Key Points
In patients with WM treated with Bruton tyrosine kinase inhibitors, major response was associated with PN symptom resolution.
Patients whose PN symptoms resolved reported improved QOL outcomes and physical functioning scores vs those without resolution.
Visual Abstract
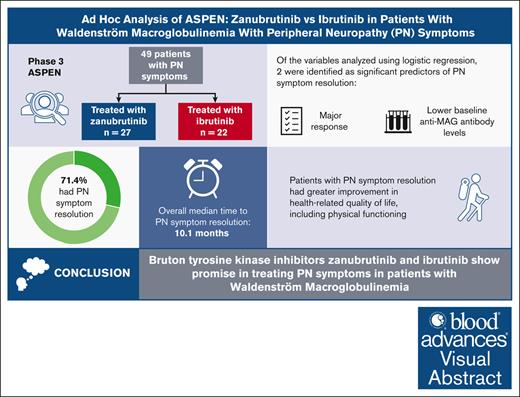
Peripheral neuropathy (PN) is a significant cause of morbidity associated with Waldenström macroglobulinemia (WM). The phase 3 ASPEN study compared the efficacy and safety of zanubrutinib with ibrutinib in patients with WM. This ad hoc analysis examined treatment outcomes with zanubrutinib or ibrutinib on PN symptoms associated with WM in patients enrolled in ASPEN. Logistic regression was performed between PN symptom resolution and several predictors. Health-related quality of life (HRQOL) was assessed using the validated European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire-Core 30. Forty-nine patients with PN symptoms were included (zanubrutinib treated, n = 27; ibrutinib treated, n = 22). Overall, 35 patients (71.4%) experienced resolution of PN symptoms, with a median time to resolution of 10.1 months (range, 1-46.8). In cohort 1 (MYD88 mutation), the median time to PN symptom resolution was 4.6 months (range, 1.1-46.8) with zanubrutinib and 14.1 months (range, 1-44) with ibrutinib. Logistic regression demonstrated a significant relationship between PN symptom resolution and both major response and lower baseline anti–myelin-associated glycoprotein antibody levels. Patients with PN symptom resolution had greater improvement in HRQOL. Physical functioning improved in patients with PN symptom resolution and was unchanged in patients without resolution. Improvements observed in PN symptoms may be in response to a reduction in immunoglobulin M. Although further investigation is required, this analysis supports the potential use and further exploration of Bruton tyrosine kinase inhibitors to treat PN symptoms in patients with WM. This trial was registered at www.clinicaltrials.gov as #NCT03053440.
Introduction
Waldenström macroglobulinemia (WM) is a rare, incurable B-cell non-Hodgkin lymphoma characterized by bone marrow infiltration by lymphoplasmacytic cells and the detection of monoclonal immunoglobulin M (IgM) protein.1 Somatic mutations in the myeloid differentiation primary response 88 (MYD88) and CXC chemokine receptor type 4 (CXCR4) genes occur in ∼95% and 30% to 40% of patients, respectively.2 One of the most significant causes of morbidity in patients with WM is peripheral neuropathy (PN), which can occur in up to 50% of patients.3-5 WM-associated PNs are heterogeneous, including demyelinating or axonal neuropathies.6 Anti–myelin-associated glycoprotein (MAG) neuropathy is the most common IgM-related neuropathy in patients with WM. Approximately 50% of patients with IgM-related PN have high titers of anti-MAG antibodies.7
The pathophysiology of IgM monoclonal gammopathy–mediated PN is not well understood. However, nerve biopsies in patients with WM have demonstrated the presence of demyelination and widened myelin lamellae, with monoclonal IgM deposits present within the widened lamellae of the myelin fibers, consistent with immune-mediated neuronal damage.8-10 In some patients without anti-MAG antibodies (up to 50% of patients11), anti-ganglioside antibodies or antibodies against ganglioside complexes may be present.12
Treating WM-associated neuropathy is challenging. In general, treatment is initiated in patients with significant symptoms or disability to halt the progression of neuropathy and improve neuropathy symptoms. Unfortunately, treatment options for patients with WM-associated neuropathy are limited. Steroids, IV immunoglobulin, and plasma exchange have not demonstrated meaningful long-term symptom improvement.13 Rituximab has been demonstrated to improve neurological disability; however, paradoxical IgM flares may limit its utility.13,14 Chemoimmunotherapy approaches can improve PN but are associated with treatment-related toxicity.7,15,16 Thus, novel treatment approaches that effectively treat the neuropathy associated with WM without increasing the risk for treatment flare are needed.
