Visual Abstract
TO THE EDITOR:
Mixed-lineage leukemia gene (MLL, also called KMT2A) rearrangements create fusion oncoproteins that couple the N-terminal portion of MLL to numerous partners, associated with a group of acute myeloid leukemia and acute lymphoblastic leukemia (ALL) with intermediate to poor clinical outcome.1 Several fusion partners, including AF4 (AFF1), ENL (MLLT1), and AF9 (MLLT3), interact with the super elongation complex or the H3K79 methyltransferase DOT1L, which regulates transcription activation.1-3 Thus, various MLL fusions can cause the aberrant activation of targets of wild-type MLL via ectopic super elongation complex recruitment and H3K79me2 deposition, leading to leukemia development through a shared pathogenesis mechanism.4,5
Despite this general leukemogenesis model, MLL-rearranged leukemia exhibits clinical heterogeneity in cellular morphologies and disease outcome.6,7 Accordingly, fusion partner–associated transcriptome heterogeneity has been observed within MLL-rearranged leukemia,8-10 although the underlying molecular mechanisms are unclear. We previously established an isogenic model of MLL-rearranged ALL by retrovirally expressing FLAG-tagged MLL-Af4 (MLL/murine-Af4 chimeric fusion) or MLL-AF9 in human CD34+ hematopoietic stem and progenitor cells from the same healthy donor, which recapitulated the fusion-specific gene signatures presented in patient samples.11 We observed that differential DNA binding of these 2 MLL fusion proteins could direct distinct gene expression at select target loci11; however, whether this instructive role of fusion partners applies to the global epigenome and transcriptome of MLL-rearranged leukemia remains to be determined. To this end, we conducted chromatin immunoprecipitation sequencing analysis in 2 pairs of isogenic ALL cells to compare the binding sites and the associated epigenetic features of the 2 MLL fusion proteins (supplemental Figure 1A).
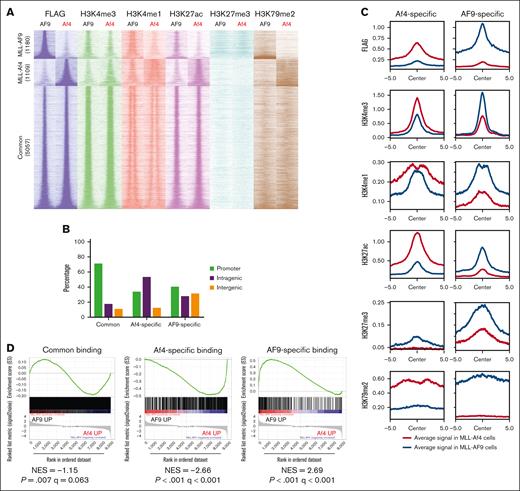
We previously showed that both fusion proteins were expressed at a comparable level in these ALL cells as detected by an anti-FLAG antibody,11 which was used here to unambiguously identify the genome-wide distribution of MLL fusion proteins. Clustering analysis based on the coordinates of all binding peaks or the peaks consistent across 2 ALL pairs can readily distinguish MLL-Af4 from MLL-AF9 samples (supplemental Figure 1B-D). A filter with false discovery rate ≤0.05 and log2FC (fold-change) ≥1 was applied to detect 1109 MLL-Af4-specific and 1180 MLL-AF9–specific binding sites with high confidence; and 5057 peaks with a log2FC <1 were considered common fusion binding sites (Figure 1A and supplemental Figure 1E). Intriguingly, although ∼70% of common peaks were detected near promoters, MLL-Af4–specific binding sites were more prevalent at intragenic regions, similar to the previous finding that MLL-AF4 binding spreads into gene bodies of a subset of targets.12 MLL-AF9-specific binding sites showed lower intragenic but higher intergenic distributions than MLL-Af4 (Figure 1B). Together, our data demonstrate that although both MLL fusions share a number of genomic targets, the fusion partners affect their genomic binding patterns.
