Chimeric antigen receptor T-cell (CAR-T) therapy has proven successful for B-cell lymphomas and leukemias. This success has inspired the development of CAR-T for T-cell malignancies. T-cell lymphomas and T-cell acute lymphoblastic leukemia (T-ALL) are highly heterogenous diseases but are united by poor prognosis in the relapsed/refractory setting and the lack of any novel, targeted therapies. CAR-T therapy is a promising solution for these diseases but carries a number of challenges, principally that target antigens are typically shared between malignant and normal T cells. This can cause issues with fratricide and T-cell aplasia. In this review we discuss the current state of CAR-T treatment for T-ALL and T-cell lymphomas, highlighting recent novel clinical data for T-cell malignancies and discuss lessons that can be learned for future research in this area.
Introduction
T-cell leukemias and lymphomas encompass a heterogenous group of aggressive hematological neoplasms, which are often chemotherapy-resistant and with poor prognosis. T-cell acute lymphoblastic leukemia (T-ALL)/T-lymphoblastic lymphoma (T-LBL) account for 25% and 10% to 15% of adult and pediatric cases of ALL, respectively.1,2 T-cell lymphomas (TCLs) encompass ∼12% of all non-Hodgkin lymphomas and are either indolent, such as most cutaneous TCL (CTCL) or aggressive such as peripheral mature TCLs (PTCLs).
Slow progress has been made in advancing immunotherapies for T-cell malignancies. The only approved mAb for TCL is mogamulizumab, a humanized anti-CCR4 immunoglobulin G antibody.3,4 In T-ALL, although there are no licensed agents, the anti-CD38 mAb daratumumab showed recent clinical efficacy.5 Antibody-drug conjugates (ADCs) represent an alternative approach. In 2022 the US Food and Drug Administration approved brentuximab vedotin, a CD30-targeting ADC, for CD30+TCLs.6 Bispecific T-cell–engaging antibodies like blinatumomab have emerged as an effective option for B-ALL but no equivalent exists for T-ALL.
Chimeric antigen receptor (CAR) T-cell (CAR-T) therapy, in which engineered T cells express a synthetic CAR that targets tumor antigens in a major histocompatibility complex (MHC)–independent manner, represent 1 of the most significant advances in treatment of B-cell malignancies in recent years.7 As such, there has been enthusiasm to develop an equivalent CAR-T therapy for T-cell malignancies. Several unique challenges have hampered this. Recent clinical breakthroughs, however, suggest the future of CAR-T in T-ALL and TCL is highly promising, with real hope that CAR-T could provide a cure for many patients. This review will focus on novel and emerging CAR-T clinical data in T-cell malignancies and discuss lessons that can be learned from initial clinical studies to guide future research in this field.
Challenges for CAR-T development in T-cell malignancies
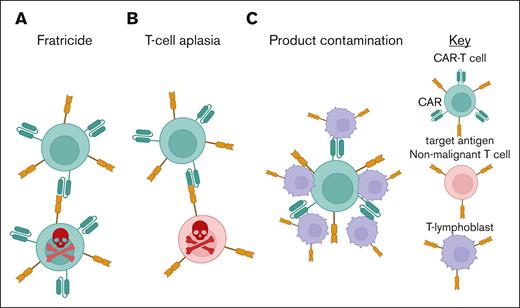
Most targetable T-cell antigens are not tumor specific, being expressed also on healthy T cells (Figure 1). One of the most significant obstacles is “fratricide,” in which expression of the target antigen on the CAR-T leads to self-directed killing, resulting in difficulties with manufacture and risking functional loss of the CAR-T product in vivo.8
T-cell aplasia is a natural consequence of pan-T antigen–targeting CAR-T therapy. Unlike B-cell aplasia in which immunoglobulin G can provide some immune protection,9,10 there is no analogous replacement therapy for T cells, leading to potentially high rates of severe opportunistic infections.10
Product contamination describes the theoretical risk that during CAR-T manufacture malignant T cells are inadvertently harvested, reinfused, and/or transduced with the CAR construct, leading to risk of relapse, as previously described with anti-CD19 CAR in B-ALL.11 This risk could be higher in T- vs B-cell malignancies because of lack of a distinguishing cell surface marker to isolate only normal T cells, in patients with often high levels of circulating tumor.
The various approaches to reduce fratricide, T-cell aplasia and product contamination have been extensively reviewed elsewhere, and are not discussed in detail in this review but are summarized in Figure 2.12-15 Success in overcoming fratricide and CAR-T manufacture has allowed progress to clinical-stage testing.
The major challenges of CAR-T therapy in T-cell malignancies. (A) Fratricide, in which CAR-T expressing the target antigen are “self killed” by fellow CAR-T. (B) T-cell aplasia, in which normal T cells that also express the target antigen are killed by the CAR-T in “on-target, off-tumor” toxicity. (C) Product contamination, the risk that T lymphoblasts contaminate the final CAR-T product.
The major challenges of CAR-T therapy in T-cell malignancies. (A) Fratricide, in which CAR-T expressing the target antigen are “self killed” by fellow CAR-T. (B) T-cell aplasia, in which normal T cells that also express the target antigen are killed by the CAR-T in “on-target, off-tumor” toxicity. (C) Product contamination, the risk that T lymphoblasts contaminate the final CAR-T product.