Bruton tyrosine kinase (BTK) inhibitors are the first US Food and Drug Administration–approved medications for treating WM. Both zanubrutinib and ibrutinib are covalent BTK inhibitors that are indicated for the treatment of WM, but data on their efficacy, specifically for WM-associated neuropathy, are limited.17,18 ASPEN was an open-label phase 3 study that compared treatment with zanubrutinib vs ibrutinib in patients with WM. This ad hoc analysis examined the efficacy of zanubrutinib and ibrutinib for PN symptoms in patients with WM.
Methods
Study design and methods of the ASPEN study (ClinicalTrials.gov identifier: NCT03053440) have been described previously, and results of the primary and the long-term follow-up analyses were published.18,19 Briefly, patients with relapsed/refractory WM or treatment-naive WM unsuitable for chemoimmunotherapy were eligible. In cohort 1, patients with mutated MYD88 were randomly assigned 1:1 to receive either zanubrutinib 160 mg twice daily or ibrutinib 420 mg once daily in 28-day cycles. In cohort 2, patients with wild-type MYD88 or undetermined MYD88 mutation status received zanubrutinib 160 mg twice daily. The ASPEN study was approved by the independent institutional review board or independent ethics committee at each site, and all patients provided written informed consent; the study was conducted following applicable regulatory requirements, the principles of the Declaration of Helsinki, and Good Clinical Practice guidelines of the International Conference on Harmonization.
Ad hoc analyses were performed using safety, efficacy, and patient-reported outcomes data from the ASPEN study as of final data cutoff date of 21 June 2022. WM responses were assessed by the investigators based on the modified International Workshop on Waldenström Macroglobulinemia (IWWM-6) criteria. Major response was defined as partial response or better.20
All enrolled patients who had symptomatic PN, assessed by the investigator as related to WM, at study enrollment were included in these ad hoc analyses. Formal objective assessments of PN, such as electromyography, or diagnosis by neurologist were not required per protocol. Resolution of treatment-precipitating symptoms (per IWWM-7 guidelines21) was a predefined secondary end point of the ASPEN study. All enrolled patients were followed up for the resolution of treatment-precipitating symptoms (defined as absence of symptoms), including PN symptoms, throughout the study treatment on day 1 of each cycle through cycle 13 and every 3 cycles after that until the end of treatment. Logistic regression models were used to assess the relationship between PN symptom resolution and several potential predictors selected based on clinical rationale, including comorbidities associated with non–WM-associated PN (medical history pertinent to PN, such as diabetes mellitus), medications associated with PN toxicity or for treatment of PN (prior antineoplastic therapy, plasmapheresis, and concomitant medications), and WM disease factors (major response, anti-MAG antibodies, IgM concentration, and IgM reduction).
Health-related quality of life (HRQOL) was assessed using the validated European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire-Core 30 (EORTC QLQ-C30). Data were analyzed by the sponsor, and all authors had access to primary clinical trial data.
Results
Patient population
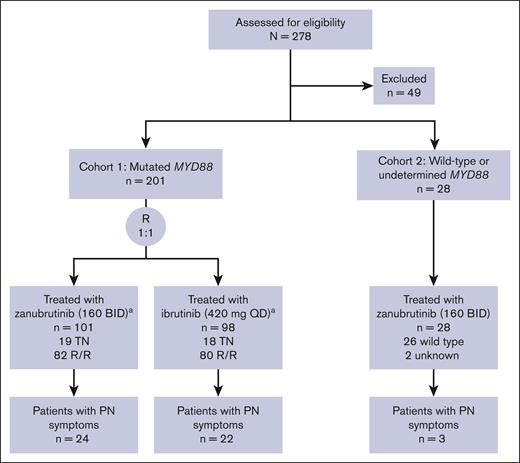
Between January 2017 and July 2018, a total of 201 patients with mutated MYD88 WM were enrolled in cohort 1 (zanubrutinib arm, n = 102; ibrutinib arm, n = 99); 28 patients were enrolled in cohort 2 (wild-type MYD88, n = 26; unknown, n = 2). At screening, PN was present as a symptom of WM in 49 patients (21.4% of the study population; cohort 1 zanubrutinib arm, n = 24; cohort 1 ibrutinib arm, n = 22; cohort 2, n = 3; Figure 1). Demographics and disease characteristics of patients with PN symptoms are shown in Table 1. The median age was 69 years, and 65% of patients with PN symptoms were male. Most patients (78%) had received prior WM-directed therapies, and 73% had CXCR4 wild-type disease. Ten patients had elevated anti-MAG antibody levels (reference range, ≤999 titer units [TU]; >70 000 TU, n = 4) at screening, and 8 had longitudinal anti-MAG antibody data.