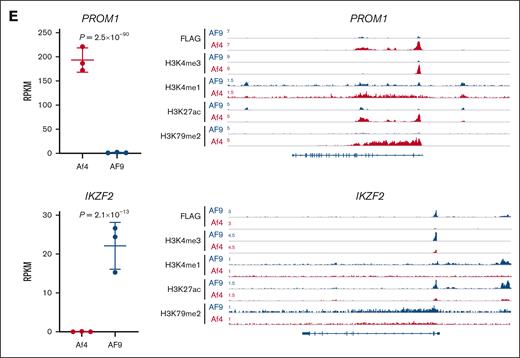
Characterization of MLL fusion type–specific chromatin binding sites. (A) Heat map showing the indicated chromatin immunoprecipitation sequencing (ChIP-seq) signals at fusion-specific and common binding sites in model ALL cells. Window shows ±5 kb from the peak center. (B) Genomic locations of fusion-specific and common binding peaks. (C) Average read density metaplots showing the indicated signals in each leukemia type at fusion-specific binding sites over a ±5 kb window from the peak center. (D) Gene set enrichment analysis (GSEA) demonstrating the association of fusion-specific binding targets with upregulated gene expression in the corresponding ALL type. (E) RNA sequence analysis showing differential expression of PROM1 and IKZF2 between paired ALLs (left). Each individual point represents one sample. P values were calculated by DESeq2. ChIP-seq tracks showing MLL-Af4 and MLL-AF9 binding and the associated histone modifications at PROM1 and IKZF2 loci (right). NES, normalized enrichment score; RPKM, reads per kilobase per million mapped reads.
Characterization of MLL fusion type–specific chromatin binding sites. (A) Heat map showing the indicated chromatin immunoprecipitation sequencing (ChIP-seq) signals at fusion-specific and common binding sites in model ALL cells. Window shows ±5 kb from the peak center. (B) Genomic locations of fusion-specific and common binding peaks. (C) Average read density metaplots showing the indicated signals in each leukemia type at fusion-specific binding sites over a ±5 kb window from the peak center. (D) Gene set enrichment analysis (GSEA) demonstrating the association of fusion-specific binding targets with upregulated gene expression in the corresponding ALL type. (E) RNA sequence analysis showing differential expression of PROM1 and IKZF2 between paired ALLs (left). Each individual point represents one sample. P values were calculated by DESeq2. ChIP-seq tracks showing MLL-Af4 and MLL-AF9 binding and the associated histone modifications at PROM1 and IKZF2 loci (right). NES, normalized enrichment score; RPKM, reads per kilobase per million mapped reads.
Next, we characterized the chromatin features around MLL fusion binding sites by profiling the “active” histone modifications H3K4me1, H3K4me3, and H3K27ac, as well as the “repressive” histone modification H3K27me3. H3K79me2 deposited by DOT1L was also profiled. Fusion-specific binding sites were associated with increased H3K4me1, H3K4me3, H3K27ac, and H3K79me2 signals in the corresponding ALL types, in line with the paradigm that MLL fusions aberrantly activate their target genes. H3K27me3 signal was in general low, although a mild elevation was observed at MLL-AF9–specific peaks (Figure 1A,C). To test whether the specific fusion binding contributed to the selective gene expression across the 2 ALL types, we performed a crossanalysis with RNA sequencing data sets of these cells. Gene set enrichment analysis revealed that the MLL-Af4–bound gene set significantly overlapped with the genes more highly expressed in MLL-Af4 cells but not with those expressed higher in MLL-AF9 cells and vice versa (Figure 1D). We validated our previous results on selected genes, such as PROM1 and IKZF2, the expression status of which were indeed associated with differential fusion occupancy and histone modifications (Figure 1E).11 Therefore, differential binding of MLL fusion proteins serves as a driving force of the global transcriptional heterogeneity in MLL-rearranged ALL.
We observed that a set of genes with comparable fusion occupancies were also differentially expressed between the 2 ALL types, indicating there are additional mechanisms regulating the selective transcription. Interestingly, among this set of genes, targets expressed higher in MLL-Af4 cells tended to display stronger H3K4me1 and H3K79me2 occupancies, spreading into their gene bodies, suggesting the activation of intragenic H3K79me2/3-marked enhancers, as described recently.13 These same targets were marked with elevated H3K27me3 in MLL-AF9 cells (supplemental Figure 2A-B). In contrast, gene targets expressed higher in MLL-AF9 cells displayed less apparent epigenetic alterations between the 2 cell types. These results reveal that even at genomic loci with similar binding propensity, different MLL fusion proteins can elicit distinct epigenetic outcomes to influence target gene expression.
