNMT3A, SRSF2, and ASXL1 (median variant allele frequency, 29%); 19 of 31 patients (68%) had high-risk variants, and 8 had multiple mutations. Mutated patients were more frequently older males and showed a shorter time from first to second-line therapy, particularly with thrombopoietin receptor agonist (TPO-RA). Additionally, clonal hematopoiesis was associated with increased thrombotic risk (26% vs 8% in NGS-negative cases; P = .01), independently from TPO-RA exposure, though with an age effect. These data demonstrated the prevalence of clonal hematopoiesis in 18% of adult patients with ITP, which is associated with older age, relapsed/refractory disease, and high risk of thrombotic complications.
Introduction
Immune thrombocytopenia (ITP) is caused by an immune-mediated attack on platelets and bone marrow precursors. ITP can be either primary or secondary to other disorders, such as systemic autoimmune and lymphoproliferative disorders, infections, or drugs, among others.1 Diagnostic boundaries between ITP and other thrombocytopenic states, such as idiopathic cytopenias of unknown significance, thrombocytopenic myelodysplastic syndromes (MDSs), and hypomegakaryocytic thrombocytopenia, may be difficult to establish. In fact, ITP is a diagnosis of exclusion, and dysplastic/dystrophic megakaryocytes may be present both in ITP and in the above-mentioned conditions.2,3 In the genomic era, the presence of somatic mutations typical of myeloid neoplasms by next-generation sequencing (NGS) is increasingly recognized in cytopenic conditions, with a possible role in clonal evolution.4 However, such mutations may be found even in the general population, namely clonal hematopoiesis of indeterminate potential (CHIP), as well as in cytopenic patients without definite myeloid neoplasms, namely clonal cytopenia of unknown significance.5 The prevalence and clinical significance of clonal hematopoiesis in ITP is not known. Here, we investigated the use of NGS myeloid panel in adult patients with ITP to evaluate the prevalence and clinical impact of clonal hematopoiesis in this setting.
Methods
This was a multicentric retrospective observational study in adult patients with ITP, followed at 13 centers in Italy, United Kingdom, and the United States from 2003 until Decembre 2023. Inclusion criteria were as follows: diagnosis of ITP as per current guidelines,1 availability of NGS data (myeloid panel of >50 genes, including hot spot genes ABL1, BRAF, CBL, CSFR3, DNMT3A, FLT3, GATA2, HRAS, IDH1, IDH2, JAK2, KIT, KRAS, MOL, MYD88, NPM1, NRAS, PTPN11, SETPB1, SF3B1, SRSF2, U2AF1, and WT1; full genes ASXL1, BCOR, CALR, CEBPA, ETV6, EZH2, IKZF1, NF1, PHF6, PRPF8, RB1, RUNX1, SH2B3, STAG2, TET2, TP53, and ZRSR2; and fusion genes ABL1, ALK, BCL2, BRAF, CCDN1, CREBBP, EGFR, ETV6, FGFR1, FGFR2, FUS, HMGA2, JAK2, KMT2A, MECOM, MET, MLLT10, MLLT3, MYBL1, MYH11, NTRK3, NUP214, PDGFRA, PDGFRB, RARA, RBM15, RUNX1, TCF3, and TFE3), and availability of hematologic and clinical data on ITP treatment and outcomes. Patients with ITP associated with a definite diagnosis of myeloid neoplasm were excluded. Patients gave informed consent, and the study was conducted according to the Declaration of Helsinki.
All centers performed NGS analysis from DNA extracted from peripheral blood granulocytes on Illumina-based platforms. The targeted gene panel included coding exons and splice sites of genes involved in myeloid malignancies based on literature data. Variants were annotated as pathogenic, likely pathogenic, or variants of unknown significance based on available literature and query of publicly available databases (Catalogue of Somatic Mutations in Cancer). Mutations with variant allele frequencies (VAFs) as low 2% were reported, and the median coverage was 500.