Disposition of patients with PN symptoms in ASPEN.aAn additional patient with R/R disease in each cohort 1 group was randomized but did not receive treatment due to acute kidney injury and central nervous system lymphoma. BID, twice daily; QD, once daily; R, randomized; R/R, relapsed/refractory; TN, treatment naive.
Disposition of patients with PN symptoms in ASPEN.aAn additional patient with R/R disease in each cohort 1 group was randomized but did not receive treatment due to acute kidney injury and central nervous system lymphoma. BID, twice daily; QD, once daily; R, randomized; R/R, relapsed/refractory; TN, treatment naive.
Demographics and disease characteristics of patients with PN symptoms at study entry
| . | Cohort 1 . | Cohort 2 . | Total (n = 49) . | |
|---|---|---|---|---|
| Zanubrutinib (n = 24) . | Ibrutinib (n = 22) . | Zanubrutinib (n = 3) . | ||
| Age, median (range), y | 69.5 (50-87) | 68 (57-83) | 70 (57-85) | 69 (50-87) |
| Male sex, n (%) | 15 (62.5) | 14 (63.6) | 3 (100) | 32 (65.3) |
| Prior lines of therapy, n (%) | ||||
| 0 | 6 (25.0) | 4 (18.2) | 1 (33.3) | 11 (22.4) |
| 1-3 | 15 (62.5) | 16 (72.7) | 2 (66.7) | 33 (67.3) |
| >3 | 3 (12.5) | 2 (9.1) | 0 | 5 (10.2) |
| Genotype by NGS, n (%) | ||||
| CXCR4WT | 18 (75.0) | 15 (68.2) | 3 (100) | 36 (73.5) |
| CXCR4MUT | 6 (25.0) | 5 (22.7) | 0 | 11 (22.4) |
| CXCR4FS | 4 (16.7) | 3 (13.6) | 0 | 7 (14.3) |
| CXCR4NS | 2 (8.3) | 2 (9.1) | 0 | 4 (8.2) |
| Unknown | 0 | 2 (9.1) | 0 | 2 (4.1) |
| Baseline IgM (central laboratory), median (range), g/L | 32.4 (6.7-68.9) | 21 (6.8-54.9) | 24 (13.8-42.5) | 26 (6.72-68.9) |
| Baseline anti-MAG Ab, median (range), TU | 70 (1 to >70 000) | 138 (9 to >70 000) | 70 (44-1545) | 85 (1 to >70 000) |
| Anti-MAG Ab elevation (>999 TU) at baseline, n (%) | 2 (8.3) | 7 (31.8) | 1 (33.3) | 10 (20.4) |
| . | Cohort 1 . | Cohort 2 . | Total (n = 49) . | |
|---|---|---|---|---|
| Zanubrutinib (n = 24) . | Ibrutinib (n = 22) . | Zanubrutinib (n = 3) . | ||
| Age, median (range), y | 69.5 (50-87) | 68 (57-83) | 70 (57-85) | 69 (50-87) |
| Male sex, n (%) | 15 (62.5) | 14 (63.6) | 3 (100) | 32 (65.3) |
| Prior lines of therapy, n (%) | ||||
| 0 | 6 (25.0) | 4 (18.2) | 1 (33.3) | 11 (22.4) |
| 1-3 | 15 (62.5) | 16 (72.7) | 2 (66.7) | 33 (67.3) |
| >3 | 3 (12.5) | 2 (9.1) | 0 | 5 (10.2) |
| Genotype by NGS, n (%) | ||||
| CXCR4WT | 18 (75.0) | 15 (68.2) | 3 (100) | 36 (73.5) |
| CXCR4MUT | 6 (25.0) | 5 (22.7) | 0 | 11 (22.4) |
| CXCR4FS | 4 (16.7) | 3 (13.6) | 0 | 7 (14.3) |
| CXCR4NS | 2 (8.3) | 2 (9.1) | 0 | 4 (8.2) |
| Unknown | 0 | 2 (9.1) | 0 | 2 (4.1) |
| Baseline IgM (central laboratory), median (range), g/L | 32.4 (6.7-68.9) | 21 (6.8-54.9) | 24 (13.8-42.5) | 26 (6.72-68.9) |
| Baseline anti-MAG Ab, median (range), TU | 70 (1 to >70 000) | 138 (9 to >70 000) | 70 (44-1545) | 85 (1 to >70 000) |
| Anti-MAG Ab elevation (>999 TU) at baseline, n (%) | 2 (8.3) | 7 (31.8) | 1 (33.3) | 10 (20.4) |
Ab, antibody; FS, frameshift; MUT, mutated; NGS, next-generation sequencing; NS, nonsense; WT, wild type.
