Key Points
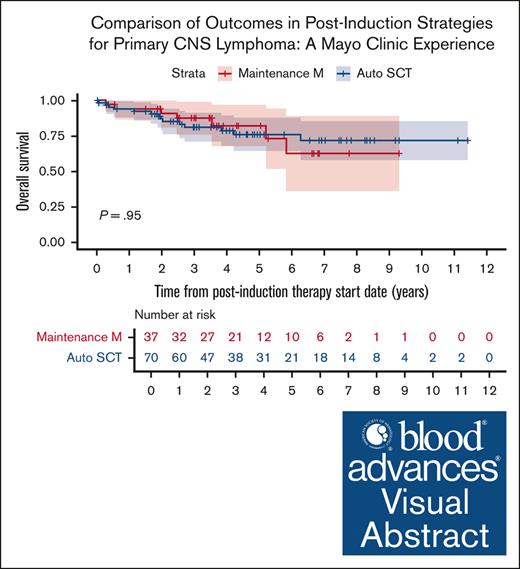
Survival outcomes in patients who received maintenance MTX were comparable with those who underwent auto-SCT.
Historical prognostic indices showed poor correlation with outcomes in our cohort, indicating a need for an updated prognostic score.
Visual Abstract
Primary central nervous system lymphoma (PCNSL) is a rare form of non-Hodgkin lymphoma involving the brain, cerebrospinal fluid, or retina/vitreous without systemic involvement. Induction with high-dose methotrexate (HD-MTX) followed by consolidation with autologous stem cell transplant (auto-SCT) has become the standard treatment paradigm for most patients. However, limited data are available regarding the efficacy of a maintenance approach with HD-MTX. Herein, we retrospectively reviewed the characteristics and outcomes of 148 patients diagnosed with PCNSL between October 2010 and June 2022, who underwent HD-MTX–based induction therapy followed by either auto-SCT consolidation (n = 70) or HD-MTX maintenance therapy (n = 37). At a median follow-up time of 4.5 years, the progression-free survival (PFS) was 8.3 years and the overall survival (OS) was not reached. Compared to patients who underwent auto-SCT, patients who received maintenance HD-MTX had a higher median age at diagnosis of 72 vs 62 years and a trend toward higher proportion of patients being Eastern Cooperative Oncology Group 2 or higher (41% vs 29%). At 5-years postinduction treatment initiation, the PFS rates in the auto-SCT cohort and HD-MTX maintenance cohort were 74.6% and 72.6%, respectively, and the OS rates were 76.0% and 82.4%, respectively. Overall, there was no significant difference in PFS or OS based on postinduction management strategy. Our data suggest that maintenance HD-MTX may be a reasonable, time-limited treatment strategy for patients with PCNSL responding to initial induction therapy.
Introduction
Primary central nervous system lymphoma (PCNSL) is an aggressive form of non-Hodgkin lymphoma involving the brain, leptomeninges, spinal cord, cerebrospinal fluid (CSF), or retina/vitreous without systemic involvement.1-4 PCNSL comprises 1.9% of all primary central nervous system (CNS) tumors and 6.1% of all malignant CNS tumors, with a reported incidence rate of 4 cases per million persons per year.5,6 The risk of PCNSL increases with age, and the disease incidence, particularly in older adults, has been rising, with a reported incidence of 40 cases per million persons per year in the 70 to 79-year-old age group.7
In recent decades, substantial progress has been made in the management of patients with PCNSL. However, this progress has mostly been seen in younger patients, and long-term survival of patients >70 years has remained relatively modest.8 Induction therapy with high-dose methotrexate (HD-MTX)–based regimens followed by consolidation with myeloablative chemotherapy and autologous stem cell transplant (auto-SCT) has become the mainstay of treatment with overall response rates (ORR) of 69% to 87% and 2-year overall survival (OS) of 66% to 70% reported in recent prospective clinical trials.9-13 However, no consensus exists regarding the optimal approach to postinduction treatment in patients who are not candidates for auto-SCT, likely explaining the lack of significant improvement in outcomes in older adults. Median OS in relapsed PCNSL is <12 months, thereby providing the rationale for maintenance therapy.14-17 In addition, as the incidence of PCNSL is rising in the older age group, patient selection is key for consideration of auto-SCT to minimize nonrelapse morbidity and mortality. Use of whole-brain radiation therapy as consolidation has decreased due to significant incidence of neurocognitive decline with memory and gait impairments, apathy, and incontinence.18-21 Recently, there has been increasing interest in evaluating the role for nonmyeloablative chemotherapy and maintenance strategies in postinduction management of PCNSL, with specific agents including temozolomide, procarbazine, lenalidomide, and ibrutinib being studied.22-26 Given the proven efficacy of HD-MTX as part of induction therapy, rationale exists for the use of HD-MTX as part of a maintenance strategy, with previous studies suggesting a potential benefit particularly in older adults with CNS lymphoma.13,27 However, these studies had limited sample sizes and many were performed prior to more recent improvement in therapeutic strategies including the standard inclusion of rituximab with induction regimens.28
In addition, there is a particular interest in prognostication of PCNSL in clinical practice to guide treatment decisions and to help better inform discussions with patients. Some of the most used indices include the International Extranodal Lymphoma Study Group (IELSG; 1980-1999) score,29 Memorial Sloan-Kettering Cancer Center (MSKCC; 1983-2003) score,30 Nottingham/Barcelona (NB; 1986-2001) score,31 and Taipei Score (TS; 2003-2015).32 However, the real-world performance of these prognostication indices has not been thoroughly evaluated. Given the rising incidence of PCNSL in older adults, the interval addition of rituximab to induction regimens, and recent improvement in postinduction management, an updated prognostication index may be needed in modern practice.