Mutations were further screened to evaluate their somatic nature and pathogenicity according to a stringent pipeline in use at the coordinating center (University of Milan). Briefly, somatic mutations were identified accepting a minimum variant coverage of 20 and a VAF of at least 0.02 and a maximum of 0.45 in concordance with established variant filtering algorithms; the only exception was JAK2 V617F mutation, for which no VAF limit was set.6-8 Prevalence of CHIP, type of mutations, and VAF were analyzed, with particular regard to variants of DNMT3A, TET2, and ASXL1 (DTA mutations) and to those considered at high risk for myeloid neoplasm transformation, high-risk CHIP (HR-CHIP). The definition of HR-CHIP was borrowed from 2 recent papers identifying high-risk and silent clonal hematopoietic genotypes in patients with nonhematologic cancer as well as in healthy people. HR-CHIP mutations as per Stonestrom et al were ASXL1, TET2, TP53, KMT2D, ERBB4, SUZ12, JAK2, RUNX1, and XP01; HR-CHIP mutations as per Weeks et al4 were SRSF2, SF3B1, ZRSR2, JAK2, RUNX1, and IDH2.9,10 The association of CHIP with clinical and laboratory features of ITP were analyzed focusing on demographics, clinical and hematologic characteristics at ITP diagnosis and at the time of NGS, thrombocytopenia severity, therapy lines (steroids, rituximab, splenectomy, thrombopoietin receptor agonists, fostamatinib, and cytotoxic immunosuppressors) and their response (attainment of platelet >50 × 109/L with no bleeding), thrombotic complications, and survival. Bone marrow trephine results were systematically collected, with a special attention to the presence of age-adjusted hypercellularity or hypocellularity, aberrant cytogenetics, dysplastic features, lymphoid infiltrates, and bone marrow fibrosis.
For statistical analysis, we calculated descriptive parameters (mean median, range, and interquartile range) for demographic, clinical, and laboratory data. Continuous variables were evaluated by Student t test or Wilcoxon Mann-Whitney test if Gaussian distribution was not present. For categorical variables, their frequency was calculated and analyzed by χ2 test or Fisher test, based on the number of samples.
Results
Demographic and hematologic features of patients at diagnosis
Among the participating centers, ∼1820 patients with ITP were on follow-up during the study period. Overall, 173 patients were screened. The reason for NGS testing in patients with chronic ITP was the differential diagnosis with myeloid neoplasms during bone marrow evaluation in the comprehensive workup of chronic cytopenia; in those patients tested at diagnosis, NGS was performed during the comprehensive workup of idiopathic cytopenia. Six patients were found to have an associated myeloid neoplasm: (1) with MDS with ring-sideroblasts, (2) with chronic myelomonocytic leukemia (SF3B1 and SRSF2 mutated), and (3) with a myeloproliferative disease (2 JAK2 and 1 CALR mutated); and they were, therefore, excluded from further analysis. Table 1 shows the clinical and hematologic features of the remaining 167 patients. At ITP diagnosis, male-to-female ratio was 1.22, and the median age was 57 years (range, 18-88). The median follow-up of the cohort was 5.5 years (range, 1-25), and the median platelet count at onset was 34 × 109/L (range, 1× 109/L to 99 × 109/L); 39% of patients had severe thrombocytopenia. Of note is that 39% of patients had a condition associated with ITP, mainly autoimmune diseases (n = 55); other conditions included Ewing sarcoma, renal transplant (n = 2), thalassemia trait, eradicated hepatitis C virus (n = 3), breast cancer, eradicated Helicobacter pylori, and type 2 diabetes mellitus. Twenty patients (12%) had a concomitant autoimmune hemolytic anemia (n = 13), namely Evans syndrome, or autoimmune neutropenia (n = 7).