Overall, 35 patients (71.4%) experienced resolution of PN symptoms, with a median time to resolution of 10.1 months (range, 1-46.8). In cohort 1, 14 of 18 patients (78%) in the zanubrutinib arm and 16 of 19 patients (84%) in the ibrutinib arm who achieved major response had resolution of PN symptoms; the median time to PN symptom resolution was 4.6 months (range, 1.1-46.8) in patients receiving zanubrutinib and 14.1 months (range, 1-44) in patients receiving ibrutinib. Major response was achieved after PN symptom resolution in 6 patients (zanubrutinib arm, n = 3; ibrutinib arm, n = 3) who had early symptom resolution (time to PN symptom resolution, <4 months). In cohort 2, both patients who achieved major response had resolution of PN symptoms, with a median time to PN symptom resolution of 28.6 months (range, 13.8-43.3).
None of the 3 patients who had plasmapheresis before ASPEN had resolution of PN symptoms, nor did either of the 2 patients who underwent plasmapheresis during ASPEN (cohort 1 ibrutinib arm, n = 1; cohort 2, n = 1). Therefore, plasmapheresis history was removed from the multivariate logistic regression model.
Predictors of PN symptom resolution
Logistic regression modeling demonstrated a relationship between major response and PN symptom resolution (with multiple variables evaluated, odds ratio [OR], 11.21; 95% confidence interval [CI], 2.06-61.15; P = .0052 and with major response as the predictor, OR, 10.67; 95% CI, 2.20-51.81; P = .0033; Table 2). A statistically significant relationship was also shown between PN symptom resolution and lower baseline anti-MAG antibody levels (10−4 TU; OR, 0.72; 95% CI, 0.52-1.00; P = .0486).
Logistic regression of predictors of PN symptom resolution
| Variable . | OR (95% CI) . | P value∗ . |
|---|---|---|
| Multivariate model | ||
| Medical history of PN | 0.65 (0.15-2.79) | .56 |
| Major response | 11.21 (2.06-61.15) | .0052 |
| Medical history pertinent to PN | 2.04 (0.34-12.35) | .44 |
| Prior antineoplastic therapy | 0.90 (0.12-6.68) | .92 |
| Pertinent concomitant medication | 0.65 (0.15-2.79) | .56 |
| Major response | 10.67 (2.20-51.81) | .0033 |
| Baseline anti-MAG antibody value, 10–4 TU | 0.72 (0.52-1.00) | .0486 |
| Binary baseline anti-MAG antibody, ≤999 TU | 1.93 (0.45-8.28) | .38 |
| Maximum IgM reduction | 0.98 (0.93-1.03) | .34 |
| Maximum IgM percent reduction | 1.03 (1.00-1.06) | .05 |
| IgM reduction at the latest measurement at or before PN symptom resolution† | 1.01 (0.97-1.06) | .52 |
| IgM reduction at the next measurement after PN symptom resolution† | 1.01 (0.96-1.06) | .71 |
| Minimum IgM | 0.98 (0.91-1.05) | .52 |
| Normalized minimum IgM less than or equal to ULN‡ | 5.05 (0.98-25.99) | .05 |
| Normalized minimum IgM ≤1.5× ULN‡ | 2.65 (0.70-10.07) | .15 |
| Normalized minimum IgM ≤2× ULN‡ | 1.50 (0.43-5.22) | .52 |
| Variable . | OR (95% CI) . | P value∗ . |
|---|---|---|
| Multivariate model | ||
| Medical history of PN | 0.65 (0.15-2.79) | .56 |
| Major response | 11.21 (2.06-61.15) | .0052 |