Herein, we report a retrospective cohort of 148 patients with newly diagnosed PCNSL treated with HD-MTX–based induction regimens at Mayo Clinic, Rochester, MN, and evaluate the relationship between baseline characteristics and patient outcomes, with particular attention to key clinical prognostic factors associated with survival.
Methods
We conducted a retrospective review of consecutive adult patients (aged ≥18 years) with newly diagnosed PCNSL treated with HD-MTX–based induction therapy between November 2010 and February 2023 at Mayo Clinic in Rochester, Minnesota. Pathological diagnosis of PCNSL was established by Mayo Clinic hematopathology expert review. Staging and treatment decisions were made by the treating physician at the time of patient presentation. The choice of using temozolomide was solely based on physician discretion but was prompted predominantly based on age cut off of <70 or >70 years and Eastern Cooperative Oncology Group (ECOG) performance status (PS) >2. Furthermore, the choice to continue temozolomide during induction and the dosing of MTX was individualized based on the tolerability of the regimen. When possible, a HD-MTX dose of 8gm/m2 was attempted both for induction and maintenance, and was adjusted based on individual patient factors or renal dysfunction, side effects, and tolerability. Patients with prior or concurrent diagnosis of systemic lymphoma were excluded. Selection guidelines for auto-SCT were based on the institutional practice requiring Eastern Cooperative Oncology Group (ECOG) PS 0-2, ejection fraction >40%, pulmonary forced expiratory volume >50% and pulmonary lung diffusion test >50%. While a normal hepatic function and creatinine clearance >50mL/min was preferred, the cutoffs for kidney and liver function were evaluated on an individual basis. We collected detailed demographic, clinicopathologic, and outcomes data using a standardized protocol through chart review. Performance status was assigned according to the ECOG or Karnofsky Performance Scale, as documented in the electronic health records by the treating physician.
When comparing outcomes between consolidative auto-SCT and maintenance HD-MTX, the index time point was defined as the postinduction therapy start date. For all other analyses, including reporting outcomes of the entire patient cohort or prognostic modeling, the index time point was defined as the date of diagnosis. Primary end points were progression-free survival (PFS) and OS. PFS was defined as index time point to time of progression, recurrence, or death due to any cause and OS was defined as index time point to time of death due to any cause or last follow-up. PFS and OS rates were estimated by Kaplan-Meier analysis and compared by risk scores using a log-rank test with hazard ratios (HRs) reported for comparisons between patient groups. Median follow-up was determined by reverse Kaplan-Meier analysis. We compared baseline characteristics by postinduction therapy using descriptive statistics. The association between baseline characteristics and survival outcomes were assessed through univariate Cox regression models. Any characteristics showing significant (P < .05) association with outcomes were combined using a multivariate Cox regression model. We evaluated the performance of each prognostic index model using Harrel concordance coefficients (C).
This study was approved by the Mayo Clinic Institutional Review Board.