Clinical and hematologic features of patients with ITP included in the study
| Demographics and hematologic parameters at diagnosis . | All patients (N = 167) . | NGS pos (n = 31) . | NGS neg (n = 136) . |
|---|---|---|---|
| Median age (IQR), y | 57 (36-69) | 68 (60-77)∗ | 55 (32-67) |
| Males, n (%) | 92 (55%) | 22 (71%)∗ | 70 (51%) |
| Females, n (%) | 75 (45%) | 9 (29%) | 66 (49%) |
| Associated conditions, n (%) | 65 (39%) | 15 (48%) | 50 (37%) |
| Median follow-up (IQR), y | 5.5 (2-14) | 5.3 (3-16) | 5.5 (2-14) |
| Platelets, median (range), ×109/L | 34 (1-99) | 34 (1-99) | 34 (1-99) |
| Hb, median (range), g/dL | 13.9 (3.8-17.2) | 13.9 (6.3-16.9) | 13.9 (3.8-17.2) |
| Neutrophils, median (range), ×109/L | 3.7 (0.1-16.2) | 3.5 (1.3-8.8) | 3.8 (0.1-16.2) |
| Severe thrombocytopenia (PLT <30 × 103/μL), n (%) | 66 (40%) | 12 (39%) | 54 (40%) |
| Demographics and hematologic parameters at NGS | All patients (N = 167) | ||
| Median age (IQR), y | 63 (52-75) | 77 (67-80)∗ | 59 (50-72) |
| Years from diagnosis to NGS, median (IQR) | 3.6 (0.6-12.8) | 4.9 (1.3-14.5) | 3.6 (0.5-11.6) |
| Platelets, median (range), ×109/L | 50 (1-164) | 37 (3-139) | 53 (1-164) |
| Hb, median (range), g/dL | 13.3 (6.2-16.6) | 12.1 (7.6-16.3) | 13.5 (6.2-16.6) |
| Neutrophils, median (range), ×109/L | 3.9 (0.1-65) | 3.6 (0.3-11.5) | 3.9 (0.1-65) |
| Reticulocytes, median (range), ×109/L | 80 (1 – 292) | 64 (0.2-292) | 83 (2-286) |
| BM evaluation | n = 148 | n = 27 | n = 121 |
| BM cellularity percent, median (IQR) | 45 (30-55) | 40 (30-55) | 45 (30-54) |
| Hypocellularity, n (%) | 31 (21%) | 2 (7%) | 29 (24%) |
| Hypercellularity, n (%) | 46 (31%) | 13 (48%) | 33 (27%) |
| Dysplasia, n (%) | 72 (49%) | 13 (48%) | 59 (49%) |
| Reticulin fibrosis MF-1, n (%) | 42 (28%) | 8 (30%) | 34 (28%) |
| Cytogenetic aberrations, n (%) | 9 (6%) | 1 (4%) | 8 (7%) |
| Demographics and hematologic parameters at diagnosis . | All patients (N = 167) . | NGS pos (n = 31) . | NGS neg (n = 136) . |
|---|---|---|---|
| Median age (IQR), y | 57 (36-69) | 68 (60-77)∗ | 55 (32-67) |
| Males, n (%) | 92 (55%) | 22 (71%)∗ | 70 (51%) |
| Females, n (%) | 75 (45%) | 9 (29%) | 66 (49%) |
| Associated conditions, n (%) | 65 (39%) | 15 (48%) | 50 (37%) |
| Median follow-up (IQR), y | 5.5 (2-14) | 5.3 (3-16) | 5.5 (2-14) |
| Platelets, median (range), ×109/L | 34 (1-99) | 34 (1-99) | 34 (1-99) |
| Hb, median (range), g/dL | 13.9 (3.8-17.2) | 13.9 (6.3-16.9) | 13.9 (3.8-17.2) |
| Neutrophils, median (range), ×109/L | 3.7 (0.1-16.2) | 3.5 (1.3-8.8) | 3.8 (0.1-16.2) |
| Severe thrombocytopenia (PLT <30 × 103/μL), n (%) | 66 (40%) | 12 (39%) | 54 (40%) |
| Demographics and hematologic parameters at NGS | All patients (N = 167) | ||
| Median age (IQR), y | 63 (52-75) | 77 (67-80)∗ | 59 (50-72) |
| Years from diagnosis to NGS, median (IQR) | 3.6 (0.6-12.8) | 4.9 (1.3-14.5) | 3.6 (0.5-11.6) |
| Platelets, median (range), ×109/L | 50 (1-164) | 37 (3-139) | 53 (1-164) |
| Hb, median (range), g/dL | 13.3 (6.2-16.6) | 12.1 (7.6-16.3) | 13.5 (6.2-16.6) |
| Neutrophils, median (range), ×109/L | 3.9 (0.1-65) | 3.6 (0.3-11.5) | 3.9 (0.1-65) |
| Reticulocytes, median (range), ×109/L | 80 (1 – 292) | 64 (0.2-292) | 83 (2-286) |
| BM evaluation | n = 148 | n = 27 | n = 121 |
| BM cellularity percent, median (IQR) | 45 (30-55) | 40 (30-55) | 45 (30-54) |
| Hypocellularity, n (%) | 31 (21%) | 2 (7%) | 29 (24%) |
| Hypercellularity, n (%) | 46 (31%) | 13 (48%) | 33 (27%) |
| Dysplasia, n (%) | 72 (49%) | 13 (48%) | 59 (49%) |
| Reticulin fibrosis MF-1, n (%) | 42 (28%) | 8 (30%) | 34 (28%) |
| Cytogenetic aberrations, n (%) | 9 (6%) | 1 (4%) | 8 (7%) |
BM, bone marrow; Hb, hemoglobin; IQR, interquartile range; MF-1, marrow fibrosis 1; neg, negative; PLT, platelet count; pos, positive.