| Medical history pertinent to PN | 2.04 (0.34-12.35) | .44 |
| Prior antineoplastic therapy | 0.90 (0.12-6.68) | .92 |
| Pertinent concomitant medication | 0.65 (0.15-2.79) | .56 |
| Major response | 10.67 (2.20-51.81) | .0033 |
| Baseline anti-MAG antibody value, 10–4 TU | 0.72 (0.52-1.00) | .0486 |
| Binary baseline anti-MAG antibody, ≤999 TU | 1.93 (0.45-8.28) | .38 |
| Maximum IgM reduction | 0.98 (0.93-1.03) | .34 |
| Maximum IgM percent reduction | 1.03 (1.00-1.06) | .05 |
| IgM reduction at the latest measurement at or before PN symptom resolution† | 1.01 (0.97-1.06) | .52 |
| IgM reduction at the next measurement after PN symptom resolution† | 1.01 (0.96-1.06) | .71 |
| Minimum IgM | 0.98 (0.91-1.05) | .52 |
| Normalized minimum IgM less than or equal to ULN‡ | 5.05 (0.98-25.99) | .05 |
| Normalized minimum IgM ≤1.5× ULN‡ | 2.65 (0.70-10.07) | .15 |
| Normalized minimum IgM ≤2× ULN‡ | 1.50 (0.43-5.22) | .52 |
Values in bold represent statistically significant associations with PN symptom resolution at P value <.05.
Or the maximum IgM reduction if the patient did not have PN symptom resolution.
ULN for IgM was defined as 2.3 g/L.
No significant relationship was demonstrated with minimum (nadir) IgM, normalization of IgM concentration at nadir (less than or equal to upper limit of normal [ULN], ≤1.5× ULN or ≤2× ULN), or IgM percent reduction from pretreatment baseline to nadir. Neither normalization of IgM (cutoff, minimum IgM less than or equal to ULN) nor IgM maximum percent reduction from pretreatment baseline was a statistically significant predictor of PN symptom resolution (P = .05 and P = .05, respectively). No relationship was demonstrated between PN symptom resolution and the extent of IgM reduction on/before resolution or after resolution (or maximum IgM reduction in patients without PN symptom resolution).
No significant relationship with the binary or log-scale baseline anti-MAG antibody level was demonstrated. A logistic regression model between PN symptom resolution and log-scale baseline anti-MAG antibody level showed a negative relationship (OR, 0.86; 95% CI, 0.68-1.08; P = .20).
Patient-reported outcomes
Patient-reported outcomes as assessed using the EORTC QLQ-C30 are shown in Table 3. Patients with and without PN symptom resolution improved from baseline in global health status/HRQOL with treatment; patients who had resolution of their PN symptoms showed an improved median score from 66.7 at baseline to 75.0 and those without resolution of PN symptoms improved from 50.0 to 54.2. Patients with PN symptom resolution had improvement in pain, with a median final pain score of 0 (19/35 patients with PN symptom resolution reported a final pain score of 0), whereas those without PN symptom resolution had worsening of pain (median final pain score of 50 from baseline of 16.7). Physical functioning scores improved from 80 to 86.7 in patients who reported PN resolution, whereas no changes in physical functioning scores were observed in patients without PN symptom resolution.