Results
We identified 148 patients (51% female, 95% White) with a median age at diagnosis of 66 years (range, 29-85), with 48 patients (32%) being >70 years. Among the 7 non-White patients, 5 were Asian, and 1 was African American and 1 was Hispanic/Latino. A total of 89 patients (60%) had multifocal disease and 100 patients (70%) had deep brain involvement identified at diagnosis. Only 15 patients (10%) had documented vitreoretinal involvement, and 9 patients (6%) had CSF involvement identified at diagnosis. Based on the MSKCC prognostic score for PCNSL, 35 patients (26%) scored class 3 (poor prognosis), 85 (62%) scored class 2 (intermediate prognosis), and 17 (12%) scored class 1 (favorable prognosis). Based on the IELSG prognostic score, 102 patients (78.5%) had score ≥2 indicating intermediate or poor prognosis (Table 1).
Patient characteristics
| . | Total (N = 148), n (%) or median [range] . | Maintenance MTX (n = 37), n (%) or median [range] . | Auto-SCT (n = 70), n (%) or median [range] . | P value . |
|---|---|---|---|---|
| Age, y | 66 [29-85] | 72 [46-85] | 62 [29-75] | .076 |
| >60 | 103 (69.6%) | 32 (86.5%) | 45 (64.3%) | |
| >70 | 48 (32.4%) | 20 (54.1%) | 11 (15.7%) | <.001∗ |
| Sex, female | 75 (50.7%) | 23 (62.2%) | 35 (50.0%) | .144 |
| Race, White | 141 (95.3%) | 37 (100.0%) | 66 (94.3%) | .070∗ |
| ECOG PS | .109 | |||
| 0-1 | 93 (62.8%) | 22 (59.5%) | 50 (71.4%) | |
| ≥2 | 55 (37.2%) | 15 (40.5%) | 20 (28.6%) | |
| KPS | .024∗ | |||
| ≥70 | 97 (71.9%) | 22 (66.7%) | 54 (83.1%) | |
| <70 | 38 (28.1%) | 11 (33.3%) | 11 (16.9%) | |
| LDH, >ULN | 73 (49.3%) | 17 (45.9%) | 27 (38.6%) | .012∗ |
| Albumin, <4 | 50 (51.5%) | 16 (64%) | 19 (36.5%) | .009∗ |
| CSF involvement | 9 (6.1%) | 4 (10.8%) | 4 (5.7%) | .419 |
| Vitreoretinal involvement | 15 (10.1%) | 6 (16.2%) | 4 (5.7%) | .144 |
| Deep brain involvement | 100 (69.9%) | 27 (75.0%) | 45 (66.2%) | .380 |
| Multifocal disease | 89 (60.1%) | 23 (62.2%) | 44 (62.9%) | .749 |
| 0-1 IELSG† (low) | .094 | |||
| 2-3 (intermediate) | 28 (21.5%) | 6 (17.6%) | 12 (18.5%) | |
| 4-5 (high) | 85 (65.4%) | 20 (58.8%) | 48 (73.8%) | |
| 17 (13.1%) | 8 (23.5%) | 5 (7.7%) | ||
| MSKCC‡ | .026∗ | |||
| Class 1 (age <50, any KPS) | 17 (12.4%) | 1 (3.0%) | 7 (10.6%) | |
| Class 2 (age ≥50, KPS ≥70) | 85 (62.0%) | 21 (63.6%) | 48 (72.7%) | |
| Class 3 (age ≥50, KPS <70) | 35 (25.5%) | 11 (33.3%) | 11 (16.7%) | |
| Induction regimen | .004∗ | |||
| MRT | 117 (79.1%) | 24 (64.9%) | 64 (91.4%) | |
| MR | 31 (20.9%) | 13 (35.1%) | 6 (8.6%) |
| . | Total (N = 148), n (%) or median [range] . | Maintenance MTX (n = 37), n (%) or median [range] . | Auto-SCT (n = 70), n (%) or median [range] . | P value . |
|---|---|---|---|---|
| Age, y | 66 [29-85] | 72 [46-85] | 62 [29-75] | .076 |
| >60 | 103 (69.6%) | 32 (86.5%) | 45 (64.3%) | |
| >70 | 48 (32.4%) | 20 (54.1%) | 11 (15.7%) | <.001∗ |
| Sex, female | 75 (50.7%) | 23 (62.2%) | 35 (50.0%) | .144 |
| Race, White | 141 (95.3%) | 37 (100.0%) | 66 (94.3%) | .070∗ |
| ECOG PS | .109 | |||
| 0-1 | 93 (62.8%) | 22 (59.5%) | 50 (71.4%) | |
| ≥2 | 55 (37.2%) | 15 (40.5%) | 20 (28.6%) | |
| KPS | .024∗ | |||
| ≥70 | 97 (71.9%) | 22 (66.7%) | 54 (83.1%) | |
| <70 | 38 (28.1%) | 11 (33.3%) | 11 (16.9%) | |
| LDH, >ULN | 73 (49.3%) | 17 (45.9%) | 27 (38.6%) | .012∗ |
| Albumin, <4 | 50 (51.5%) | 16 (64%) | 19 (36.5%) | .009∗ |
| CSF involvement | 9 (6.1%) | 4 (10.8%) | 4 (5.7%) | .419 |
| Vitreoretinal involvement | 15 (10.1%) | 6 (16.2%) | 4 (5.7%) | .144 |
