Key Points
Fc mutations enhance the ADCC activity of anti-RhD mAbs.
Fc mutations improve the function of anti-RhD mAbs without glycoengineering.
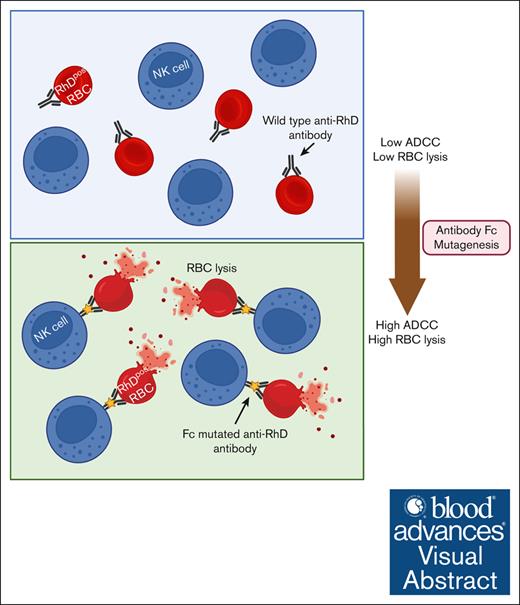
Visual Abstract
Hemolytic disease of the fetus and newborn (HDFN) due to Rhesus D (RhD) antigen mismatch between the mother and fetus has been a significant cause of neonatal jaundice, recurrent miscarriage, and stillbirth throughout history. Polyclonal anti-RhD immunoglobulin G (RhD-pIgG), derived from the plasma of RhD-negative donors immunized with RhD-positive red blood cells (RBCs), has reduced the incidence of HDFN, but this approach is currently restricted to developed countries. Monoclonal antibodies (mAbs) offer a promising alternative to address this pressing need, but prior attempts to develop effective anti-RhD mAbs have failed, in some cases, due to differences in fucosylation patterns between mAbs produced in cell lines and RhD-pIgG. Chinese hamster ovary (CHO) cell lines, commonly used for pharmaceutical protein production, induce high levels of fucosylation, reducing the antibody-dependent cellular cytotoxicity (ADCC) activity crucial for clearing RhD-positive RBCs. In contrast, RhD-pIgG has lower fucosylation levels, which enhances ADCC activity. Regulating the glycan levels of mAbs during production requires specialized cell lines and culture conditions. In this study, we took an alternative approach through antibody engineering. The Fragment crystallizable (Fc) regions of 2 existing anti-RhD mAbs (Brad3 and Fog1) were subjected to mutagenesis to introduce ADCC-enhancing mutations and then expressed in CHO cells under standard conditions. We demonstrate that targeted Fc mutagenesis significantly enhanced ADCC compared with the wild-type mAbs, while preserving RhD binding and efficient production in CHO cells. Furthermore, these Fc variants achieved comparable efficacy with RhD-pIgG, suggesting a new strategy for producing anti-RhD mAbs with improved functionality, without the need for glycoengineering.
Introduction
Rhesus D (RhD) is a blood group found on human red blood cells (RBCs).1 When an RhD-negative (RhDneg) mother carries an RhD-positive (RhDpos) fetus, fetal RBCs can enter the maternal circulation, sensitizing the mother and provoking an antibody (Ab) response to the RhD molecule.2 This alloimmune response increases with each RhDpos pregnancy and can cause destruction of the baby’s RBCs, known as hemolytic disease of the fetus and newborn (HDFN). HDFN can lead to miscarriage, stillbirth, and neonatal jaundice. HDFN has been a major cause of infant mortality throughout history and is still so in the developing world.3 In the 1960s, it was suggested that administration of preformed anti-RhD Abs to RhDneg women during pregnancy could avoid sensitization of the mother’s immune system.4,5 This proved to be remarkably successful, and since then, the standard of care clinically has been to treat RhDneg women postpartum and at 28 and 34 weeks of pregnancy with polyclonal anti-RhD immunoglobulin G (RhD-pIgG). As a result, RhD-related HDFN is now rare in developed countries, and perinatal mortality has reduced from ∼10% to 0.02%.6
However, many countries have inadequate anti-RhD prophylaxis programs due to cost and insufficient public health care resources.7-9 In low- and middle-income countries, HDFN still affects thousands of babies, with >160 000 perinatal deaths and >100 000 cases of associated disability annually.10 Additionally, recruiting blood donors and repeated immunization to maintain anti-RhD Ab titer is resource intensive and expensive.11 In the United Kingdom, donor plasma is sourced from North America to minimize the risk of transmission of variant Creutzfeldt-Jakob disease.12 Although most Abs in RhD-pIgG are against the major RhD antigen, low titers of other blood group Abs can be present. It is thought that these Abs are responsible for rare but sometimes severe complications seen with RhD-pIgG.11 For all these reasons, the development of a non–plasma-derived source of prophylactic anti–RhD immunoglobulin G (IgG) is highly desirable.
Although the mechanism of HDFN prevention is not fully understood, the development of alternative anti-RhD monoclonal Abs (mAbs) has focused on ensuring Ab-dependent phagocytosis (ADP)13,14 and Ab-dependent cellular cytotoxicity (ADCC) by natural killer (NK) cells.15 Several anti-RhD mAbs have been produced and evaluated in clinical trials,16,17 but none have yet had equivalent activity to RhD-pIgG obtained from blood donors. It has been hypothesized that this problem could be due to differing glycosylation profiles of anti-RhD mAbs produced in cell lines.15 Glycosylation and, more specifically, afucosylation (in this context reduction of core fucose) of Ab-constant regions can enhance ADCC, both in vitro and in vivo,18,19 by strengthening the binding of IgG to Fragment crystallizable (Fc) gamma receptors (FcγRs) on NK cells.20 Introducing point mutations in the mAb Fc domain is another promising approach to improve ADCC.21 for example, mAbs containing the GASDALIE mutation set (G236A/S239D/A330L/I332E)22 or other related point mutations (eg, GASDIE/GAALIE) show increased binding to activating FcγRs23,24 and exhibit superior ADCC and ADP activities compared with wild-type (WT) IgG1 Fc.25
In this study, we aimed to enhance ADCC mediated by 2 existing anti-RhD mAbs (Brad3 and Fog1) through targeted Fc domain mutagenesis. We assessed Fc mutagenesis and afucosylation and compared the functional effects of these modified mAb variants with RhD-pIgG.