Summary of potential strategies for overcoming fratricide, T-cell aplasia, and product contamination. G-D, gamma-delta.
Summary of potential strategies for overcoming fratricide, T-cell aplasia, and product contamination. G-D, gamma-delta.
CAR-T clinical trial data in T-cell malignancies
Clinical testing of CAR-T for T-cell malignancies is in its infancy. However, particularly in T-ALL, there have been recent exciting clinical developments. Below we discuss relevant ongoing trials, focusing on those with reported results (Table 1; supplemental Table 1).
Summary of all currently reported trials of CAR-T cell therapy in T-cell malignancies
| Cell source . | Target . | Fratricide prevention . | Patients enrolled . | Patients who received infusion . | CR rate at day 30 . | Toxicity . | Median follow-up . | Survival . | Reference . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CRS . | ICANS . | ICAHT . | Infection . | GVHD . | PFS . | OS . | ||||||||
| Auto | CD7 | Natural selection | 65 (35 T-ALL, 25 T-LBL) | 60 | 94% | Gd 1-2 (80.0%), Gd 3-4 (11.7%) | Gd 1 (3.3%), Gd 4 (1.7%) | Gd ≥3 (61.7%) | 36.7% (any Gd), Gd 3 (16%) | 3/11 (27%) mild | 368.5 d | 2 y 53.7% | 2 y 63.5% | 21 |
| CD7 | PEBL | 30 | 27 | 85% | Gd 1-2 (71%), Gd 3-4 (8%) | Gd 1 (7%) | Gd ≥3 (100%) | Gd 2 (8%) | Gd 2 (4%) | 12 mo | 1 y 22% | 1 y 79% | 22 | |
| CD7 | PEBL | 8 (1 T-ALL, 1 MPL, 6 T-LBL) | 8 | 75% | Gd 1-2 (87.5%), Gd 4 (12.5%) | Nil | Any Gd (100%) | Gd 5 (12.5%) | Nil | N/A | N/A | N/A | 23 | |
| CD7 | PEBL | 20∗ | 17 | 94% | Gd 1-2 (77%) | Gd 1 (12%) | Gd ≥2 (100%) | Gd 1-2 (18%), Gd 3 (29%) | N/A | 15 mo | N/A | N/A | 24 | |
| Allo | CD7 | PEBL | 20 | 20 | 85% | Gd 1-2 (90%), Gd ≥3 (10%) | Gd 1-2 (15%) | Gd ≥3 (100%) | Gd 3-4 (5%), Gd 5 (20%) | Gd 1-2 (60%) | 27 mo | 2 y 36.8% | 2 y 42.3% | 26 |
| CD7 | CRISPR/Cas9 CD7 KO | 28 | 26 | 52% | 88.5% (any Gd), Gd ≥3 (19%) | Gd 1 (7.7%) | Neutropenia (19.2%) | Gd 3-4 (19.2%) | Gd 2 (3.8%) | N/A | N/A | N/A | 27 | |
| CD7 | CRISPR/Cas9 CD7 KO | 12 (7 T-ALL, 4 T-LBL, 1 AML) | 12 | 64% | Gd 1-2 (83%) | Nil | Gd 4 neutropenia (100%) | Gd 5 (8%) | Nil | 10.5 mo | N/A | N/A | 30 | |
| CD7 | CRISPR/Cas9 CD7 KO | 12 (11 T-ALL, 1 T-LBL) | 12 | 92% | 83% (any Gd), Gd 3 (67%) | Nil | N/A | Gd 5 (8%) | N/A | N/A | N/A | N/A | 29 | |
| CD7 | Base-editing CD7 | 3† | 3 | 67% | Gd 2 (66%) | Gd 1 (33%) | Gd ≥4 (66%) | Gd 5 (33%) | Gd 2 (33%) | N/A | N/A | N/A | 31 | |
| Auto | CD5 | None | 17 (TCL) | 9 | 22% | Gd 1-2 (44%) | Gd 2 (11%) | Any Gd (56%) | Gd ≥3 (11%) | N/A | N/A | N/A | N/A | 32 |
| Allo | CD5 | CRISPR/Cas9 CD5 KO | 19 (T-ALL) | 16 | 94% | Gd 1-2 (75%) | Gd 1-2 (25%) | Gd ≥3 (100%) | Gd 3 (12%), Gd 5 (25%) | Gd 1 (69%) | 14.3 mo | N/A | NR (cohort B), 4.6 mo (cohort A) | 33 |
| Auto | TRBC1 | None | 12 (TCL) | 12 | 50% | Any Gd (50%) | Nil | Gd 3-4 neutropenia (80%) | None Gd ≥3 | N/A | N/A | N/A | N/A | 34 |