| Deep brain involvement | 100 (69.9%) | 27 (75.0%) | 45 (66.2%) | .380 |
| Multifocal disease | 89 (60.1%) | 23 (62.2%) | 44 (62.9%) | .749 |
| 0-1 IELSG† (low) | .094 | |||
| 2-3 (intermediate) | 28 (21.5%) | 6 (17.6%) | 12 (18.5%) | |
| 4-5 (high) | 85 (65.4%) | 20 (58.8%) | 48 (73.8%) | |
| 17 (13.1%) | 8 (23.5%) | 5 (7.7%) | ||
| MSKCC‡ | .026∗ | |||
| Class 1 (age <50, any KPS) | 17 (12.4%) | 1 (3.0%) | 7 (10.6%) | |
| Class 2 (age ≥50, KPS ≥70) | 85 (62.0%) | 21 (63.6%) | 48 (72.7%) | |
| Class 3 (age ≥50, KPS <70) | 35 (25.5%) | 11 (33.3%) | 11 (16.7%) | |
| Induction regimen | .004∗ | |||
| MRT | 117 (79.1%) | 24 (64.9%) | 64 (91.4%) | |
| MR | 31 (20.9%) | 13 (35.1%) | 6 (8.6%) |
ECOG PS, ECOG performance score; KPS, Karnofsky performance score; LDH, lactate dehydrogenase; ULN, upper limit of normal.
Statistically significant difference between maintenance MTX and auto-SCT cohorts.
Missing data n = 18.
Missing data n = 11.
Induction therapy: MRT vs MR
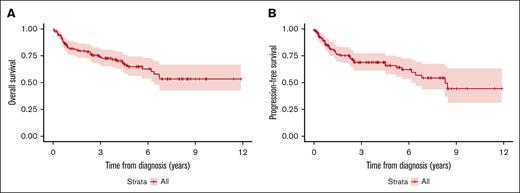
In total, 117 patients (79%) received MTX, rituximab, and temozolomide (MRT) for induction therapy, and the remaining 31 patients (21%) received MTX and rituximab (MR) induction therapy. The median number of induction cycles for both MRT and MR groups was 8 (range, 1-18 for MRT; range, 1-12 for MR). The decision for omission of temozolomide was based on the treating physician’s discretion but was most often attributed to patient’s age, risk of cytopenias, and/or concern about myelodysplasia risk. The ORR to MRT and MR induction regimens was 91% and 81%. At a median follow-up of 4.5 years for the entire cohort, 35.3% had progressed and 32.4% had died. The median PFS from diagnosis for all patients was 8.3 years and the median OS for all patients was not reached (Figure 1).
Postinduction therapy: auto-SCT vs maintenance HD-MTX
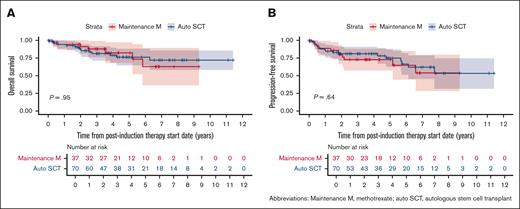
Of the 148 patients included in our initial cohort, 107 patients (72%) received postinduction therapy with 70 patients (65%) receiving consolidation with auto-SCT and 37 patients (35%) receiving maintenance MTX (without auto-SCT). Within the auto-SCT cohort all patients received carmustine/thiotepa as conditioning regimen except 2 patients that received carmustine, etoposide, cytarabine and melphalan regimen. The median number of maintenance cycles of MTX in our cohort was 11 (range, 2-14). Compared to patients who underwent consolidation with auto-SCT, the patients who received maintenance MTX had a higher median age at diagnosis of 72 vs 62 years, higher proportion of patients older than 70 (54% vs 16%; P < .001), and a higher proportion of MSKCC prognostic score class 3 patients (33% vs 17%; P = .026). A higher proportion of patients who underwent auto-SCT received MRT (vs MR) induction (n = 64, 91%) than those who received maintenance MTX alone (n = 24, 65%; P = .004; Table 1). Before initiating maintenance MTX, 30 patients (81%) were in a complete response and 7 were in a partial response. At 5-years postinduction treatment initiation, the PFS rates from start of postinduction therapy in the auto-SCT cohort and HD-MTX maintenance cohort were 74.6% (95% confidence interval [95% CI], 63.3-87.9) and 72.6% (95% CI, 58.8-89.8), respectively, and the OS rates were 76.0% (95% CI, 65.0-89.0) and 82.4% (95% CI, 69.0-98.4), respectively. Overall, there was no significant difference in PFS (HR, 0.8; 95% CI, 0.4-1.8; P = .64) or OS (HR, 1.0; 95% CI, 0.4-2.6; P = .95) based on postinduction management strategy (Figure 2).