Methods
Enrichment of RhD-specific Abs from RhD-pIgG
Therapeutic preparations of RhD-pIgG contain a mixture of both RhD-specific and non-RhD Abs. To directly compare biological activities, RhD-specific Abs were enriched. For this purpose, 1.5 mL (625 IU) of commercially available RhD-pIgG-1 (derived from Australian donors) was diluted with phosphate-buffered saline (PBS) and incubated with 8 mL of packed RhDpos RBCs (R0r variant). The RBCs were then washed at least 6 times with PBS to remove nonspecific or weak-binding Abs. To elute the RBC-bound Abs, 16 mL of 50-mM glycine, 0.9% NaCl, and 2-mM EDTA (pH 2.7) were added, and the mixture was vortexed for 5 seconds and incubated on ice for 2 minutes. The RBCs were immediately centrifuged at 2500 g for 5 minutes, and the supernatant was collected. The acidic pH was neutralized by adding 2.1 mL of 1 M Tris and 5.2% NaCl (pH 8), producing a pool of eluted RhD-specific Abs. To remove potential RBC protein contaminants, further enrichment was achieved by affinity chromatography using protein G agarose followed by buffer exchange to PBS. Ultraviolet absorbance at 280 nm was used to measure the concentration of purified Abs.
Preparation of mAbs
Heavy and light variable genes of Brad3 anti-RhD mAb (GenBank X64149.1 and X64162.1), Fog1 anti-RhD mAb (GenBank X64150.1 and X64163.1), and VRC03 non-RhD mAb (GenBank GU980707.1 and GU980706.1) were codon optimized, synthesized, and cloned into human IgG1 expression plasmids. To make Fc-mutated mAbs, the following mutations were introduced: G236A, S239D, A330L, and I332E (GASDALIE variant) or G236R and L328 (GRLR variant) using the primers listed in Table 1.25 The purified mutated constant genes were substituted with a WT constant gene in heavy chain expression plasmids. Ab heavy and light chain expression plasmids were cotransfected into Expi–Chinese hamster ovary (Expi-CHO) cells using the Expi-CHO expression system kit (Thermo Fisher Scientific; catalog no. A29133) according to the manufacturer’s instructions. Supernatants were harvested, and the mAbs purified using Protein G Agarose Fast Flow (Merck Millipore) according to the manufacturer’s instructions. To produce afucosylated (AFUC) mAbs, heavy (WT and GASDALIE variants) and light chain expression plasmids were cotransfected into Expi-CHO cells in the presence of 50-μM Fucostatin I, which was synthesized in accordance with established protocols.26,27 mAbs were harvested, purified, and assessed for fucose reduction and the expression of heavy and light chains by western blot as follows.
Oligonucleotides used for mAb heavy chain Fc mutagenesis.
| Oligo ID . | Sequence (5’-3’) . | . | Reaction . | mAb variant . | Mutation . |
|---|---|---|---|---|---|
| BH110 | TCCTCAGCGTCGACCAAGGGCCCATCGGTCT | Forward | A | GASDALIE | — |
| BH113 | AGAGGAAGACGTCCGGTCCCGCCAGGAGTTCAGGTGCTGGG | Reverse | A | GASDALIE | G236A, S239D |
| BH112 | GAACTCCTGGCGGGACCGGACGTCTTCCTCTTCCCCCCAAA | Forward | B | GASDALIE | G236A, S239D |
| BH115 | TGGAGATGGTTTTCTCTTCGGGGAGTGGGAGGGCTTTGTTGGAGACC | Reverse | B | GASDALIE | I332E, A330L |
| BH114 | AAAGCCCTCCCACTCCCCGAAGAGAAAACCATCTCCAAAGCCAAAGG | Forward | C | GASDALIE | I332E, A330L |
| BH111 | CAGATCTGGATCCTCATCATTTACCCGGAGA | Reverse | C | GASDALIE | — |
| BH110 | TCCTCAGCGTCGACCAAGGGCCCATCGGTCT | Forward | A | GRLR | — |
| BH117 | GAGGAAGACTGACGGTCCCCTCAGGAGTTCAGGTGCTGGG | Reverse | A | GRLR | G236R |
| BH116 | GAACTCCTGAGGGGACCGTCAGTCTTCCTCTTCCCCCCAAA | Forward | B | GRLR | G236R |
| BH119 | CTCGATGGGGGCTGGGCGGGCTTTGTTGGAGACCTTGCACTTGTA | Reverse | B | GRLR | L328R |
| BH118 | AGGTCTCCAACAAAGCCCGCCCAGCCCCCATCGAGAAAACCATC | Forward | C | GRLR | L328R |
| BH111 | CAGATCTGGATCCTCATCATTTACCCGGAGA | Reverse | C | GRLR | — |
| Oligo ID . | Sequence (5’-3’) . | . | Reaction . | mAb variant . | Mutation . |
|---|---|---|---|---|---|
| BH110 | TCCTCAGCGTCGACCAAGGGCCCATCGGTCT | Forward | A | GASDALIE | — |
| BH113 | AGAGGAAGACGTCCGGTCCCGCCAGGAGTTCAGGTGCTGGG | Reverse | A | GASDALIE | G236A, S239D |
| BH112 | GAACTCCTGGCGGGACCGGACGTCTTCCTCTTCCCCCCAAA | Forward | B | GASDALIE | G236A, S239D |
| BH115 | TGGAGATGGTTTTCTCTTCGGGGAGTGGGAGGGCTTTGTTGGAGACC | Reverse | B | GASDALIE | I332E, A330L |
| BH114 | AAAGCCCTCCCACTCCCCGAAGAGAAAACCATCTCCAAAGCCAAAGG | Forward | C | GASDALIE | I332E, A330L |
| BH111 | CAGATCTGGATCCTCATCATTTACCCGGAGA | Reverse | C | GASDALIE | — |
| BH110 | TCCTCAGCGTCGACCAAGGGCCCATCGGTCT | Forward | A | GRLR | — |
| BH117 | GAGGAAGACTGACGGTCCCCTCAGGAGTTCAGGTGCTGGG | Reverse | A | GRLR | G236R |
| BH116 | GAACTCCTGAGGGGACCGTCAGTCTTCCTCTTCCCCCCAAA | Forward | B | GRLR | G236R |
| BH119 | CTCGATGGGGGCTGGGCGGGCTTTGTTGGAGACCTTGCACTTGTA | Reverse | B | GRLR | L328R |