| Allo | CD70 | CRISPR/Cas9 CD70 KO | 18 (TCL) | 18 | 22% | Gd 1-2 (56%) | Gd 1-2 (17%) | N/A | Gd 1-2 (28%), Gd ≥3 (22%) | Nil | N/A | N/A | N/A | 35 |
| Cell source . | Target . | Fratricide prevention . | Patients enrolled . | Patients who received infusion . | CR rate at day 30 . | Toxicity . | Median follow-up . | Survival . | Reference . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CRS . | ICANS . | ICAHT . | Infection . | GVHD . | PFS . | OS . | ||||||||
| Auto | CD7 | Natural selection | 65 (35 T-ALL, 25 T-LBL) | 60 | 94% | Gd 1-2 (80.0%), Gd 3-4 (11.7%) | Gd 1 (3.3%), Gd 4 (1.7%) | Gd ≥3 (61.7%) | 36.7% (any Gd), Gd 3 (16%) | 3/11 (27%) mild | 368.5 d | 2 y 53.7% | 2 y 63.5% | 21 |
| CD7 | PEBL | 30 | 27 | 85% | Gd 1-2 (71%), Gd 3-4 (8%) | Gd 1 (7%) | Gd ≥3 (100%) | Gd 2 (8%) | Gd 2 (4%) | 12 mo | 1 y 22% | 1 y 79% | 22 | |
| CD7 | PEBL | 8 (1 T-ALL, 1 MPL, 6 T-LBL) | 8 | 75% | Gd 1-2 (87.5%), Gd 4 (12.5%) | Nil | Any Gd (100%) | Gd 5 (12.5%) | Nil | N/A | N/A | N/A | 23 | |
| CD7 | PEBL | 20∗ | 17 | 94% | Gd 1-2 (77%) | Gd 1 (12%) | Gd ≥2 (100%) | Gd 1-2 (18%), Gd 3 (29%) | N/A | 15 mo | N/A | N/A | 24 | |
| Allo | CD7 | PEBL | 20 | 20 | 85% | Gd 1-2 (90%), Gd ≥3 (10%) | Gd 1-2 (15%) | Gd ≥3 (100%) | Gd 3-4 (5%), Gd 5 (20%) | Gd 1-2 (60%) | 27 mo | 2 y 36.8% | 2 y 42.3% | 26 |
| CD7 | CRISPR/Cas9 CD7 KO | 28 | 26 | 52% | 88.5% (any Gd), Gd ≥3 (19%) | Gd 1 (7.7%) | Neutropenia (19.2%) | Gd 3-4 (19.2%) | Gd 2 (3.8%) | N/A | N/A | N/A | 27 | |
| CD7 | CRISPR/Cas9 CD7 KO | 12 (7 T-ALL, 4 T-LBL, 1 AML) | 12 | 64% | Gd 1-2 (83%) | Nil | Gd 4 neutropenia (100%) | Gd 5 (8%) | Nil | 10.5 mo | N/A | N/A | 30 | |
| CD7 | CRISPR/Cas9 CD7 KO | 12 (11 T-ALL, 1 T-LBL) | 12 | 92% | 83% (any Gd), Gd 3 (67%) | Nil | N/A | Gd 5 (8%) | N/A | N/A | N/A | N/A | 29 | |
| CD7 | Base-editing CD7 | 3† | 3 | 67% | Gd 2 (66%) | Gd 1 (33%) | Gd ≥4 (66%) | Gd 5 (33%) | Gd 2 (33%) | N/A | N/A | N/A | 31 | |
| Auto | CD5 | None | 17 (TCL) | 9 | 22% | Gd 1-2 (44%) | Gd 2 (11%) | Any Gd (56%) | Gd ≥3 (11%) | N/A | N/A | N/A | N/A | 32 |
| Allo | CD5 | CRISPR/Cas9 CD5 KO | 19 (T-ALL) | 16 | 94% | Gd 1-2 (75%) | Gd 1-2 (25%) | Gd ≥3 (100%) | Gd 3 (12%), Gd 5 (25%) | Gd 1 (69%) | 14.3 mo | N/A | NR (cohort B), 4.6 mo (cohort A) | 33 |
| Auto | TRBC1 | None | 12 (TCL) | 12 | 50% | Any Gd (50%) | Nil | Gd 3-4 neutropenia (80%) | None Gd ≥3 | N/A | N/A | N/A | N/A | 34 |
| Allo | CD70 | CRISPR/Cas9 CD70 KO | 18 (TCL) | 18 | 22% | Gd 1-2 (56%) | Gd 1-2 (17%) | N/A | Gd 1-2 (28%), Gd ≥3 (22%) | Nil | N/A | N/A | N/A | 35 |
Allo, allogeneic; AML, acute myeloid leukemia; auto, autologous; Gd, grade; KO, knockout; MPL, mixed pjenotype leukemia; N/A, not available; NR, not reached; OS, overall survival.
Only 1 of 17 enrolled in a clinical trial.
Only first 3 patients reported.