Survival outcomes by postinduction treatment (maintenance MTX vs auto-SCT in years). (A) OS and (B) PFS.
Survival outcomes by postinduction treatment (maintenance MTX vs auto-SCT in years). (A) OS and (B) PFS.
Six of the 7 patients in the maintenance arm that had died at the time of data cutoff, were due to disease progression and one from neurocognitive decline with dementia after receiving whole-brain radiation for relapsed disease. None of the patients in this arm had died from toxicity from treatment. In the auto-SCT arm 14 of the 70 patients had died at the time of data cutoff. Cause of death included disease progression (n = 7), auto-SCT toxicity within 3 months of transplant (n = 2, sepsis), unknown cause (n = 2), and 2 from late events, pulmonary hypertension, and unrelated antisynthetase syndrome, >1 year after transplant.
Of the 41 patients (28%) in our cohort that were not included in our final analysis of postinduction therapy, 14 patients (34%) were observed after induction for various reasons (patient preference, medical comorbidities, performance status, etc), 9 patients (22%) died during induction therapy or before postinduction therapy (cause of death: primary refractory PCNSL, n = 4; toxicity due to treatment, n = 3; relapsed PCNSL, n = 1; unknown n = 1), 6 patients (15%) received alternative postinduction therapy (temozolomide, n = 4; CNS radiation therapy, n = 1; cytarabine/etoposide consolidation, n = 1), and 12 patients (29%) had no or limited follow-up data available.
Prognostication
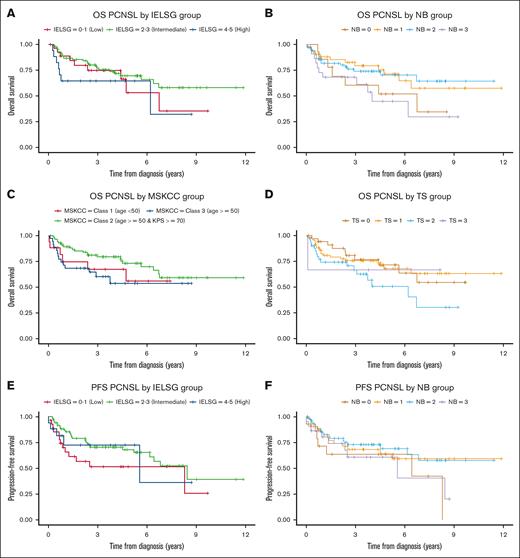
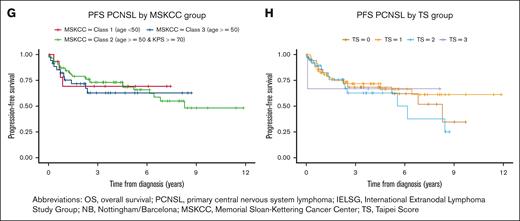
We evaluated previous published prognostic indices in the entire patient cohort (148 patients). There was no association with PFS from time of diagnosis for the IELSG (score 4-5: HR, 0.7; 95% CI, 0.3-2.0; C = 0.58), MSKCC (score 3: HR, 1.3; 95% CI, 0.4-4.0; C = 0.53), NB (score 3: HR, 0.9; 95% CI, 0.4-2.5; C = 0.56), or TS (score 3: HR, 0.9; 95% CI, 0.1-7.2; C = 0.52) prognostication indices. Similarly, OS from diagnosis was not associated with the IELSG (score 4-5: HR, 1.5; 95% CI, 0.6-4.0; C = 0.56), MSKCC (score 3: HR, 1.2; 95% CI, 0.5-3.2; C = 0.60), NB (score 3: HR, 1.2; 95% CI, 0.5-3.2; C = 0.58), or TS (score 3: HR, 1.3; 95% CI, 0.2-9.8; C = 0.58) prognostication indices (Figure 3).