P < .05.
Demographic and hematologic features of patients at NGS evaluation
Patients underwent NGS evaluation after a median of 3.6 years from ITP onset, at a median age of 64 years (range, 53-75). Overall, 40 patients (24%) were tested within 6 months of diagnosis (newly diagnosed ITP), of whom 14 (8%) were tested within the first month. At the time of NGS testing, the median platelet count was 50 × 109/L (range, 1× 109/L to 164 × 109/L), and 46 patients (27%) had severe thrombocytopenia.
Bone marrow features
Overall, 148 patients received a bone marrow evaluation within 1 year from NGS test (Table 1). The median cellularity was 45% (range, 8%-90%), with 31 (21%) showing hypocellularity and 50 (31%) showing hypercellularity; 72 (49%) had dysplastic features, and 42 (28%) had a fibrosis grade marrow fibrosis 1. Dysplastic features mainly involved erythroid and megakaryocytic lineages; however, all interested <10% of each lineage; otherwise, the patients would have been diagnosed as MDS and excluded from the study. A lymphoid infiltrate was present in 93 patients (63%), with a median size of 7% (range, 2%-30%), mostly with a T-cell phenotype (56%), followed by mixed B/T phenotype (42%) and B-cell phenotype (3%). Nine patients displayed cytogenetic alterations: 5 with delY, 2 with del20q, 1 with t(X;20), and 1 with 47, XXY.
Treatments performed at the time of NGS evaluation
At the time of NGS testing, 83% of patients had received at least 1 therapy line, with a median of 2 lines (range, 0-9), for their ITP, including steroids, rituximab, thrombopoietin receptor agonists, splenectomy, fostamatinib, and cytotoxic immunosuppressors. Number of treatments and relative responses are depicted in Table 2. Overall, response rates were comparable with those reported in the literature for each treatment.
Therapies performed for ITP at the time of NGS evaluation
| . | All patients (N = 167) . | NGS pos (n = 31) . | NGS neg (n = 136) . |
|---|---|---|---|
| No. of therapy lines, median (range) | 2 (0-9) | 2 (0-6) | 2 (0-9) |
| Untreated, n (%) | 29 (17%) | 6 (19%) | 23 (17%) |
| 1 line, n (%) | 28 (17%) | 3 (10%) | 25 (18%) |
| 2 lines, n (%) | 46 (28%) | 11 (35.5%) | 35 (26%) |
| ≥3 lines, n (%) | 64 (38%) | 11 (35.5%) | 53 (39%) |
| Corticosteroids, n (%) | 138 (83%) | 25 (81%) | 113 (83%) |
| CR/PR | 64 (46%)/33 (24%) | 9 (36%)/6 (24%) | 55 (49%)/27 (24%) |
| Eltrombopag, n (%) | 75 (45%) | 15 (48%) | 60 (44%) |
| CR/PR | 40 (53%)/15 (20%) | 5 (33%)/5 (33%) | 35 (58%)/10 (17%) |