| BH118 | AGGTCTCCAACAAAGCCCGCCCAGCCCCCATCGAGAAAACCATC | Forward | C | GRLR | L328R |
| BH111 | CAGATCTGGATCCTCATCATTTACCCGGAGA | Reverse | C | GRLR | — |
In brief, the purified mAbs were run on NuPAGE 4% to 12% Bis-Tris Gel (Invitrogen; catalog no. NP0336) Bolt MES SDS running buffer (Invitrogen; catalog no. B000202), blotted onto nitrocellulose using Novex NuPAGE Transfer buffer (Thermo Fisher Scientific; catalog no. NP0006-1), and blocked with 3% bovine serum albumin (BSA) in Tris-Buffered Saline (TBS). To assess the core fucosylation levels of the AFUC mAb variants, the blot was incubated with 5 μg/mL biotinylated recombinant Pholiota squarrosa lectin (PhoSL; prepared in accordance with established protocols28,29) in 3% BSA-TBS for 1 hour at room temperature (RT). To confirm the expression of heavy/light chains, the blot was stripped and reprobed with 1:5000 dilutions of Goat anti-Human (H+L) horseradish peroxidase conjugate (Bio-Rad; catalog no. 1721050) in 3% BSA-TBS for 1 hour at RT. ECL Western Blotting Substrate (Thermo Fisher Scientific; catalog no. 32209) was used to detect the signal.
RBC preparation
RhDpos (R0r phenotype, Dce/dce) and RhDneg (r’r phenotype, dCe/dce) O blood types were collected and centrifuged, and packed RBCs were washed with Dulbecco's PBS (DPBS) containing 2-mM EDTA. For long-term storage, RBCs were glycerolized as previously described.30 Washed RBCs were resuspended in DPBS and treated with bromelain (Sigma-Aldrich; catalog no. B4882-25G) to enzymatically modify surface proteins, enhancing Ab binding and increasing the sensitivity of subsequent in vitro assays. Briefly, 2% RBC in DPBS was mixed with 0.5% bromelain (weight-to-volume ratio) with (1:2 ratio) and incubated at 37°C for 10 minutes. The cells were centrifuged at 1500g for 5 minutes and washed with DPBS twice more.
Agglutination assay
A total of 50-μL bromelain-treated RhDpos or RhDneg RBCs (1% hematocrit), 25 μL of serial dilutions of mAbs, and 25-μL anti-human globulin colour (Bio-Rad Laboratories; catalog no. 804115) were added to each well of a U-bottom 96-well plate and incubated for 90 minutes at RT. Agglutination was visualized by the naked eye. Titers were assessed as the highest dilution at which RBC clumping (agglutination) was still observed.
RBC binding
Flow cytometry analysis was performed to evaluate the effect of mAb engineering on RhD binding. In brief, serial dilutions of mAbs (50 μL) were incubated with 50 μL (2 × 105) of bromelain-treated RhDpos or RhDneg RBCs in U-bottom 96-well plates at 37°C for 30 minutes. The cells were washed with wash buffer (DPBS containing 0.5% BSA and 2-mM EDTA) and centrifuged at 1500g for 5 minutes. A 1:1000 dilution of Fab goat anti-human IgG Texas Red (Rockland Antibodies and Assays; catalog no. 809-1902) was added, and the plate was incubated at RT for 30 minutes and centrifuged at 1500g for 5 minutes. The plate was washed wash buffer, and the cells resuspended in 50-μL wash buffer for flow cytometry.
ADP
The monocytic THP-1 cell line (ATCC) was grown in RPMI culture medium + 10% fetal calf serum (FCS). A total of 10 000 cells were incubated with CD16 (FcγRIII)–Fluorescein Isothiocyanate (FITC) (BD Biosciences; catalog no. 555406), CD32 (FcγRII)–R-phycoerythrin (PE) (BD Biosciences; catalog no. 552884), and CD64 (FcγRI)–PE Cy7 (BD Biosciences; catalog no. 561191) for 30 minutes on ice to assess the expression of FcγRs. The cells were washed with DPBS; then flow cytometry data were acquired on a Fortessa instrument (Becton Dickinson) and analyzed using FlowJo software, v10.6.2.
For phagocytic assays, monocytic THP-1 cells and bromelain-treated RBCs were labeled with CellTrace Violet (CTV; Invitrogen; catalog no.C34571) and pHrodo Red, succinimidyl ester (Thermo Fisher Scientific; catalog no. P36600), respectively, according to the manufacturer’s instruction. The cells were washed and resuspended in RPMI + 10% FCS.
To examine ADP, various concentrations of mAb variants, RhD-pIgG or "no-Ab" control (25 μL) were incubated with 2 × 105 pHrodo Red–labeled RhDpos or RhDneg RBCs (25 μL) and 20 000 CTV-labeled THP-1 monocytes (50 μL) for 16 hours at 37°C in a 96-well U-bottom plate. The plate was washed, followed by the addition of cold RBC lysis buffer (156-mM ammonium chloride, 11.9-mM sodium bicarbonate, and 0.097-mM EDTA). The plate was again washed with DPBS twice, and the flow cytometry data were acquired as described before. The ADP score was calculated using the following formula: (% CTV+/pHrodo red+ cells × mean fluorescence intensity)/1000. The phagocytosis score of “no-Ab” control was subtracted from all samples.