Kaplan-Meier analysis of OS using published prognostic indices. (A) IELSG, (B) NB, (C) MSKCC, (D) TS. Kaplan-Meier analysis of PFS using published prognostic indices. (E) IELSG, (F) NB, (G) MSKCC, (H) TS.
Kaplan-Meier analysis of OS using published prognostic indices. (A) IELSG, (B) NB, (C) MSKCC, (D) TS. Kaplan-Meier analysis of PFS using published prognostic indices. (E) IELSG, (F) NB, (G) MSKCC, (H) TS.
Univariate analysis of all patients in our study identified 2 variables predicting an inferior OS from time of diagnosis: ECOG score of ≥2 (HR, 2.4; 95% CI, 1.35-4.24) and use of an MR induction regimen vs MRT (HR, 2.4; 95% CI, 1.26-4.51; Table 2). Notably, use of auto-SCT vs maintenance HD-MTX did not confer predictive value for PFS (HR, 0.8; 95% CI, 0.39-1.8) or OS (HR, 1.03; 95% CI, 0.42-2.6). Upon multivariate analysis after adjusting for pretreatment baseline characteristics, ECOG score of ≥2 and use of an MR induction regimen vs MRT were also found to be associated with inferior OS (Table 2).
Univariate and multivariate analysis for OS and PFS
| Univariate analysis . | PFS HR∗ . | 95% CI . | P value . | OS HR∗ . | 95% CI . | P value . |
|---|---|---|---|---|---|---|
| ECOG PS ≥2 | 1.786 | 1.011-3.155 | .046 | 2.397 | 1.353-4.248 | .003 |
| KPS ≥70† | 0.803 | 0.402-1.603 | .534 | 0.438 | 0.233-0.824 | .010 |
| Induction regimen, MR | 1.819 | 0.940-3.520 | .076 | 2.384 | 1.259-4.514 | .008 |
| Age >70 y | 1.903 | 1.082-3.345 | .025 | 1.731 | 0.978-3.063 | .060 |
| Sex, female | 0.780 | 0.444-1.373 | .389 | 0.910 | 0.516-1.603 | .743 |
| Postinduction therapy, auto-SCT | 0.835 | 0.394-1.774 | .640 | 1.031 | 0.415-2.560 | .948 |
| Multivariate analysis | PFS HR∗ | 95% CI | P value | OS HR∗ | 95% CI | P value |
| ECOG PS ≥2 | 1.458 | 0.672-3.161 | .340 | 1.907 | 1.014-3.588 | .045 |
| Induction regimen, MR | 2.411 | 0.574-10.122 | .229 | 2.118 | 1.047-4.286 | .037 |
| Age >70 y | 1.323 | 0.392-4.464 | .651 | 0.886 | 0.193-4.054 | .876 |
| Postinduction therapy, auto-SCT | 0.884 | 0.250-3.126 | .848 | 0.914 | 0.205-4.070 | .906 |
| Univariate analysis . | PFS HR∗ . | 95% CI . | P value . | OS HR∗ . | 95% CI . | P value . |
|---|---|---|---|---|---|---|
| ECOG PS ≥2 | 1.786 | 1.011-3.155 | .046 | 2.397 | 1.353-4.248 | .003 |
| KPS ≥70† | 0.803 | 0.402-1.603 | .534 | 0.438 | 0.233-0.824 | .010 |
| Induction regimen, MR | 1.819 | 0.940-3.520 | .076 | 2.384 | 1.259-4.514 | .008 |
| Age >70 y | 1.903 | 1.082-3.345 | .025 | 1.731 | 0.978-3.063 | .060 |
| Sex, female | 0.780 | 0.444-1.373 | .389 | 0.910 | 0.516-1.603 | .743 |
| Postinduction therapy, auto-SCT | 0.835 | 0.394-1.774 | .640 | 1.031 | 0.415-2.560 | .948 |
| Multivariate analysis | PFS HR∗ | 95% CI | P value | OS HR∗ | 95% CI | P value |
| ECOG PS ≥2 | 1.458 | 0.672-3.161 | .340 | 1.907 | 1.014-3.588 | .045 |
| Induction regimen, MR | 2.411 | 0.574-10.122 | .229 | 2.118 | 1.047-4.286 | .037 |
| Age >70 y | 1.323 | 0.392-4.464 | .651 | 0.886 | 0.193-4.054 | .876 |
| Postinduction therapy, auto-SCT | 0.884 | 0.250-3.126 | .848 | 0.914 | 0.205-4.070 | .906 |
HR >1 indicates a variable associated with poor prognosis.