T-ALL/T-LBL
CD7
CD7 is a type 1 transmembrane glycoprotein with high and uniform surface expression in 95% of T-ALL, a subset of TCL, and mature T and natural killer (NK) cells but no other nonhematopoeitic cells.16,17 The role of CD7 in T cells is not clear, although CD7-deficient mice have no significant immunodeficiency.18 Less than 5% of T cells are CD7−.19
AUTOLOGOUS CD7 CAR-T PRODUCTS
In a phase 1 trial (ClinicalTrials.gov identifier: NCT04572308), T cells were unmanipulated before CD7 CAR transduction, undergoing “natural selection” of CD7− cells during manufacture.20 Overall, 95% of patients (14 with T-ALL, 6 with T-LBL) achieved measurable residual disease (MRD)–negative bone marrow complete response (CR), and 25% achieved extramedullary CR. Eighteen patients experienced grade ≤2 cytokine release syndrome (CRS), with 1 grade 3 CRS and 2 grade 1 neurotoxicity (immune effector cell–associated neurotoxicity syndrome [ICANS]).20 In the phase 2 follow-up (35 patients with T-ALL and 25 with T-LBL), 2-year overall survival and progression free survival (PFS) were 63.5% and 53.7%, respectively, with PFS significantly higher in the 37 CR patients consolidated with allogeneic hematopoietic stem cell transplant (allo-HSCT). Overall, 8 of 10 patients without transplant relapsed, largely with CD7−disease. Any-grade infections, (sepsis, lung infections, and cytomegalovirus [CMV] or Epstein-Barr virus [EBV]), occurred in 36.7%.21
In another study (NCT04840875), surface CD7 was downregulated using anti-CD7 protein expression blocker (PEBL). Only patients without detectable peripheral lymphoblasts were enrolled and CAR-T products were only released after being confirmed leukemia free. Hematotoxicity (immune effector cell–associated hematotoxicity [ICAHT]) was the most common adverse event but was grade ≤2 in 63% by day 30. There were 2 grade 3/4 CRS but only 2 grade 1 ICANS events. Overall, 20 of 27 underwent allo-HSCT. All 7 without allo-HSCT progressed with either CD7+ or CD7−disease. Of transplant recipients, 15 of 20 remained MRD negative at a median follow-up of 14.1months. Four relapsed after HSCT (3 with CD7+ and 1 with CD7− disease) and 1 died of sepsis.22
In a trial (NCT04004637) of autologous nanobody-derived, PEBL CD7 CAR-T, 6 of 8 patients (75%) achieved CR by day 28. There was 1 grade 4 CRS and no ICANS events; 1 patient in CR died of an abdominal infection.23
In a case series of patients treated with a PEBL-blocked CD7 CAR, 16 of 17 were treated on a hospital-exemption basis and 1 enrolled in an ongoing clinical trial (NCT05043571). All 17 starting products had leukemic cell contamination (0.01%-60.3%) but none was detected after manufacture. There was no grade ≥3 CRS or ICANS; however, all 17 had ICAHT grade ≥2. Overall, 16 of 17 achieved MRD-negative CR. At 15-month median follow-up, 9 of 9 patients who received consolidative allo-HSCT were alive and leukemia free compared with 2 of 8 without transplant. Of the remaining 6, there were 3 relapses (2/3 with CD7− disease). None of the relapsed T-ALL cases showed CAR expression. There were 3 deaths in remission: 1 fungal infection and 2 neurological complications.24
ALLOGENEIC CD7 CAR-T PRODUCTS
In a phase 1/2 trial of PEBL-blocked CD7 CAR-T (NCT04689659), 20 patients with T-ALL were treated with CAR-T manufactured from a previous HLA-matched or haploidentical HSCT donor, or new compatible donor. Overall, 17 of 20 achieved MRD-negative CR and 7 proceeded to allo-HSCT. There was 10% grade 3/4 CRS and 60% grade 1/2 acute graft-versus-host disease (GVHD). There were 4 viral reactivations. One patient without transplant died of fungal pneumonia at 5.5 months.25 At 2-year follow-up, PFS and overall survival rates were 36.8% and 42.3%, respectively. Overall, 6 of 7 patients who received transplant relapsed (CD7− in 4/6; NCT04689659). Long-term serious adverse events in 12 patients without allo-HSCT included 4 grade 5 infections and 1 grade 4 gut GVHD.26
In the phase 2 WU-CART-007 trial (NCT04984356) CD7 and T-cell receptor α constant (TRAC) were edited. Overall, 82% (9/11) of evaluable patients achieved CR. At 2.7-month median follow-up, 6 of 11 remained in remission. CRS occurred in all (grade ≥3 in 4). Grade ≥3 infections occurred in 5 of 13. There was no GVHD. There were 2 grade 5 events (fungal sepsis and CRS/disease progression).27
In a phase 1 trial (NCT04538599) in 12 patients (11 with T-ALL/T-LBL and 1 with acute myeloid leukemia), CD7 CAR-T were manufactured from healthy donor peripheral blood mononuclear cells, using clustered regularly interspaced short palindromic repeats (CRISPR) and CRISPR-associated protein 9 (Cas-9) editing of CD7, T-cell receptor (TCR), and HLA class II, and to introduce an NK cell inhibitor. Overall, 63.6% (7/11) achieved CR. Three responders underwent allo-HSCT. Four patients remained in remission at 10.5-month median follow-up. No dose-limiting toxicities (DLTs), GVHD, or ICANS were reported. Grade 1 to 2 CRS occurred in 10 patients. CMV and EBV reactivations were observed, and 1 patient died from EBV+ diffuse large B-cell lymphoma.28
GC027 is a CD7 CAR-T using CRISPR-Cas9 disruption of CD7 and TRAC. Overall, 11 of 12 patients with relapsed/refractory (R/R) T-ALL/T-LBL achieved CR (ChiCTR1900025311). One patient was consolidated with allo-HSCT but died from GVHD. Four patients relapsed, with either CD7+ or CD7− disease.29