NK-cell activation
Intracellular interferon gamma (IFN-γ) and CD107a expression were used as measures of NK-cell activation after incubation with mAb variants.25 To prepare effector cells, peripheral blood mononuclear cells (PBMCs) were purified from the whole blood of healthy donors on a Ficoll gradient (GE Healthcare; 17144003), followed by the enrichment of NK cells using an NK Cell Isolation kit (Miltenyi Biotec; catalog no. 130-092-657), according to the manufacturer’s instruction. Purified NK cells (1 million/mL) were cultured overnight in RPMI-1640 supplemented with penicillin-streptomycin, 1% minimum essential medium nonessential amino acids (Thermo Fisher Scientific; catalog no. 11140050), 1% sodium pyruvate (Thermo Fisher Scientific; catalog no. 11360070), 1% Glutamax (Thermo Fisher Scientific; catalog no. 35050061), recombinant human interleukin-2 100 IU/mL (In Vitro Technologies; catalog no. RDS202IL010), and 10% FCS. Finally, NK cells, bromelain-treated RBCs, and Ab dilutions were prepared in RPMI-1640 + 10% FCS.
Briefly, 2 × 105 bromelain-treated RBCs (50 μL) were incubated with 2-μg Abs (50 μL) in 96-U-bottom plate for 30 minutes at RT. A total of 2 × 105 cultured NK cells (100 μL) were added along with 20 μL inhibitor cocktail prepared in RPMI-1640 + 10% FCS (1-μL CD107a APC H7 [BD Biosciences; catalog no. 561343], 100 μg/mL Brefeldin A [Sigma; catalog no. B7651-5MG], and GolgiStop [BD Biosciences; catalog no. 554724]). Cells were incubated for 4 hours at 37°C. Plates were kept at 4°C overnight, then stained with 20-μL surface markers prepared in DPBS + 2% FCS (1-μL V500 CD3 [BD Biosciences; catalog no. 561416], 2.5-μL PE-Cy7 anti-human CD56 [BD Biosciences; catalog no. 335791], 0.1-μL Fixable Viability Stain 620 [BD Biosciences; catalog no. 564996], and 0.8 μL of 0.25-M EDTA) at RT for 30 minutes. Cytofix/Cytoperm Fixation/Permeabilization solution (50 μL; BD Biosciences; catalog no. 554714) was added and incubated for 20 minutes at 4°C. The plate was washed with DPBS + 2-mM EDTA + 1% FCS. The supernatant was discarded, 50 μL of intracellular stain (0.5-μL AF700 anti-human IFN-γ [BD Biosciences; catalog no. 557995] in 49.5-μL Perm/Wash buffer [BD Biosciences; catalog no. 554714]) was added and incubated at RT for 1 hour (in the dark). A total of 150-μL Perm/Wash buffer was again added, and the plate was centrifuged to discard the supernatant. DPBS was added to the wells, and flow cytometry data were acquired as described before. NK-cell activation was calculated as the percentage of cells that were positive for one or both markers. No-Ab control values were subtracted.
ADCC
To prepare effector cells, monocytes were depleted from Ficoll-gradient purified PBMCs by incubating the cells in tissue culture flasks containing RPMI and 10% FCS for 2 hours at 37°C. The nonadherent cells were then resuspended in RPMI media containing 3% human serum and 7% FCS at a concentration of 12 million cells per mL. Bromelain-treated or untreated RhDpos RBCs were resuspended at 80 000 cells per mL in RPMI media containing 3% human AB serum and 7% FCS. Combinations of 25-μL RhDpos RBC (20 000 cells per well), 25-μL purified Ab or RhD-pIgG (100 ng/well or 1 μg/mL in RPMI + 3% human AB serum + 7% FCS), and 50-μL effector cells (600 000 monocyte-depleted PBMCs per well) were incubated overnight at 37°C. 2μL Triton X-100 (Thermo Fisher Scientific; catalog no. 28314) was then added to the control well (no-Ab control) and incubated for 5 minutes at 37°C to achieve maximal hemoglobin (Hb) release. The cells were then centrifuged at 1500g for 2 minutes, and the supernatants were collected. A 1:100 dilution of the supernatant was used to detect the free Hb content in each well using the Human Hb Enzyme-linked immunosorbent assay (ELISA) Kit (Elabscience catalog no. E-EL-H0415) according to the manufacturer's instructions. Percentage of specific RBC lysis (% ADCC) was calculated using the following formula: 100 × (experimental release – spontaneous release)/(maximum release – spontaneous release).
Results
In this study, we generated various engineered anti-RhD mAb variants to determine the most effective approach for enhancing ADCC. WT mAbs served as the baseline, with no Fc modifications. The GASDALIE variant included Fc domain mutations (G236A/S239D/A330L/I332E) specifically designed to improve ADCC. Additionally, we produced AFUC mAbs with reduced core fucose levels to enhance ADCC, following established strategies.26,27 To assess the combined effects of these modifications, we created the AFUC GASDALIE variant, which incorporates both Fc domain mutations and fucose reduction. The GRLR variant was included as a negative control in functional assays. This mutation hinders the mAb's interaction with FcγRs activating the immune responses.25 By evaluating these different variants, we were able to compare the effects of Fc mutagenesis and afucosylation on ADCC enhancement, providing a comprehensive assessment of their relative efficacy. This approach allowed us to determine whether Fc mutagenesis alone could achieve functional improvements comparable with those achieved with afucosylation strategies, which have previously been shown to enhance ADCC in anti-RhD mAbs.
Agglutination and RBC binding is maintained in Fc modified anti-RhD mAbs
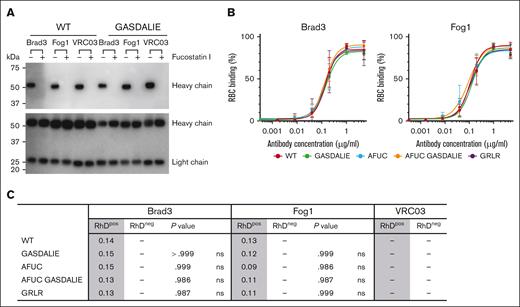
The mAb variants were expressed in Expi-CHO cells in the presence or absence of Fucostatin I, and the fucosylation levels of secreted mAbs were evaluated by lectin-probed western blot using the core fucose-specific lectin Pholiota squarrosa lectin. As demonstrated in Figure 1A (top blot), Fucostatin I abrogated core fucosylation in the purified AFUC and AFUC GASDALIE mAbs compared with the WT mAbs, which were expressed in the absence of Fucostatin I. Heavy and light chain expression was not affected by afucosylation (Figure 1A, bottom blot).