Patient-reported outcomes in patients with and without resolution of PN symptoms
| EORTC QLQ-C30 score, median (IQR) . | PN symptoms resolved (n = 35)∗ . | PN symptoms not resolved (n = 14)† . |
|---|---|---|
| Global health status/QOL | ||
| Baseline | 66.7 (47.9-83.3) | 50.0 (33.3-66.7) |
| First time point after PN symptom resolution | 66.7 (50.0-83.3) | |
| Final | 75.0 (66.7-87.5) | 54.2 (50.0-66.7) |
| Pain | ||
| Baseline | 16.7 (0-33.3) | 16.7 (0-16.7) |
| First time point after PN symptom resolution | 16.7 (0-33.3) | |
| Final | 0 (0-33.3) | 50.0 (0-50.0) |
| Physical functioning | ||
| Baseline | 80.0 (65.0-93.3) | 66.7 (60.0-80.0) |
| First time point after PN symptom resolution | 86.7 (70.0-100) | |
| Final | 86.7 (76.7-100) | 66.7 (43.3-80.0) |
| EORTC QLQ-C30 score, median (IQR) . | PN symptoms resolved (n = 35)∗ . | PN symptoms not resolved (n = 14)† . |
|---|---|---|
| Global health status/QOL | ||
| Baseline | 66.7 (47.9-83.3) | 50.0 (33.3-66.7) |
| First time point after PN symptom resolution | 66.7 (50.0-83.3) | |
| Final | 75.0 (66.7-87.5) | 54.2 (50.0-66.7) |
| Pain | ||
| Baseline | 16.7 (0-33.3) | 16.7 (0-16.7) |
| First time point after PN symptom resolution | 16.7 (0-33.3) | |
| Final | 0 (0-33.3) | 50.0 (0-50.0) |
| Physical functioning | ||
| Baseline | 80.0 (65.0-93.3) | 66.7 (60.0-80.0) |
| First time point after PN symptom resolution | 86.7 (70.0-100) | |
| Final | 86.7 (76.7-100) | 66.7 (43.3-80.0) |
IQR, interquartile range.
Patients with PN symptom resolution who completed the EORTC QLQ-C30 questionnaire at baseline (n = 32), the first time point after PN symptom resolution (n = 31), and the final time point (n = 35).
Patients without PN symptom resolution who completed the EORTC QLQ-C30 questionnaire at baseline (n = 13) and the final time point (n = 14).
Discussion
In patients with WM, PN is a frequent and often debilitating symptom. Approximately 25% of patients have PN at diagnosis, but up to 50% of patients will be affected by PN during their disease.3,5 The current IWWM consensus guidelines include symptomatic PN as an indication for initiation of treatment in patients with WM.22,23 However, in patients whose sole indication for treatment is PN, treatment frequently may be delayed until neuropathy symptoms are significant, given the concern about the lack of effective treatments, as well as potential side effects of therapy, including the risk of exacerbating/worsening neuropathy.16,23 BTK inhibitors have demonstrated promising efficacy and a tolerable safety profile in patients with either treatment-naive or relapsed/refractory WM. Previous reports on ibrutinib for treating patients with WM suggested that BTK inhibitors may effectively improve WM-associated neuropathy.24
In this ad hoc analysis of the ASPEN trial, treatment with zanubrutinib or ibrutinib led to PN symptom resolution in most patients (71.4%), based on patient-reported outcomes. PN symptom resolution occurred quickly, with a median time to resolution of 10.1 months (range, 1-47). WM-associated PN typically takes several months to improve. In this study, PN symptoms were resolved more rapidly in patients receiving zanubrutinib than those receiving ibrutinib (4.6 vs 14.1 months, respectively). These data support the use of BTK inhibitors as a treatment option for patients with WM with PN symptoms. In addition, although patients with anti-MAG PN mostly have the same bone marrow infiltration range as IgM monoclonal gammopathy of undetermined significance, data can likely be extrapolated due to their similar molecular characteristics.25,26
Logistic regression analysis demonstrated that patients who achieved a major response after treatment with either zanubrutinib or ibrutinib were more likely to have PN symptom resolution. The relationship between PN symptom resolution and WM major response is consistent with that in patients treated with chemoimmunotherapy-based regimens, who were more likely to experience symptomatic improvement if they achieved at least a major response.7,27 Other factors associated with improvement in PN symptoms include rituximab combination treatment, non–amyloid-related PN, and earlier time to initial treatment.7,27 No relationship was demonstrated between PN symptom resolution and the extent of IgM reduction at the time of PN symptom resolution. In patients with symptomatic improvement before attaining a major response, especially those with neurolymphomatosis, improvement may reflect the redistribution/mobilization of WM cells after treatment with BTK inhibitors.