We next investigated the biological pathways enriched in differentially regulated targets associated with fusion-specific or common binding sites (supplemental Figure 3A-B). Intriguingly, the signature of myeloid cell differentiation specifically scored in MLL-AF9 targets, which is in accordance with the clinical and experimental observations that MLL-AF9–expressing cells have a stronger propensity for myeloid lineage.6,14 In addition, MLL-Af4 ALL displayed a signature of elevated ribosome biogenesis, which is required for maintaining the rapid growth of several cancer types.15,16
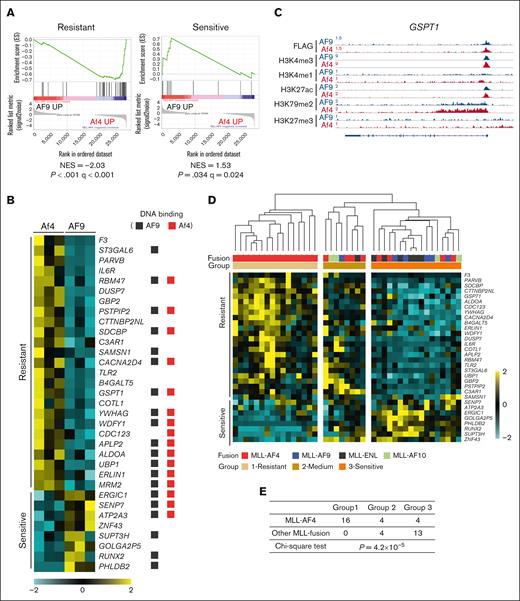
We also noticed several alternatively expressed gene targets that may affect therapeutic outcome. For instance, high BAALC expression was reported as an adverse prognostic marker for chemoresistance in ALL.17 Although both fusion proteins bound to the BAALC promoter, H3K79me2 over the gene body was increased only in MLL-Af4 cells, in which the gene expression was specifically upregulated. By contrast, an increased H3K27me3 signal was observed in MLL-AF9 cells (supplemental Figure 4A). Accordingly, gene signatures identified in ALL patient samples with high or low BAALC expression levels were significantly enriched in Af4 cells and AF9 cells, respectively (supplemental Figure 4B).17 It was reported that MLL-AF4 ALL cells showed increased resistance to prednisolone compared with other MLL-rearranged ALL, although the mechanism is unclear.18 Intriguingly, a published gene signature correlating with prednisolone resistance or sensitivity was significantly overrepresented in MLL-Af4 and MLL-AF9 cells, respectively (Figure 2A).19 Of note, more than half of the prednisolone response–related genes appeared to be the common targets of the 2 fusions but were differentially regulated (Figure 2B-C).
Prednisolone response genes are differentially regulated between MLL-Af4 and -AF9 ALL. (A) GSEA to assess enrichment of prednisolone-resistant and -sensitive signatures in MLL-Af4 compared with MLL-AF9 cells. (B) Heat map of resistant and sensitive signature genes from the leading-edge subsets of GSEA analysis in panel A. The genes with MLL-Af4 or -AF9 binding peaks were designated with red and black squares, respectively. (C) ChIP-seq tracks at GSPT1 loci as an example of resistant genes upregulated in MLL-Af4 ALL. (D) Unsupervised hierarchical clustering of MLL fusion ALL patient samples based on the expression of prednisolone response genes shown in panel B. Samples were clustered into 3 groups according to the expression pattern. Patient sample data was from the Andersson data set.8 (E) Distribution of MLL-AF4 and other MLL fusion patient samples in 3 clustering groups described in panel D. P value was calculated by χ2 test. NES, normalized enrichment score.
Prednisolone response genes are differentially regulated between MLL-Af4 and -AF9 ALL. (A) GSEA to assess enrichment of prednisolone-resistant and -sensitive signatures in MLL-Af4 compared with MLL-AF9 cells. (B) Heat map of resistant and sensitive signature genes from the leading-edge subsets of GSEA analysis in panel A. The genes with MLL-Af4 or -AF9 binding peaks were designated with red and black squares, respectively. (C) ChIP-seq tracks at GSPT1 loci as an example of resistant genes upregulated in MLL-Af4 ALL. (D) Unsupervised hierarchical clustering of MLL fusion ALL patient samples based on the expression of prednisolone response genes shown in panel B. Samples were clustered into 3 groups according to the expression pattern. Patient sample data was from the Andersson data set.8 (E) Distribution of MLL-AF4 and other MLL fusion patient samples in 3 clustering groups described in panel D. P value was calculated by χ2 test. NES, normalized enrichment score.