| Romiplostim, n (%) | 40 (24%) | 9 (29%) | 31 (23%) |
| CR/PR | 19 (48%)/11 (28%) | 3 (33%)/2 (22%) | 16 (52%)/9 (29%) |
| Avatrombopag, n (%) | 14 (8%) | 2 (6%) | 12 (9%) |
| CR/PR | 3 (21%)/0 (0%) | 1 (50%)/0 (0%) | 2 (17%)/0 (0%) |
| Rituximab, n (%) | 39 (23%) | 6 (19%) | 33 (24%) |
| CR/PR | 13 (33%)/7 (18%) | 6 (100%)/0 (0%) | 7 (21%)/7 (21%) |
| Splenectomy, n (%) | 24 (14%) | 3 (10%) | 21 (15%) |
| CR/PR | 9 (38%)/5 (21%) | 0 (0%)/1 (33%) | 9 (43%)/4 (19%) |
| Fostamatinib, n (%) | 22 (13%) | 5 (16%) | 17 (13%) |
| CR/PR | 8 (36%)/2 (9%) | 3 (60%)/1 (20%) | 5 (29%)/1 (6%) |
| Immunosuppressors, n (%) | 38 (23%) | 4 (13%) | 34 (25%) |
| CR/PR | 6 (16%)/10 (26%) | 0 (0%)/0 (0%) | 6 (18%)/10 (29%) |
| IV immunoglobulin, n (%) | 88 (53%) | 20 (6%) | 68 (50%) |
| CR/PR | 40 (45%)/23 (26%) | 7 (35%)/6 (30%) | 33 (49%)/17 (25%) |
| Other therapies, n (%) | 12 (7%) | 3 (10%) | 9 (7%) |
| . | All patients (N = 167) . | NGS pos (n = 31) . | NGS neg (n = 136) . |
|---|---|---|---|
| No. of therapy lines, median (range) | 2 (0-9) | 2 (0-6) | 2 (0-9) |
| Untreated, n (%) | 29 (17%) | 6 (19%) | 23 (17%) |
| 1 line, n (%) | 28 (17%) | 3 (10%) | 25 (18%) |
| 2 lines, n (%) | 46 (28%) | 11 (35.5%) | 35 (26%) |
| ≥3 lines, n (%) | 64 (38%) | 11 (35.5%) | 53 (39%) |
| Corticosteroids, n (%) | 138 (83%) | 25 (81%) | 113 (83%) |
| CR/PR | 64 (46%)/33 (24%) | 9 (36%)/6 (24%) | 55 (49%)/27 (24%) |
| Eltrombopag, n (%) | 75 (45%) | 15 (48%) | 60 (44%) |
| CR/PR | 40 (53%)/15 (20%) | 5 (33%)/5 (33%) | 35 (58%)/10 (17%) |
| Romiplostim, n (%) | 40 (24%) | 9 (29%) | 31 (23%) |
| CR/PR | 19 (48%)/11 (28%) | 3 (33%)/2 (22%) | 16 (52%)/9 (29%) |
| Avatrombopag, n (%) | 14 (8%) | 2 (6%) | 12 (9%) |
| CR/PR | 3 (21%)/0 (0%) | 1 (50%)/0 (0%) | 2 (17%)/0 (0%) |
| Rituximab, n (%) | 39 (23%) | 6 (19%) | 33 (24%) |
| CR/PR | 13 (33%)/7 (18%) | 6 (100%)/0 (0%) | 7 (21%)/7 (21%) |
| Splenectomy, n (%) | 24 (14%) | 3 (10%) | 21 (15%) |
| CR/PR | 9 (38%)/5 (21%) | 0 (0%)/1 (33%) | 9 (43%)/4 (19%) |
| Fostamatinib, n (%) | 22 (13%) | 5 (16%) | 17 (13%) |
| CR/PR | 8 (36%)/2 (9%) | 3 (60%)/1 (20%) | 5 (29%)/1 (6%) |
| Immunosuppressors, n (%) | 38 (23%) | 4 (13%) | 34 (25%) |
| CR/PR | 6 (16%)/10 (26%) | 0 (0%)/0 (0%) | 6 (18%)/10 (29%) |
| IV immunoglobulin, n (%) | 88 (53%) | 20 (6%) | 68 (50%) |
| CR/PR | 40 (45%)/23 (26%) | 7 (35%)/6 (30%) | 33 (49%)/17 (25%) |
| Other therapies, n (%) | 12 (7%) | 3 (10%) | 9 (7%) |
CR, complete response; neg, negative; pos, positive; PR, partial response.