Missing value n = 18, removed from multivariate analysis.
Discussion
This report provides a longitudinal assessment of patients with newly diagnosed PCNSL treated with HD-MTX and rituximab-based induction therapy and adds important data regarding outcomes with consolidative auto-SCT vs time-limited maintenance with single-agent HD-MTX. There are limited comparative data regarding optimal maintenance nonmyeloablative chemotherapy strategies in the current literature. The PRIMAIN study reported a 2-year OS of 47% in 107 patients older than 65 years treated with 6 months of maintenance procarbazine after initial induction with rituximab, MTX, and procarbazine.33 The Nordic Lymphoma Group evaluated a temozolomide maintenance regimen in 27 patients with ages ranging from 66 to 75, resulting in a 2-year OS of 60%.23 Another retrospective study out of University of California in San Francisco reported 13 patients treated with maintenance lenalidomide showed that a median PFS was not reached after a median follow-up of 30 months.22 Most recently, a multicenter retrospective study reported outcomes on 90 patients aged 60 and older who received maintenance with nonmyeloablative chemotherapy.34 The maintenance regimens used were heterogeneous, with temozolomide (n = 28, 31%) and lenalidomide (n = 21, 23%) being the most common. A total of 15 patients received maintenance HD-MTX. The 3-year PFS and OS of all 90 patients who received any maintenance therapy was 65% and 84%, respectively. Outcomes based on specific maintenance agents were not parsed out. Tatarczuch et al reported comparable outcomes to our study in older patients with PCNSL treated with rituximab, methotrexate, procarbazine and vincristine/cytarabine without whole-brain radiotherapy or auto-SCT.35 However, number of patients in the older cohort (>60 years old) was relatively small (n = 27), 3 patients underwent consolidative radiation, maximum dose of HD-MTX was 3.5 gm/m2 and patients, despite being in the older cohort were perceived to be fit enough to receive combination chemotherapy and high-dose cytarabine.
The overall outcomes in our patient cohort were significantly better than historically reported outcomes in the studies utilizing nonmyeloablative chemotherapy maintenance approaches noted above. This is likely attributable in part due to modern advances in therapeutic and supportive management such as availability of glucarpidase, dedicated pharmacy staff for monitoring and dosing adjustments, but also primarily due to our cohort being a homogeneous demographically and highly selective population of patients receiving a HD-MTX and rituximab-based regimen. When possible a 8 gm/m2 was attempted for the patients, and a relatively higher dose intensity could also have contributed to better outcomes as has been previously suggested.36 Patient demographics likely play a role in this with Mayo Clinic being a tertiary center with predominantly White patient cohort. The PFS and OS in our patient cohort at 2 years after diagnosis of PCNSL was 75% and 79%, respectively, which are comparable to previously reported outcomes in studies evaluating the use of auto-SCT in PCNSL including IELSG3237 and the PRECIS trial.38
To our knowledge, our data set represents the largest PCNSL postinduction maintenance HD-MTX cohort reported to date in the literature in the recent era. A retrospective study out of Massachusetts General Hospital published in 2009 reported on a cohort of 31 patients ≥70 years of age treated with single-agent HD-MTX induction followed by HD-MTX maintenance between 1992 and 2006.27 Median PFS and OS were 7.1 months and 37 months, respectively. However, this study was performed before the routine inclusion of rituximab in most standard of care induction regimens, as well as before the interval improvements in supportive care and management of toxicities associated with HD-MTX treatment. In our study, patients who went on to receive auto-SCT were, not surprisingly, younger but also were induced with more MRT (compared to MR alone) and had superior predicted outcomes by historical prognostic indices compared to the maintenance HD-MTX cohort. Despite these differences, and after a rigorous adjusted survival analysis, our study demonstrates comparable outcomes between consolidation auto-SCT and maintenance HD-MTX. This suggests that maintenance HD-MTX should be considered as a time-limited strategy for patients with PCNSL responding to initial induction therapy, particularly in patients who are not candidates for consolidation with auto-SCT.