RBC binding is maintained in Fc-mutated and glycoengineered anti-RhD mAbs. (A) Detection of fucosylation levels (top blot) and Ab heavy/light chains (bottom blot) in purified AFUC mAbs. (B) Binding of mAb variants to RBCs was analyzed in flow cytometry. Serial dilutions of mAbs were incubated with RhDpos or RhDneg RBCs, and the binding was detected with Fab goat anti-human IgG (H&L) Texas Red. (C) EC50 binding of mAb variants. P values show the significance of RhDpos RBC binding of mAb variants vs WT format in each group. Statistical analyses were conducted using 1-way analysis of variance (ANOVA), followed by Dunnett multiple comparison test (GraphPad Prism v9). Data are representative of 3 replicates. ns, not significant.
RBC binding is maintained in Fc-mutated and glycoengineered anti-RhD mAbs. (A) Detection of fucosylation levels (top blot) and Ab heavy/light chains (bottom blot) in purified AFUC mAbs. (B) Binding of mAb variants to RBCs was analyzed in flow cytometry. Serial dilutions of mAbs were incubated with RhDpos or RhDneg RBCs, and the binding was detected with Fab goat anti-human IgG (H&L) Texas Red. (C) EC50 binding of mAb variants. P values show the significance of RhDpos RBC binding of mAb variants vs WT format in each group. Statistical analyses were conducted using 1-way analysis of variance (ANOVA), followed by Dunnett multiple comparison test (GraphPad Prism v9). Data are representative of 3 replicates. ns, not significant.
Agglutination is a highly sensitive assay, and the end point concentration for both Brad3 and Fog1 variants was 5 ng/mL against RhDpos RBCs, indicating that the engineered anti-RhD mAbs retained their agglutination activity. RhDneg RBC were not agglutinated by any of the mAbs, confirming the specificity of Brad3 and Fog1 against RhD antigen. As expected, no agglutination was detected for VRC03 (negative control mAb).
As shown in Figure 1B-C, modifications in the Fc region of anti-RhD mAbs did not affect RhD antigen binding. EC50 values were also maintained across mAb variants. None of the anti-RhD mAb variants showed binding to RhDneg RBC. As expected, VRC03 mAbs did not bind RhDpos RBCs.
ADP activity is maintained in Fc-engineered anti-RhD mAbs
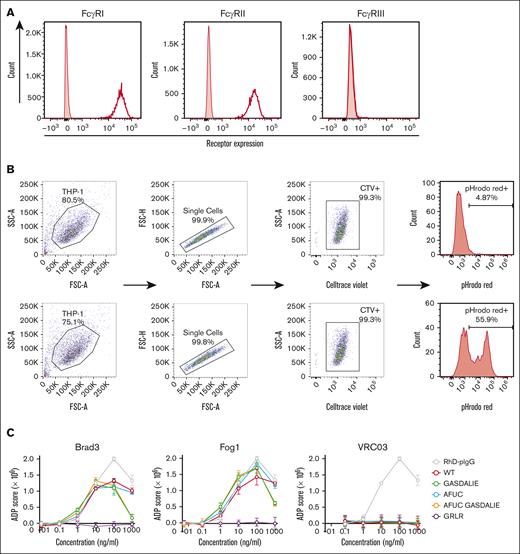
FACS analysis confirmed that monocytic THP-1 cells express CD64 (FcγRI) and CD32 (FcγRII) receptors but not CD16 (FcγRIII) (Figure 2A). Phagocytosis was evaluated using the pHrodo red signal in THP-1 cells in the presence of mAb variants (Figure 2B). As shown in Figure 2C, maximal phagocytocytic activity of the anti-RhD mAbs was achieved at a concentration of 100 ng/mL, after which a reduction in activity was observed, suggesting a prozone-like effect at higher concentrations. Brad3 and Fog1 mAb variants induced phagocytosis against RhDpos RBCs but not RhDneg RBCs. Brad3-WT mAb generated an average phagocytosis score of 114. Brad3-GRLR mutation reduced the phagocytosis score to 11. All other Fc-engineered Brad3 mAbs maintained ADP activity, with phagocytosis scores of 110, 106, and 120 for GASDALIE, AFUC, and AFUC GASDALIE variants, respectively (Figure 2D). The same effect was observed for Fog1 variants with no significant change in phagocytic activity upon Fc engineering (ADP scores of 136, 156, 153, and 164 for Fog1-WT, Fog1-GASDALIE, Fog1-AFUC, and Fog1-AFUC GASDALIE, respectively). However, as expected, the GRLR mutation in Fog1 reduced the phagocytosis score to 8. VRC03 had no ADP activity against either RhDpos RBCs or RhDneg RBCs (Figure 2C-D).
ADP is maintained in Fc-engineered anti-RhD mAbs. (A) The expression levels of FcγRI, FcγRII, and FcγRIII of THP-1 cells were measured by flow cytometry. (B) The gating strategy used for analysis of in vitro phagocytosis assays in the presence of non-RhD (top) or anti-RhD mAbs (bottom). (C) Phagocytic activity of mAbs at various concentrations. The phagocytosis score was calculated using the following formula: (% CTV+/pHrodo red+ cells × mean fluorescent intensity)/1000. The phagocytosis score of “no-Ab” control was subtracted from all samples. (D) Bar graphs depict the AUC of phagocytosis scores across varying mAb concentrations. The data presented show Ab concentrations ≤100 ng/mL. All the mAbs were tested alongside RhD-pIgG, but each group of mAbs is presented in a separate figure to allow for a clear comparison. Statistical analyses were conducted using 2-way ANOVA, followed by Dunnett multiple comparison test (GraphPad Prism v9). ∗∗∗∗P < .0001. Data are the average of 3 replicates, and error bars denote standard error of the mean (SEM). AUC, area under the curve.