Results of NGS analysis
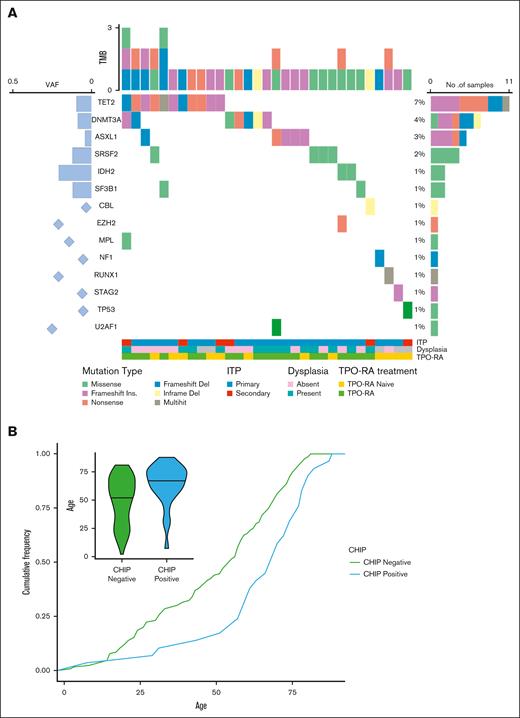
By NGS analysis, 51 of 167 patients had at least 1 mutation (30%). After exclusion of germ line variants and polymorphisms, 31 of 167 (18.5%) were defined as having clonal hemopoiesis (supplemental Table 1). As shown in Figure 1, most commonly mutated genes were TET2, DNMT3A, SRSF2, and ASXL1, with a median VAF of 29% (range, 2.6%-39%); 19 of 31 patients (68%) had a HR-CHIP; 8 patients had multiple mutations, and 2 harbored 3 mutations. Notably, no germ line variants known to be causative for inherited thrombocytopenic diseases were detected (variants with VAF >40% were removed). Additionally, no gene fusions were detected. NGS-positive patients were significantly older than NGS-negative ones (median age, 77 years [range, 18-89] vs 59 years [range, 18-84]; P = .05) and were more frequently males (22/31 [70%]). No differences were noted according to the presence of associated conditions, bone marrow (BM) features (ie, hypercellularity, dysplasia, or reticulin fibrosis), or exposure to thrombopoietin receptor agonist (TPO-RA) at the time of NGS.
Prevalence and type of somatic mutations in patients with ITP. (A) Oncoplot showing mutated genes, frequency, and allelic burden. (B) Association of clonal hemopoiesis with age in terms of cumulative incidence over age. The detail shows the age distribution in patients bearing CHIP mutations. TMB, total mutation burden.
Prevalence and type of somatic mutations in patients with ITP. (A) Oncoplot showing mutated genes, frequency, and allelic burden. (B) Association of clonal hemopoiesis with age in terms of cumulative incidence over age. The detail shows the age distribution in patients bearing CHIP mutations. TMB, total mutation burden.
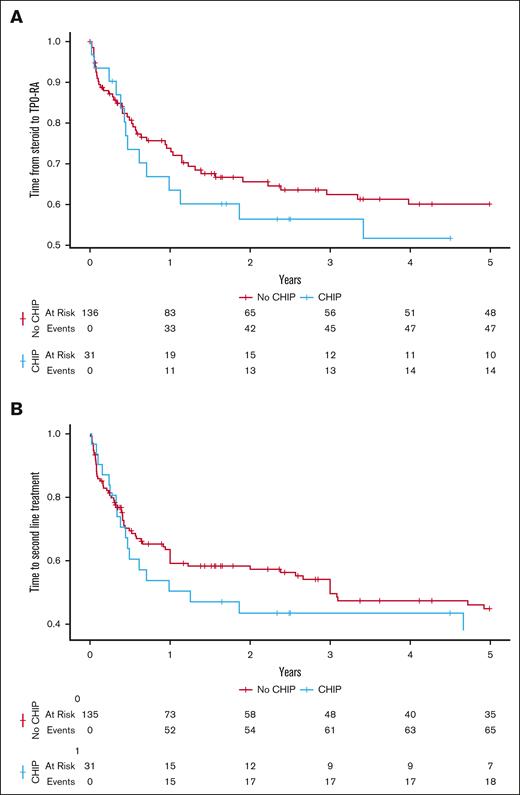
Interestingly, the time from first-line steroid therapy to TPO-RA treatment was shorter in NGS-positive vs NGS-negative patients, although it was not significant. Similarly, NGS-positive patients experienced a shorter time from diagnosis to second-line therapy, although this was not significant (Figure 2). Only younger age and platelet count <30 × 109/L at diagnosis had an impact by multivariable analysis. Finally, no associations with treatment type, responses, or the cumulative number of therapy lines at NGS testing were observed.