A novel strategy of CD7 CAR-T therapy followed by haploidentical HSCT, without myeloablation or GVHD prophylaxis recently reported 100% CR with incomplete count recovery rates in 10 patients. Six remained in remission at a median 15.1-month follow-up, whereas 2 had relapse with CD7−disease, and 1 died of sepsis.30
In initial reports using anti-CD7 allogeneic CAR-T, base-edited to inactivate CD52, CD7, and TCR β constant (TRBC), 2 children with R/R T-ALL sustained molecular remission and immunologic reconstitution after CAR-T followed by allo-HSCT consolidation. One further patient achieved MRD-positive remission but died of an opportunistic fungal infection.31
CD5
CD5 is a transmembrane glycoprotein expressed in ∼80% of T-ALL and 87.5% of PTCL.36,37 Normal expression is limited to T cells and a small B-cell population.38
Initial preclinical reports of unmanipulated anti-CD5 CAR-T showed limited and transient fratricide, with downregulation of CAR-T CD5 expression.39,40 A recent report found that this was because of rapid and complete internalization of CD5.41
The Baylor group has published preliminary data using anti-CD5 CAR-T in T-ALL. Initial response rates using a conventional manufacturing process were low (1/8 remissions), which was associated with terminally differentiated phenotype of CAR-T. Responses were improved when the tyrosine kinase inhibitors dasatinib and ibrutinib were added during manufacture. In the tyrosine kinase inhibitor–manufactured cohort, 4 of 7 patients achieved MRD-negative CR. Two patients developed EBV viremia and died from posttransplant lymphoproliferative disorder.32
In a phase 1 trial of an allogeneic gene-edited CD5 CAR-T (NCT05032599), 19 patients with R/R T-ALL were recruited, with successful manufacture in 16. CAR-T products were derived from previous transplant donors (cohort A, n = 11) or newly matched donors (cohort B, n = 5). Overall, 100% (16/16) of patients achieved CR/CR with incomplete count recovery. Patients were heavily pretreated including 10 of 16 who had received prior CD7 CAR-T. All experienced grade 3/4 ICAHT, which did not recover in 7 of 11 from cohort A; 75% had grade 1/2 CRS and 25% had grade 1/2 ICANS. Overall, 69% developed grade 1 skin GVHD. Of 4 patients with consolidative allo-HSCT, 3 remained in remission at median follow-up of 14.3 moths. Of 12 patients who did not undergo transplant, 2 patients remained in remission, whereas 3 relapsed (CD5−), 5 died of infection and 2 died of thrombotic microangiopathy. Cohort A had a high rate of fatal late-onset infections resulting in trial termination; 2 grade 3 and 4 grade 5 infections occurred including bacterial, fungal, EBV, and CMV infections.33
TCL
CD5
The MAGENTA trial (NCT03081910) reported results from 17 patients with TCL. CAR-T were successfully manufactured for 13 and administered to 9. No grade >2 CRS or ICANS was observed. There was a 44% overall response rate and 2 CRs.42 This study persists with an updated manufacturing process as described above.
Three patients with angioimmunoblastic TCL and subcutaneous panniculitis-like TCL received CD5 CAR-T with an engineered truncated human epidermal growth factor receptor safety switch (NCT04767308). One patient achieved CR but died from sepsis. The remaining 2 patients achieved partial response (PR), with 1 receiving allo-HSCT and 1 relapsing on day 102. All 3 patients developed grade 1 CRS and all developed grade 3/4 rash of uncertain etiology.43
TRBC1/2
In αβTCR+ T cells the TRBC gene encodes for the β-chain in the TCR. Human mature T cells are a mixture of TRBC1+ or TRBC2+ TCRs, whereas clonal TCLs are positive for 1 or the other.44 More than 95% of PTCLs and >30% of T-ALL cases express αβTCR,45,46 suggesting it may be a target for CAR-T therapies with limited risk of fratricide or T-cell aplasia. Anti-TRBC1 CAR-T were potent in preclinical models of T-ALL, with preservation of the healthy TRBC2+ compartment.47 A complementary TRBC2-specific antibody was developed using computational biology.48,49
The AUTO4 (NCT03590574) clinical trial of anti-TRBC1 CAR-T in PTCL has reported; 5 of 10 (50%) patients who received infusion achieved complete metabolic response (CMR), with 2 further PRs. Three patients (33%) did not respond. Only 33% (n = 3) of patients experienced CRS (grade ≤3), with no ICANS or DLTs. Three of 4 patients receiving the highest dose (450 × 106 CAR-T) obtained CMR. CAR-T were not detected in the peripheral blood beyond 1 week but were identified in lymph nodes.34 “Reverse-kill” of CAR-T by recovering normal TRBC1+ T cells may be responsible for short CAR-T persistence.
CD4
CD4 is a transmembrane glycoprotein present on a subset of T cells that serves as a coreceptor for the TCR.50 CD4 is expressed in ∼50% of PTCL45 and in some T-ALL.51
Results from 3 patients from an ongoing trial of CD4 CAR-T in TCL and leukemia (NCT03829540) have been presented. Manufacture was feasible and there were no DLTs. Two patients (PTCL and T-ALL) achieved CR, and 1 (mycosis fungoides) achieved hematological CR.52
Three patients with TCL have been treated with CD4-IL15/IL15sushi CAR-T (NCT03829540). One patient (Sézary syndrome) achieved CMR, ongoing at 15 months. A second (mycosis fungoides) achieved PR. The third (angioimmunoblastic TCL) achieved CR. CD4 T-cell aplasia was seen but CD3+ CD8+ T cells and NK cells expanded, with no significant infectious toxicity and no grade ≥3 CRS or ICANS.53
CD30
CD30 is expressed in Hodgkin lymphoma and in some TCLs, particularly anaplastic large cell lymphoma (ALCL), in which CD30-targeting ADC brentuximab vedotin has been effective.54-56 CD30 expression in normal tissues is limited to activated T cells.