We validated the fusion-directed gene expression pattern in a published patient data set of MLL-rearranged ALL.8 Indeed, upregulation of genes encoding ribosomal proteins and BAALC was evident in a set of MLL-AF4 but not MLL-AF9 patient samples (supplemental Figure 4C-D). Furthermore, patient samples can be segregated into 3 groups based on the expression levels of prednisolone response genes, and the majority of MLL-AF4 samples were found in the “resistant” group. In contrast, all other MLL fusion types were assigned into the “medium” and “sensitive” groups (Figure 2D-E). Taken together, these results suggest that the differentially regulated targets of MLL fusions may affect disease physiology and therapy response.
Previous studies in established leukemia cell lines and patient samples have suggested that MLL fusion proteins differ in their chromatin binding capacities. However, the interpretation of these findings can be confounded by the distinct genomic backgrounds associated with those cells and the use of different fusion partner–specific antibodies for profiling each fusion.20,21 Leveraging our isogenic ALL models, we are able to directly compare the chromatin binding of 2 MLL fusion proteins with the same anti-FLAG antibody and clearly demonstrate the instructive role of fusion partners in directing this process. Our models are based on retroviral transduction in CD34+ hematopoietic stem and progenitor cells, which reliably recapitulate many critical molecular features of primary disease as validated across multiple independent biological replicates.11,14,22 However, due to the complexity of the CD34+ population, the exact cell of origin for each ALL subtype remains elusive and may differ. Indeed, we previously showed that MLL-Af4 and -AF9 cells are blocked at distinct stages of B-cell development, which may also contribute to variations in gene expression.11 Overall, our study highlights that the transcriptional heterogeneity instructed by fusion partners potentially affects the prognostic biomarkers and therapeutic response of MLL-rearranged ALL. Although therapies targeting the shared mechanisms of MLL fusion pathology hold clinical promise,3,23 customizing treatment strategies for each fusion type may help maximize the therapeutic efficacy.
Acknowledgments: The authors thank members of the Mulloy, Bonifer and Barski laboratories for experimental support.
This work was supported by National Institutes of Health (NIH), National Cancer Institute grant CA215504 (J.C.M.), an Institutional Clinical and Translational Science award from NIH, National Center for Research Resources (1UL1RR026314-01), a Translational Trials Development and Support Laboratory award (United States Public Health Service, grant MO1 RR 08084), a CancerFree KIDS Research award (S.L.), and the Academic and Research Committee of Cincinnati Children’s Hospital Medical Center (J.C.M.). S.L. was a Fellow of the Leukemia and Lymphoma Society. Work in the Bonifer laboratory was funded by a program grant from Bloodwise (15001).
Contribution: S.L. and J.C.M. conceived and designed the study, analyzed data, and wrote the manuscript; M.K. and A.B. analyzed data and wrote the manuscript; S.L., S.V., A.P., and M.W. performed experiments; S.A.A. and C.B. analyzed data; and all authors read, edited, and approved the final manuscript.
Conflict-of-interest disclosure: A.B. is a cofounder of Datirium, LLC, a company developing SciDAP data analysis platform used in this manuscript and is on the scientific advisory board of Liposomal Disease Research and Training Center, LLC. M.K. developed CWL-Airflow, licensed to Datirium, LLC. The remaining authors declare no competing financial interests.
The current affiliation for C.B. is Murdoch Institute, University of Melbourne, Melbourne, Australia.
Correspondence: Shan Lin, Seattle Children's Research Institute, Cure 9, 1920 Terry Ave, Seattle, WA 98101; email: shan.lin@seattlechildrens.org; James C. Mulloy, Cincinnati Children’s Hospital Medical Center, S7.603, 240 Albert Sabin Way, Cincinnati, OH 45229; email: james.mulloy@cchmc.org; and Artem Barski, Cincinnati Children’s Hospital Medical Center, MLC7028, 240 Albert Sabin Way, Cincinnati, OH 45229; email: artem.barski@cchmc.org.
References
Author notes
S.L. and M.K. contributed equally to this work.
RNA sequencing data are available at Gene Expression Omnibus (accession number GSE76978); chromatin immunoprecipitation sequencing data are available at BioProject (PRJNA1048517).
The full-text version of this article contains a data supplement.