Normalization of IgM at its nadir and maximum percent reduction from pretreatment baseline to nadir trended toward an association with PN symptom resolution; however, analysis was limited by the small sample size. Although the ASPEN trial represents, to our knowledge, the largest data set on the efficacy of BTK inhibitors for treating PN symptoms in patients with WM, relatively few patients had PN symptoms (n = 49). PN symptom resolution occurred in 71.4% of patients treated with zanubrutinib and ibrutinib, demonstrating that BTK inhibitors may potentially treat multiple neuropathy causes in patients with WM and PN symptoms. Because the etiology of PN symptoms was not formally evaluated, it is possible that some patients in this analysis could have had concomitant non–WM-related immune PN, which responded to BTK inhibition as in other autoimmune diseases.28
This study has several limitations. The study had an open-label design and small sample size. Electromyography and nerve conduction studies, which are important in better elucidating the cause of neuropathy in patients with WM, were not required for study entry. Formal diagnosis of anti-MAG neuropathy was not made. The percentage of patients whose neuropathy resulted from alternative causes, such as neurolymphomatosis, cryoglobulinemia, vasculitis, amyloidosis, or causes unrelated to WM, is unclear. It is also unclear whether neuropathy resulting from any of these causes might have had greater symptomatic improvement after treatment with BTK inhibitors. Moreover, neuropathy is a frequent comorbidity seen in older patients without WM.29 Thus, there is the potential that a patient’s neuropathy may not have been primarily caused by WM IgM. However, as defined by the ASPEN study procedures, the investigator assessed all enrolled patients for PN symptoms. The association of PN symptom resolution with major response suggests but does not prove that the etiology of PN was WM. Further investigation of novel treatments in WM should include systematic evaluation of PN symptoms using prospective neurophysiological evaluation, including validated scales and patient-reported outcome measures to thoroughly characterize the effect of novel treatments on these important symptoms.
In the ASPEN trial, patient-reported outcomes assessed by EORTC QLQ-C30 demonstrated that after BTK inhibitor treatment, patients whose PN resolved experienced significantly improved HRQOL and pain scores. Those who did not respond to treatment had further worsening of pain and no improvement in physical functioning. These findings are consistent with previous reports, in which patients with either IgM-associated PN or WM-associated PN had decreased QOL.30,31 Although QOL assessments are valuable, the Inflammatory Rasch-Built Overall Disability Scale (I-RODS) is a recommended and validated disability assessment scale used to capture temporal changes in function in patients with inflammatory neuropathies, such as WM-associated PN.32 This type of assessment is better able to capture changes over time, as well as the magnitude of change, especially when assessing physical function,33 thus offering a more sensitive measure than patient-reported outcomes or QOL surveys. Future studies on the utility of BTK inhibitors for treating WM-associated PN should use I-RODS.
This ad hoc analysis of the efficacy of BTK inhibitors for the treatment of PN symptoms in patients with WM supports prospective interventional clinical trials. A single-arm phase 2 study of acalabrutinib and rituximab (NCT05065554) currently treats patients with anti-MAG antibody– or WM-associated PN. Preliminary results have been promising, with hematologic responses occurring in 86% of patients and improvement in I-RODS score in 57%.34 A prospective phase 2 study of zanubrutinib in patients with anti-MAG antibody neuropathy (the MAGNAZ trial; NCT05939037) is also underway.35
In conclusion, in the phase 3, international ASPEN study, BTK inhibitors effectively treated PN symptoms in patients with WM. PN symptom resolution was correlated with the depth of disease response, and zanubrutinib led to faster resolution of PN symptoms than ibrutinib. The PN improvements may be in response to a reduction in IgM levels. Although further investigation of BTK inhibitors in PN-specific studies incorporating detailed neurophysiological investigations is required, this analysis supports the potential use of BTK inhibitors to treat PN symptoms in patients with WM.
Acknowledgments
Shanen Perumal, a medical writer at Nucleus Global, an Inizio company, provided editorial assistance to the authors during the preparation of this manuscript, funded by BeiGene.
This study was funded by BeiGene.
Authorship
Contribution: B.M.H., A.T., C.S.T., C.B., R.G.O., V.L., J.T., G.B., W.Y.C., J.S., H.A., A.C., and J.V.M. contributed to conception and design; A.T., C.S.T., C.B., R.G.O., and V.L. served on the steering committee; B.M.H., S.S.O., B.E.W., M.-A.A.D., J.J.C., A.T., C.S.T., C.B., R.G.O, V.L., J.T., G.B., W.Y.C., H.A., A.C., and J.V.M. contributed to data acquisition; and all authors contributed to data analysis and data interpretation and approved of the submission of this manuscript.