ADP is maintained in Fc-engineered anti-RhD mAbs. (A) The expression levels of FcγRI, FcγRII, and FcγRIII of THP-1 cells were measured by flow cytometry. (B) The gating strategy used for analysis of in vitro phagocytosis assays in the presence of non-RhD (top) or anti-RhD mAbs (bottom). (C) Phagocytic activity of mAbs at various concentrations. The phagocytosis score was calculated using the following formula: (% CTV+/pHrodo red+ cells × mean fluorescent intensity)/1000. The phagocytosis score of “no-Ab” control was subtracted from all samples. (D) Bar graphs depict the AUC of phagocytosis scores across varying mAb concentrations. The data presented show Ab concentrations ≤100 ng/mL. All the mAbs were tested alongside RhD-pIgG, but each group of mAbs is presented in a separate figure to allow for a clear comparison. Statistical analyses were conducted using 2-way ANOVA, followed by Dunnett multiple comparison test (GraphPad Prism v9). ∗∗∗∗P < .0001. Data are the average of 3 replicates, and error bars denote standard error of the mean (SEM). AUC, area under the curve.
Fc engineering enhances anti-RhD mAb–mediated NK-cell activation
To evaluate the in vitro effect of Fc engineering on anti-RhD mAb activity, NK-cell activation was assessed. Both RhDpos and RhDneg RBCs were incubated with mAb variants, and NK-cell activation was measured by the production of intracellular IFN-γ and upregulation of CD107a (Figure 3A). As shown in Figure 3B and Table 2, all engineered mAbs enhanced CD107, IFN-γ production, and NK-cell activation compared with WT variants. NK-cell activation with Brad3-WT was 25.08%, whereas GRLR mutation did not mediate any activation (–3.57%), as expected. Both Fc mutation and glycoengineering increased NK-cell activation (averages of 63.07%, 60.48%, and 62.13% in GASDALIE, AFUC, and AFUC GASDALIE Brad3 variants, respectively). Similar trends were observed for Fog1 mAbs, with average NK-cell activation of 24.18% for WT mAb, increasing to 63.42%, 64.13%, and 66.98% in GASDALIE, AFUC, and AFUC GASDALIE mAb formats, respectively. Fog1-GRLR showed no NK-cell activation (–3.57%). In general, there was no significant difference between Fc mutagenesis and afucosylation for enhancing NK-cell activation.
Fc modification augments NK-cell activation. (A) Gating strategy for the evaluation of NK-cell activation. RhDpos RBCs were incubated with mAbs. NK cells obtained from 3 to 4 healthy donors were added to opsonized RBCs in the presence of brefeldin A and GolgiStop, anti-CD107a (APC H7), anti-CD3 (V500), anti-CD56 (PE-Cy7), and anti- IFN-γ (AF700). Activation was measured by evaluating intracellular IFN-γ production and/or the CD107a degranulation marker expression in NK cells (CD3– CD56dim). (B) The bar graphs show percentage of CD107+ cells, IFNγ+, or total activated NK cells. C107+ cells are represented by the combined populations of Q2 and Q3, IFNγ+ cells by Q1 and Q2, and total activated NK cells by Q1, Q2, and Q3 combined. Data are the average of 3 replicates, and error bars denote SEM. Total NK-cell activation was calculated with subtraction of “no-Ab” control background. Data show the average of 3 replicates, and error bars denote SEM. Statistical analyses were conducted using 1-way ANOVA, followed by Dunnett multiple comparison test (GraphPad Prism v9). ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. Data are the average of 3 replicates, and error bars denote SEM. FSC-A: Forward scatter area; Q: Quarter; SSC-A: Side scatter area.
Fc modification augments NK-cell activation. (A) Gating strategy for the evaluation of NK-cell activation. RhDpos RBCs were incubated with mAbs. NK cells obtained from 3 to 4 healthy donors were added to opsonized RBCs in the presence of brefeldin A and GolgiStop, anti-CD107a (APC H7), anti-CD3 (V500), anti-CD56 (PE-Cy7), and anti- IFN-γ (AF700). Activation was measured by evaluating intracellular IFN-γ production and/or the CD107a degranulation marker expression in NK cells (CD3– CD56dim). (B) The bar graphs show percentage of CD107+ cells, IFNγ+, or total activated NK cells. C107+ cells are represented by the combined populations of Q2 and Q3, IFNγ+ cells by Q1 and Q2, and total activated NK cells by Q1, Q2, and Q3 combined. Data are the average of 3 replicates, and error bars denote SEM. Total NK-cell activation was calculated with subtraction of “no-Ab” control background. Data show the average of 3 replicates, and error bars denote SEM. Statistical analyses were conducted using 1-way ANOVA, followed by Dunnett multiple comparison test (GraphPad Prism v9). ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. Data are the average of 3 replicates, and error bars denote SEM. FSC-A: Forward scatter area; Q: Quarter; SSC-A: Side scatter area.