Few early-phase clinical trials have targeted CD30 exclusively in TCLs,57 although several have included some patients with PTCL (NCT04083495, NCT04526834, and NCT02917083). In 1 trial involving 2 patients with ALCL, there was 1 CR that persisted 9 months after the fourth infusion of CAR-T.58 Another trial of CD30 CAR-T as consolidation after autologous transplant included 6 patients with TCL, with no DLTs and acceptable safety profile. At a median follow-up of 48.2 months, all patients with TCL had died, 5 from relapse and 1 from unrelated lung cancer.59
CD70
CD70 is a type 2 transmembrane glycoprotein that has limited expression in normal tissues, apart from activated T cells.60 CD70 expression has been described in most TCLs apart from anaplastic lymphoma kinase–positive ALCLs.61
In a phase 1 study of allogeneic CD70 CAR-T with multiplexed CRISPR-Cas9 deletion of TRAC, beta-2-microglobulin, and CD70 in 18 patients with R/R TCL (COBALT-LYM; NCT04502446) CTX130 showed 22% CR rate, with no DLTs. There was 1 grade ≥3 infection, and 1 death from an unrelated pneumonia. Median duration of response was only 1.8 months with short CAR-T persistence.35
CD37
CD37 is a 4-pass transmembrane protein that is expressed in CTCL and PTCL. Normal expression is limited to lymphoid cells.62-64
A phase 1 trial of CD37 CAR-T in B- and T-cell cancers has reported results from (NCT04136275) an initial 5 patients.65 Only 1 patient had a nasal NK cell lymphoma/TCL. This patient had a mixed response followed by rapid CD37 loss after treatment. They died from disease progression on day 32 after CAR infusion.
What have we learned about CAR-T for T-cell malignancies thus far?
Emerging clinical data in CAR-T for T-cell malignancies clearly shows that it is feasible and may be effective. These first early-phase trials do however raise many questions, the answers to which will guide future rational CAR-T and clinical trial design.
Is product contamination a concern for autologous manufacture?
Although lymphoblasts have been detected in some initial apheresis products, none have been detected in final manufactured products to date.22 In addition, no CAR transgenes have been reported in relapses after CD7 and CD5 CAR-T, suggesting clinically significant inadvertent blast transduction has not occurred.22,24,33
Varying approaches to mitigate product contamination have been used. Some trials have enrolled only patients with MRD undetectable/low disease, whereas others have chosen to test apheresis and final CAR-T products before release.22 In a recent case series, CAR-T products were manufactured even in the presence of high levels of circulating disease with no postmanufacture malignant cells detected, suggesting “self-purging” of malignant cells.24
Thus, although there is no evidence that manufacturing from patients with high levels of circulating disease increases contamination risk, it seems prudent to reduce tumor bulk with bridging therapies before apheresis when possible, particularly given that outcomes after CAR-T are improved in the setting of low-burden disease. Further study is required to determine optimal and safe inclusion/exclusion criteria for future clinical trials, and these may vary according to target and disease.
Is an autologous or allogeneic approach preferable?
Allogeneic CAR-T have some theoretical advantages when treating T-cell malignancies, including eliminating concerns about product contamination and T-cell fitness. Off-the-shelf products could also provide rapid access to treatment. Allogeneic CAR-T also have important potential limitations. Donor cells may cause GVHD. In an anti-CD7 study using previous or new HLA-matched allogeneic donors, 60% developed GVHD.26 To reduce GVHD risk and to allow use of off-the-shelf CAR-T, investigators have deleted genes involved in GVHD (TCR), as well as fratricide (CD7, CD70) with/without immune rejection (CD52/beta-2-microglobulin), using CRISPR-Cas9 or base editing. Although this has eliminated GVHD risk in studies to date, genome engineering introduces other challenges. These include the current logistical difficulty of manufacture with potential impact on T-cell product quality, risk of translocations, and unknown long-term safety profile of genetically edited cells, plus risk of rapid immune rejection.
Most allo-CAR-T tested in T-cell malignancies have been manufactured from HLA-matched/haploidentical donor cells and are not truly off-the-shelf.26 HLA-matched CD7- and CD5-targeting CAR-T have shown high initial response rates in T-ALL, equivalent to autologous CAR-T. Encouragingly, expansion and long-term persistence in the absence of allo-HSCT has typically been seen.