There exist multiple prognostication indices in PCNSL, of which the IELSG and MSKCC indices are most commonly used in clinical practice.29,30,39 Both indices were created before the inclusion of rituximab in induction regimens for PCNSL. Using our single-center patient cohort that received HD-MTX and rituximab-based induction, we demonstrated that PFS and OS were not accurately predicted using the IELSG or MSKCC indices (Figure 3), suggesting that an updated prognostic index may be needed. Univariate and multivariate analyses of multiple disease- and treatment-specific characteristics were unable to identify enough variables to propose a prognostic model. This is likely explained by the small sample size due to the rarity of PCNSL, single-center treatment experience, and homogeneous patient population, and suggests that a larger, multicenter study is required to develop a clinically useful and updated prognostic model. Furthermore, it may suggest a need for novel prognostic approaches such as circulating tumor DNA in CSF and plasma.40,41
Limitations of our study include the retrospective nature of the study design with inherent selection bias. As this study is primarily focused on the comparison of postinduction management strategies, that analysis is selective for chemotherapy-sensitive patients who responded to induction HD-MTX–based regimens. In addition, many of the variables were collected through review of electronic clinical documentation, which can inherently have inaccuracies at time of input affecting the data and final analyses. We attempted to abate some of these limitations by including consecutive patients in a preselected time period, rigorous data analysis to account for identifiable confounding variables, and having multiple authors perform a review of the electronic medical records. Our study also does not capture the total cumulative dose of MTX in the patient cohorts, even though our standard practice was to attempt 8 gm/m2 dose of HD-MTX when feasible, both during induction and maintenance. Lastly, this cohort lacks the racial and ethnic diversity that could play a role in the MTX tolerability.42
The major strengths of our study include a homogeneous and relatively large patient cohort all receiving HD-MTX– and rituximab-based induction therapy, all treated at single academic institution with standardized supportive care practices.
Altogether, our data demonstrate comparable outcomes in the use of consolidative auto-SCT and maintenance MTX after initial induction with HD-MTX and rituximab-based regimens in PCNSL. This was despite favorable baseline characteristics in the auto-SCT group, suggesting that maintenance HD-MTX should be considered as a time-limited strategy in patients responding to initial induction therapy. Maintenance HD-MTX may be a favorable postinduction treatment strategy in patients who are not candidates for auto-SCT but also can be entertained in fit patients who wish to reserve novel, more aggressive treatment strategies for the relapsed setting. Future prospective studies should focus on these exploratory findings to better understand the optimal postinduction management strategy. Larger international collaborations are needed to identify better prognostic markers in a rare disease to account for geographic, racial/ethnic and treatment practice differences. In addition, we demonstrate that historical prognostication indices performed poorly with our cohort, suggesting that an updated prognostic index based on patients treated in the modern era is clinically needed.
Authorship
Contribution: S.R.H. and A.K. designed the research; S.R.H. performed the research; S.R.H. and R.C.G. collected the data; S.R.H., M.J.M., and A.K. analyzed and interpreted the data; B.J.N., R.M., and M.J.M. performed statistical analysis; and S.R.H., R.C.G., A.N.N., J.N.B., I.N.M., S.M.A., L.P., U.D., G.T., T.M.H., M.J.M., P.B.J., and A.K. wrote the manuscript.
Conflict-of-interest disclosure: S.M.A. reports research funding from ADC Therapeutics, AstraZeneca, Bristol Myers Squibb, Pfizer, Regeneron, Seagen, and Takeda. G.T. discloses participation in advisory boards for Seattle Genetics and Novartis, all funds have been routed to a research fund (no personal remuneration). T.M.H. reports being on the data monitoring committees of Seagen, Tess Therapeutics, and Eli Lilly & Co; being on the scientific advisory boards of Eli Lilly & Co, MorphoSys, Incyte, BeiGene, and Loxo Oncology; and receiving research support from Genentech and Sorrento (all payments were made to the institution). M.J.M. reports research funding from Roche/Genentech, Bristol Myers Squibb, and Genmab, and serves on the advisory boards of Genmab and Adaptive Biotechnologies. The remaining authors declare no competing financial interests.
Correspondence: Arushi Khurana, Mayo Clinic, 200 First St SW, Rochester, MN 55905; email: khurana.arushi@mayo.edu.
References
Author notes
Original data are available on request from corresponding author, Arushi Khurana (khurana.arushi@mayo.edu).