Flow cytometry data of NK cell activation mediated by mAb variants (frequency of CD3-CD56dim cells)
| mAb . | Variant . | IFN-γ+ . | IFN-γ+/CD107a+ . | CD107a+ . | Total activation∗ . | P value† . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| RhDPos . | RhDNeg . | RhDPos . | RhDNeg . | RhDPos . | RhDNeg . | RhDPos . | RhDNeg . | |||
| Brad3 | WT | 15.20 | 1.18 | 12.09 | 0.85 | 27.40 | 3.91 | 25.08 | 1.95 | |
| GASDALIE | 45.51 | 2.68 | 37.88 | 2.11 | 60.80 | 6.28 | 63.07 | 4.75 | .0023 | |
| AFUC | 41.47 | 1.07 | 35.12 | 0.72 | 59.52 | 5.03 | 60.48 | 4.50 | .0040 | |
| AFUC GASDALIE | 44.18 | 2.76 | 37.55 | 2.24 | 60.92 | 10.33 | 62.13 | 6.90 | .0028 | |
| GRLR | 0.43 | 1.19 | 0.21 | 0.35 | 1.61 | 1.75 | −3.57 | −0.05 | .0171 | |
| Fog1 | WT | 13.30 | 0.60 | 10.59 | 0.31 | 26.85 | 1.77 | 24.18 | 0.00 | |
| GASDALIE | 43.50 | 0.87 | 38.50 | 0.58 | 63.82 | 3.65 | 63.42 | 2.85 | .0002 | |
| AFUC | 43.49 | 0.60 | 38.23 | 0.44 | 64.30 | 3.26 | 64.13 | 2.00 | .0001 | |
| AFUC GASDALIE | 45.36 | 2.06 | 39.12 | 1.35 | 66.20 | 7.25 | 66.98 | 9.55 | <.0001 | |
| GRLR | 0.46 | 1.10 | 0.21 | 0.34 | 1.58 | 1.70 | −3.57 | −0.10 | .0029 | |
| VRC03 | WT | 0.96 | 1.17 | 0.39 | 1.90 | 2.35 | 2.89 | −2.50 | 0.10 | — |
| GASDALIE | 0.66 | 1.64 | 0.38 | 2.60 | 2.76 | 4.67 | −2.38 | −0.20 | — | |
| AFUC | 2.23 | 0.58 | 0.90 | 0.67 | 3.66 | 1.66 | −0.43 | 0.30 | — | |
| AFUC GASDALIE | 3.83 | 0.37 | 1.92 | 0.86 | 4.54 | 1.83 | 1.05 | 0.30 | — | |
| GRLR | 0.44 | 1.49 | 0.14 | 1.36 | 1.36 | 4.72 | −3.75 | −0.10 | — | |
| No Ab | 1.43 | 0.54 | 0.68 | 0.30 | 3.76 | 1.67 | — | — | — | |
| mAb . | Variant . | IFN-γ+ . | IFN-γ+/CD107a+ . | CD107a+ . | Total activation∗ . | P value† . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| RhDPos . | RhDNeg . | RhDPos . | RhDNeg . | RhDPos . | RhDNeg . | RhDPos . | RhDNeg . | |||
| Brad3 | WT | 15.20 | 1.18 | 12.09 | 0.85 | 27.40 | 3.91 | 25.08 | 1.95 | |
| GASDALIE | 45.51 | 2.68 | 37.88 | 2.11 | 60.80 | 6.28 | 63.07 | 4.75 | .0023 | |
| AFUC | 41.47 | 1.07 | 35.12 | 0.72 | 59.52 | 5.03 | 60.48 | 4.50 | .0040 | |
| AFUC GASDALIE | 44.18 | 2.76 | 37.55 | 2.24 | 60.92 | 10.33 | 62.13 | 6.90 | .0028 | |
| GRLR | 0.43 | 1.19 | 0.21 | 0.35 | 1.61 | 1.75 | −3.57 | −0.05 | .0171 | |
| Fog1 | WT | 13.30 | 0.60 | 10.59 | 0.31 | 26.85 | 1.77 | 24.18 | 0.00 | |
| GASDALIE | 43.50 | 0.87 | 38.50 | 0.58 | 63.82 | 3.65 | 63.42 | 2.85 | .0002 | |
| AFUC | 43.49 | 0.60 | 38.23 | 0.44 | 64.30 | 3.26 | 64.13 | 2.00 | .0001 | |
| AFUC GASDALIE | 45.36 | 2.06 | 39.12 | 1.35 | 66.20 | 7.25 | 66.98 | 9.55 | <.0001 | |
| GRLR | 0.46 | 1.10 | 0.21 | 0.34 | 1.58 | 1.70 | −3.57 | −0.10 | .0029 | |
| VRC03 | WT | 0.96 | 1.17 | 0.39 | 1.90 | 2.35 | 2.89 | −2.50 | 0.10 | — |
| GASDALIE | 0.66 | 1.64 | 0.38 | 2.60 | 2.76 | 4.67 | −2.38 | −0.20 | — | |
| AFUC | 2.23 | 0.58 | 0.90 | 0.67 | 3.66 | 1.66 | −0.43 | 0.30 | — | |
| AFUC GASDALIE | 3.83 | 0.37 | 1.92 | 0.86 | 4.54 | 1.83 | 1.05 | 0.30 | — | |
| GRLR | 0.44 | 1.49 | 0.14 | 1.36 | 1.36 | 4.72 | −3.75 | −0.10 | — | |
| No Ab | 1.43 | 0.54 | 0.68 | 0.30 | 3.76 | 1.67 | — | — | — | |
Brad and Fog1 are anti-RhD mAbs while VRC03 is a non-RhD mAb (negative control).
Total activation was calculated with subtraction of No-Ab control background.
P values show the significance of total NK cell activation of mAb variant versus IgG1 format in each group. Statistical analyses were conducted using one-way ANOVA followed by Dunnett’s multiple-comparison test (GraphPad Prism v9). Data are average of 3 replicates. In each replicate, a pool of NK cells from 3-4 independent donors was used.
ADCC is augmented in engineered anti-RhD mAbs
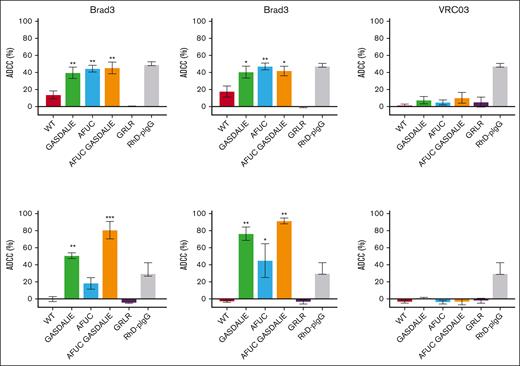
ADCC of bromelain-treated RBCs was assessed with a Hb ELISA for RBC lysis. Brad3-WT displayed 14% ADCC activity, whereas engineering of the Fc region increased ADCC to 40%, 44%, and 45% for the GASDALIE, AFUC, and AFUC GASDALIE variants, respectively. A similar degree of enhancement was seen in the Fog1 variants, with ADCC activity rising from 18% in the WT to 42%, 49%, and 43% for the GASDALIE, AFUC, and AFUC GASDALIE variants, respectively. Notably, the GRLR variants showed no ADCC activity, whereas RhD-pIgG achieved 49% ADCC activity (Figure 4A). The ADCC enhancements observed in the AFUC, GASDALIE, and AFUC GASDALIE variants indicate that both glycoengineering and Fc mutagenesis are effective approaches for enhancing effector cell engagement of anti-RhD mAbs when using bromelain-treated RBCs in vitro.