Only limited data are available for off-the-shelf CAR-T. The largest study of CRISPR-Cas9 TRAC/CD7-deleted anti-CD7 CAR-T involved 12 patients with T-ALL. Although 11 of 12 attained CR, only 2 of 12 remained alive in CR at 12 months. Persistence was short with no detectable cells beyond 1 month. Of note, patients did not receive mandatory allo-HSCT consolidation.29 The Great Ormond Street Hospital group is testing base-edited anti-CD7 CAR-T as a bridge to mandatory allo-HSCT, an approach that addresses the challenge of short CAR-T persistence, and has demonstrated efficacy in a case report.31
Thus, although HLA-matched anti-CD7 donor cells appear to have similar efficacy to autologous CAR-T, they require matched donor availability and bespoke manufacture and have a significant GVHD risk. Off-the-shelf CAR-T are in their infancy, and it remains to be proven that they can be as effective as autologous CAR-T, can function as a stand-alone therapy or are safe in the long term.
Is CAR-T for T-cell malignancies prohibitively toxic?
Rates of CAR-T–specific toxicities in clinical trials (CRS and ICANS) have generally been low and of mild-to-moderate severity across a range of antigens such as CD7, CD5, and TRBC1. By contrast, significant and often prolonged hematotoxicity has been reported in trials of both CD7 and CD5 CAR-T.26,34,42,53 It remains unclear whether such hematotoxicity can be attributed to the CAR-T itself, because patients were often heavily pretreated and had undergone bridging chemotherapy as well as lymphodepletion. Overall, rates of these CAR-related toxicities appear broadly comparable with those of CD19 CAR-T in B-cell malignancies.
In all CD7 CAR trials to date, CD7+ T and NK cells have been rapidly and completely eliminated. In parallel, the usually small subset of naturally CD7− T and NK cells has expanded, although total T and NK cells have remained lower than normal. CD7− cells, although displaying restricted TCR diversity, could react against viral and fungal pathogens.25 Grade ≥3 events from bacterial, viral, and fungal infections have been reported across all CD7 trials. However, although the data is confounded by the high numbers of patients undergoing transplantation after CAR-T, anti-CD7 CAR-T appear less immunosuppressive than initially expected.
In CD5 CAR-T trials the impact on normal T cells is less clear. In the largest reported study in T-ALL, there was loss of normal CD5+ T cells with some recovery of naturally occurring or gene-edited CD5− T cells,33 which may be partially protective. However, viral reactivation has been seen, with multiple infectious deaths including 4 due to EBV+ posttransplant lymphoproliferative disorder from 23 reported patients.
It remains unclear what the long-term impact of the loss of CD7+/CD5+ T cells and NK cells on the immune repertoire and infectious toxicity will be. This will be critical to understand whether these therapies can be used as curative treatments without allo-HSCT.
Can anti-CD7 or anti-CD5 CAR-T be a stand-alone therapy?
Before clinical testing, it was broadly expected that CAR-T targeting pan–T-cell antigens would require mandatory allo-HSCT to rescue the profound immune suppression expected to occur. Moreover, HSCT is an effective modality in R/R T-ALL, and, in B-ALL, short persistence of anti-CD19 CAR-T is associated with almost inevitable relapse. However, the unexpected recovery of functional T/NK cells in these patients challenges this proposed paradigm. Given the toxicity associated with allo-HSCT, it would be beneficial whether CAR-T could be curative without allo-HSCT.
Complete remission rates for autologous anti-CD7 CAR-T have been >90% to 95%. However, remissions have often been short-lived in those without allo-HSCT. In a phase 2 study of CD7 CAR-T, PFS was significantly longer in patients who received consolidative transplant than in patients who did not; 80% of those who relapsed developed CD7− disease.21 Furthermore, in a recent case series, 9 of 9 patients who received consolidative allo-HSCT were alive and leukemia free at median follow-up of 15 months, compared with 3 of 8 patients who did not receive transplant.24 A similar pattern was seen in a recent trial of allogeneic CD5 CAR-T, with only 2 of 12 patients without transplant remaining in remission at last follow-up compared with 3 of 4 who did.33
It should be noted that none of these studies randomized patients to allograft or not, and there may be important differences between these groups (eg, fitness for transplant). However, based on the high rates of antigen-negative relapses seen, it seems likely that initial responses to anti-CD7 or -CD5 CAR-T may require allo-HSCT consolidation for long-term remission, which also enables recovery or normal T-cell populations. However, HSCT is not a viable approach for many, especially in T-ALL in which patients often received transplant early because of high-risk features. Thus, further approaches, which may enable stand-alone CAR-T, are desirable.
What other targets are under investigation?
Although pan-T antigens have the advantage of high and near-ubiquitous tumor expression, their corresponding high normal T-cell expression poses considerable challenges.
An ideal target would be 1 with expression limited to neoplastic T cells. Targeting such antigens could avoid fratricide, T-cell aplasia, and product contamination, allowing uncomplicated manufacture of autologous CAR-T as well as potentially avoiding consolidative allo-HSCT.
Research to identify further novel tumor-selective targets in T-ALL and TCL using multiomic techniques is warranted. A few such antigens are already under investigation and are discussed below.
CCR9
C-C chemokine receptor type 9 (CCR9) has roles in thymic T-cell development and intestinal homing. It is expressed on thymocytes and small intestinal lymphoid cells.66 CCR9 is expressed on >70% of T-ALL, (>85% of R/R cases) but only on <5% of mature T cells and no nonlymphoid tissues.67 Anti-CCR9 CAR-T proliferated without fratricide and were potent in preclinical models of T-ALL.67 A phase 1 clinical trial of anti-CCR9 CAR-T for T-ALL opens in the United Kingdom soon.