Conflict-of-interest disclosure: B.M.H. reports research funding from AbbVie, Century Therapeutics, Oncternal Therapeutics, and Roche; and advisory board fees from AstraZeneca, BeiGene, and Epizyme, Inc. S.S.O. reports consulting fees from AbbVie, Antengene, AstraZeneca, BeiGene, Bristol Myers Squibb (BMS), CSL Behring, Gilead, Merck, Novartis, Janssen, Roche, and Takeda; research funding from AbbVie, AstraZeneca, BeiGene, BMS, Gilead, Janssen, Merck, Novartis, Pharmacyclics, Roche, and Takeda; honoraria from AbbVie, AstraZeneca, BeiGene, BMS, Gilead, Janssen, Merck, Novartis, Roche, and Takeda; and membership on an entity’s board of directors or advisory committees in AbbVie, AstraZeneca, BeiGene, BMS, Gilead, Janssen, Merck, Novartis, Roche, and Takeda outside the submitted work. B.E.W. reports stocks in Genmab. M.-A.A.D. reports advisory board fees and honoraria from Amgen, AbbVie, BMS, BeiGene, Janssen, GSK, Menarini, Takeda, Regeneron, and Sanofi. J.J.C. reports research funding from AbbVie, AstraZeneca, BeiGene, Cellectar, Loxo, and Pharmacyclics; and consultancy fees from AbbVie, AstraZeneca, Avilar, BeiGene, Cellectar, Janssen, Kite, Loxo, Mustang Bio, and Pharmacyclics. A.T. reports consultancy fees from BeiGene, AstraZeneca, AbbVie, and Janssen; honoraria from BeiGene, AstraZeneca, AbbVie, and Janssen; speakers bureau fees from BeiGene, AstraZeneca, AbbVie, and Janssen; and travel, accommodations, and expenses from BeiGene, AstraZeneca, AbbVie, and Janssen. C.S.T. reports research funding from Janssen, AbbVie, and BeiGene; and honoraria from Janssen, AbbVie, BeiGene, Loxo, and AstraZeneca. C.B. reports consultancy, honoraria, advisory board, and travel expenses from Roche/Genentech, Janssen, BeiGene, Novartis, Pfizer, Incyte, AbbVie, Gilead, Celltrion, MorphoSys, Regeneron, Sobi, and Lilly. R.G.O. reports consultancy fees from BeiGene and Janssen; honoraria from BeiGene, Janssen, and AstraZeneca; and meetings/travel expenses from BeiGene. V.L. reports consultancy fees from BeiGene, AbbVie, Janssen, and Merck Sharp & Dohme; and honoraria from Pfizer, BeiGene, AstraZeneca, and Amgen. J.T. reports research funding from BeiGene, Janssen, Pharmacyclics, Roche, Celgene/BMS, and Cellectar; and advisory board fees from BeiGene. G.B. and J.S. report employment with BeiGene. W.Y.C. reports employment with BeiGene; and equity in BeiGene and BMS. H.A. reports employment and is an equity holder in a publicly traded company with BeiGene and Nkarta Therapeutics; and patents and royalties with St Jude Children’s Research Hospital. A.C. reports consultancy and is an equity holder in BeiGene. J.V.M. reports consultancy fees from Waldenström macroglobulinemia advisory committee of BeiGene.
Correspondence: Benjamin M. Heyman, Medicine, University of California San Diego, 9500 Gilman Drive, La Jolla, CA 92093; email: bheyman@health.ucsd.edu.
References
Author notes
BeiGene voluntarily shares anonymous data on completed studies responsibly and provides qualified scientific and medical researchers access to anonymous data and supporting clinical trial documentation for clinical trials in dossiers for medicines and indications after submission and approval in the United States, China, and Europe. Clinical trials supporting subsequent local approvals, new indications, or combination products are eligible for sharing once corresponding regulatory approvals are achieved. BeiGene shares data only when permitted by applicable data privacy and security laws and regulations. In addition, data can only be shared when it is feasible to do so without compromising the privacy of study participants. Qualified researchers may submit data requests/research proposals for BeiGene review and consideration through BeiGene’s clinical trials webpage available at https://www.beigene.com/our-science-and-medicines/our-clinical-trials/.