Impact of clonal hematopoiesis on the time from diagnosis to thrombopoietin receptor agonist (TPO-RA) and to second-line treatment. (A) Time to thrombopoietin receptor agonists treatment according to NGS positivity for CHIP mutations. (B) Time to second-line treatment (any treatment option) in patients with ITP according to NGS positivity for CHIP mutations.
Impact of clonal hematopoiesis on the time from diagnosis to thrombopoietin receptor agonist (TPO-RA) and to second-line treatment. (A) Time to thrombopoietin receptor agonists treatment according to NGS positivity for CHIP mutations. (B) Time to second-line treatment (any treatment option) in patients with ITP according to NGS positivity for CHIP mutations.
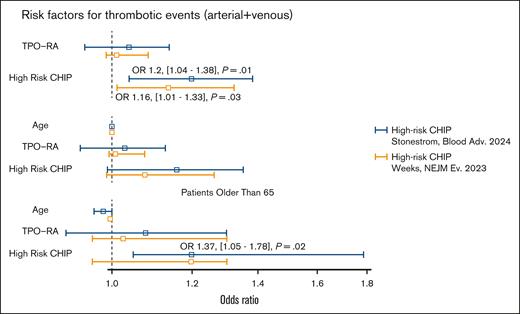
During a median follow-up of 5 years (similar for NGS-positive/negative cases), 17 patients (10%) experienced a thrombotic complication, including 6 arterial and 11 venous. Among arterial events, we observed 3 myocardial infraction, 2 strokes, and 1 femoral artery thrombosis. Among venous events, we registered 11 deep venous thrombosis (10 thrombosis of lower limbs, 2 of which complicated by pulmonary embolism; and 1 thrombosis of splanchnic veins). Notably, patients with HR-CHIP were at higher risk of developing thrombosis than those without (5/19 [26%] vs 12/148 [8%]; P = .01; odds ratio [OR], 1.2; 95% confidence interval [CI], 1.04-1.38, considering HR-CHIP by Stonestrom et al; 5/17 [29%] vs 12/148 [8%]; P = .03; OR, 1.16; 95% CI, 1.01-1.33, considering HR-CHIP by Weeks et al4,9; Figure 3). Multivariable analysis showed that the occurrence of thrombosis in patients with HR-CHIP was independent from TPO-RA treatment. However, when considering age and TPO-RA treatment in the model, the impact of HR-CHIP on thrombosis risk was lost. Conversely, when analyzing only patients aged ≥65 years, we confirmed the independent effect of HR-CHIP on thrombotic risk (OR, 1.37; 95% CI, 1.05-1.78; P = .02, considering HR-CHIP by Stonestrom et al).9 During the follow-up, 13 patients died, none because of ITP. No associations were observed between mortality and NGS positivity.
Association of high-risk clonal hemopoiesis with thrombosis in patients with ITP by multivariable analysis. High-risk clonal hemopoiesis as interpreted according to Stonestrom et al9 (blue lines) and Weeks et al4 (orange lines).
Discussion
In this study, we evaluated, to our knowledge, for the first time the prevalence and possible clinical impact of clonal hematopoiesis in adult patients with ITP. We found a not negligible prevalence of 18%, being associated with older age and male gender. Interestingly, NGS-positive patients showed a trend toward a shorter interval from the first to further therapy lines, particularly TPO-RA, suggesting a possible role of clonal hematopoiesis in the development of more refractory/relapsing disease.