CD1a
CD1a is a transmembrane glycoprotein that presents lipid-derived antigens to T cells.68 Expression is limited to cortical thymocytes and Langerhans cells.69,70 Anti-CD1a CAR-T expanded without fratricide and eradicated disease in a primary cortical–T-ALL mouse model.71 However, CD1a is expressed only in cortical T-ALL, which has good prognosis.72,73 Analysis of 103 R/R T-ALL cases found only 8 of 103 had high/uniform CD1a expression.72 A phase 1 study of anti-CD1a CAR-T in T-ALL is open (NCT05679895).
CD38
CD38 is a type II transmembrane glycoprotein that mediates signal transduction in immune cells. In T-ALL, 75% of cases had high expression.72 CD38 is also present on activated T cells, B cells, dendritic cells, and NK cells. Daratumumab, an anti-CD38 mAb, showed efficacy in patient-derived T-ALL xenograft models,74 and the DELPHINUS study (NTC03384654) showed an overall response rate of 83.3% and 60% in pediatric and adult patients, respectively,5 demonstrating potential of CD38-targeting therapies.
CD38 CAR-T have shown activity against NK/TCL in preclinical models75 and variable activity against 5 pediatric patient-derived T-ALL xenograft models with prolonged survival in 4 of 5.76 CD38 expression on activated T cells did not impair expansion or function. However, in a xenograft model of normal human hematopoiesis they did deplete hematopoietic precursors, a potential concern for clinical translation.77 No anti-CD38 CAR-T trial data are yet available.
CD21
CCR4
CCR4 is a C-X-C chemokine G protein-coupled receptor expressed on regulatory T cell, T helper 2 cell, T helper 17 cell, and platelet cells and plays roles in signaling.80 CCR4 expression is high in most adult T cell leukemia/lymphoma and CTCL and in 30% to 40% of PTCL. CCR4–CAR-T were potent against patient-derived cell lines and mouse models of TCL, with selective fratricide preserving the T helper 1 cell and CD8+ T-cell compartments.81
Overall, these neoplastic T-cell restricted targets hold promise but clinical development of them lags behind pan-T antigens. Signals of initial efficacy are awaited from ongoing clinical trials.
How can antigen-negative relapses be prevented?
Rates of antigen-negative relapses appear to be high with CD7 CAR-T and pose a considerable therapeutic challenge. Multiantigen-targeted CAR-T are under investigation to prevent antigen-negative relapses in B-cell malignancies, predominantly targeting CD19 and CD22.82-85
In T-ALL, CRISPR-edited CD5/CD7 dual-CARs displayed potent antileukemic activity in vivo. Loss of either CD5 or CD7 had no impact on antileukemic activity.86
Base-edited dual CD3/CD7 CAR-T exhibited potent and specific cytotoxicity against cell lines and pediatric T-ALL samples with varying CD3 and CD7 expression. In in vivo models, dual CD3/CD7 CAR-T exhibited the highest tumor clearance.87
Targeting 2 antigens highly expressed on normal T cells will likely require more complex CAR engineering to avoid fratricide and may result in high infectious toxicity because the ablation of >1 T-cell subset. It may be that such therapies will require mandatory allo-HSCT consolidation.
Recently, a CAR targeting 2 T-cell restricted antigens, CCR9 and CD1a, has been described. Overall, 86% (155/186) of T-ALL cases tested expressed either target. This dual-CAR was more effective in pre-clinical models of T-ALL than respective single-antigen targeting CARs.88
Multiantigen CAR-T in T-ALL has not yet reached clinical testing, but it is likely, taking lessons from B-ALL, that such clinical data will emerge in the future. It remains to be seen whether this approach can overcome antigen-negative escape and improve long-term survival.
Conclusion
Although the development of CAR-T for T-cell cancers is about a decade behind that for their B-cell counterparts, the countless preclinical and clinical studies targeting pan-T and tumor-selective antigens provide optimism.
In T-ALL, CD7- and CD5-targeting CAR-T have shown impressive response rates with some durable remissions, especially when consolidated with allo-HSCT. These data provide evidence that CAR-T can be highly effective against T-ALL. Manufacturing has been feasible, using various strategies to overcome fratricide, with no reports of lymphoblast contamination. In mature TCLs, response rates have generally been less impressive, and antigen heterogeneity makes identification of an ideal CAR-T target here more difficult. However, some durable responses have been obtained with both anti-CD5 and anti-TRBC1 CAR-T.
Significant hurdles remain: how to improve long-term survival without transplantation, how to reduce rates of antigen-negative relapse, how to minimize infectious toxicity, and the optimal approach to develop effective allogeneic products. Despite these challenges, it seems likely that cellular therapies may indeed provide a cure for more patients with T-cell malignancies in the near future.
Authorship
Contribution: N.M. conceived the topic, and wrote and edited the manuscript; B.W. wrote and edited the manuscript; and P.M. edited the manuscript.
Conflict-of-interest disclosure: P.M. owns stock in a publicly traded company, Autolus Ltd. The remaining authors declare no competing financial interests.
Correspondence: Nicola Maciocia, Cancer Institute, University College London, 72 Huntley St, London WC1E 6DD, United Kingdom; email: n.maciocia@ucl.ac.uk.
References
Author notes
The full-text version of this article contains a data supplement.