The most novel finding is the association of somatic mutations of CHIP with thrombotic risk in ITP, independently from TPO-RA use, although with an age effect. In particular, carriers of HR-CHIP had more than threefold rate of arterial and venous thrombosis than noncarriers, highlighting the possible impact of clonal hematopoiesis on the clinical severity of the disease. These data are also consistent with CHIP being associated with increased cardiovascular risk in the general population.10 Given that ITP is considered a thrombophilic disease and that prothrombotic treatments such as TPO-RA and splenectomy may be used, the identification of predictors of thrombosis, including NGS, deserves further investigation.11
The question arises as to whether NGS analysis might be useful in informing the differential diagnosis between ITP and myelodysplastic/aplastic conditions. In fact, no test is sensitive/specific enough for ITP diagnosis, and BM evaluation is not always diagnostic. In this analysis, two-third of patients had BM dysplasia, and one-third had reticulin fibrosis; and no associations were found with NGS data. However, the 5 patients excluded due to a concomitant diagnosis of myeloid neoplasms harbored typical mutations by NGS. Therefore, in line with the latest international consensus,1 older patients or those not responding to corticosteroids or IV immunoglobulin may benefit from NGS analysis to unravel underlying myeloid neoplasm.
Given the association with older age, one may speculate whether patients with ITP with clonal hemopoiesis are “early MDS” or just harbor “age-related clonal hemopoiesis.” The type of mutations was similar, most of the cases harbored HR-CHIP, and VAFs were also heterogeneous; thus, a longer and prospective follow-up, as well as a dynamic hematologic, histopathologic, and molecular re-evaluation, would be required to answer this question.
With NGS panels becoming increasingly available, their correct interpretation is becoming an issue. In our analysis that used NGS reports by several laboratories, 113 mutations were reported in 51 patients, and after applying a stringent pipeline, 41 mutations in 31 patients only were considered as clonal hematopoiesis, claiming the need for standardization.
From a pathogenic point of view, it may be speculated that, in ITP, somatic mutations may arise through a clonal selection related to the chronic autoimmune attack on marrow precursors, similar to the model of aplastic anemia, in which clonal hematopoiesis may be demonstrated in up to half of patients.12 In fact, in a similar study focusing on autoimmune neutropenia, a frequency similar to that of our cohort (12%) of somatic myeloid drivers was found when looking at NGS results.13,14
The role of myelopoiesis-stimulating agents might also be hypothesized, although this study did not find an association of treatment types with clonal hematopoiesis, as also demonstrated in patients with aplastic anemia.15 Similarly, no association of NGS positivity with cytotoxic immunosuppressant treatment was observed, although larger patient numbers and a prolonged follow-up might be needed to unravel a toxic effect.
We understand that our study has several limitations, particularly the retrospective design and the limited number of patients. The latter is in keeping with the absence of formal indication for NGS testing in ITP as well as with the limited availability of the tests until the last years. The indication for NGS testing might represent a bias, because of being primarily performed at the time of BM evaluation in the comprehensive workup of chronic cytopenia. However, even newly diagnosed patients have been included in the study and were found to harbor clonal hematopoiesis at a similar rate. In any case, only a prospective systematic evaluation of patients with ITP at diagnosis will possibly clarify the exact prevalence and clinical significance of clonal hematopoiesis in this setting.
In conclusion, our study demonstrated the prevalence of clonal hematopoiesis in 18% of adult patients with ITP, being associated with older age, relapsed/refractory disease, and high risk of thrombotic complications, which identify a more severe disease.
Acknowledgment
Article processing charge publication fees were partially covered by the Italian Ministry of Health, current research grant.
Authorship
Contribution: B.F., M. Capecchi, and W.B. conceived the study; B.F., A.M., and W.B. wrote the article; and all authors followed patients, collected data, and revised the article for the important intellectual content.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Bruno Fattizzo, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, via Francesco Sforza 35, 20100, Milan, Italy; email: bruno.fattizzo@unimi.it.
References
Author notes
All data have been included in the manuscript. Further information may be obtained upon reasonable request from the corresponding author, Bruno Fattizzo (bruno.fattizzo@unimi.it).
The full-text version of this article contains a data supplement.