Fc modification enhances RBC clearance through ADCC. (A-B) ADCC assays were performed using monocyte-depleted PBMCs in the presence bromelain-treated (A) or untreated (B) RhDpos RBCs opsonized with anti-RhD Abs. The release of Hb, indicating RBC lysis, was measured by enzyme-linked immunosorbent assay (ELISA). ADCC was calculated with subtraction of “no Ab” control background. All the mAbs were tested alongside RhD-pIgG, but each group of mAbs is presented in a separate figure to allow for a clear comparison. Data are the average of 2 to 4 replicates, and error bars denote SEM. Statistical analyses were conducted using 1-way ANOVA, followed by Dunnett multiple comparison test (GraphPad Prism v9). ∗P < .05; ∗∗P < .01.
Fc modification enhances RBC clearance through ADCC. (A-B) ADCC assays were performed using monocyte-depleted PBMCs in the presence bromelain-treated (A) or untreated (B) RhDpos RBCs opsonized with anti-RhD Abs. The release of Hb, indicating RBC lysis, was measured by enzyme-linked immunosorbent assay (ELISA). ADCC was calculated with subtraction of “no Ab” control background. All the mAbs were tested alongside RhD-pIgG, but each group of mAbs is presented in a separate figure to allow for a clear comparison. Data are the average of 2 to 4 replicates, and error bars denote SEM. Statistical analyses were conducted using 1-way ANOVA, followed by Dunnett multiple comparison test (GraphPad Prism v9). ∗P < .05; ∗∗P < .01.
However, to assess the mAbs’ ADCC capabilities under physiological conditions, native untreated RhDpos RBCs were used instead of bromelain-treated cells. As shown in Figure 4B, although RhD-pIgG mediated moderate ADCC (30.5%), no ADCC activity was detected for Brad3-WT and Fog1-WT. In contrast, the Brad3-GASDALIE and Brad3-AFUC GASDALIE variants demonstrated significant ADCC enhancement (with values of 52.5% and 83.5%, respectively), surpassing both Brad3-WT and RhD-pIgG. Similar trends were observed for the Fog1-GASDALIE and Fog1-AFUC GASDALIE variants, which mediated 79% and 94.5% ADCC, respectively. The Brad3-AFUC variant showed 19% ADCC, which was not statistically significant compared with Brad3-WT. The Fog1-AFUC variant exhibited a 46.5% ADCC increase over Fog1-WT, although its activity remained lower than the GASDALIE and AFUC GASDALIE variants. AFUC GASDALIE showed some enhancement over GASDALIE, but this difference was not statistically significant. These results highlight the effectiveness of Fc mutagenesis (GASDALIE and AFUC GASDALIE variants) to enhance ADCC and the importance of assessing ADCC using native, untreated RBCs.
Discussion
Preventing maternal alloimmunization during pregnancy and after childbirth by administering RhD-pIgG has proven efficacy. However, the supply of this life-saving therapy is currently restricted to wealthy countries. Synthetically produced anti-RhD mAbs could offer a standardized and sustainable alternative to donor-derived RhD-pIgG for the prevention of HDFN. However, despite the production of multiple anti-RhD mAbs, none have demonstrated activity equivalent to that of RhD-pIgG.15 A further challenge is that the mechanism of immune suppression mediated by RhD-pIgG remains incompletely understood.31
It has been shown that RhDpos RBCs opsonized with anti-RhD Abs are sequestered by splenic macrophages,32 which express all 3 activating FcγRs (FcγRI, FcγRIIa, and FcγRIIIa).33 NK cells (expressing FcγRIIIa) and monocytes (expressing FcγRI and FcγRIIa) are, therefore, widely used for in vitro functional assays to mimic the clearance of RhDpos RBCs by splenic macrophages and to predict the suppression of the anti-RhD immune response during pregnancy.34,35 Anti-RhD mAbs produced in heterohybridoma or Epstein-Barr virus–transformed B-lymphoblastoid cell lines typically induce RBC phagocytosis but exhibit minimal ADCC, likely due to insufficient FcγRIIIa binding.36,37 This is a major limitation because effective clearance of RBCs is correlated with robust ADCC activity, particularly through FcγRIIIa engagement.38,39 Notably, anti-RhD mAbs produced in rat myeloma cells showed significantly greater ADCC and RBC clearance than RhD-pIgG.40-43 This enhanced activity is thought to be due to the reduced core fucosylation levels of mAbs produced in rat cell lines (<35%, compared with ∼80% fucosylation in RhD-pIgG), thereby facilitating higher ADCC.42 However, roledumab, an anti-RhD mAb produced in rat YB2/0 cells, caused adverse events in 38% of pregnant participants due to immune responses triggered by nonhuman glycosylation patterns.44
To overcome these limitations, CHO cell lines are commonly used for large-scale production of recombinant therapeutic mAbs, because their posttranslational modifications, such as glycosylation, are comparable with human cells.45 CHO cells also pose less risk of spreading human pathogenic viruses.46 High Fc galactosylation, together with low core fucosylation, is found in RhD-pIgG,47 and these features are thought to facilitate rapid RBC clearance by anti-RhD mAbs.42 However, CHO-produced anti-RhD mAbs typically exhibit high core fucosylation levels (74%-93%) with reduced mAb binding to FcγRIIIa receptors, thereby diminishing ADCC activity and impairing RBC clearance.42 To our knowledge, Rhoclone is the only CHO-expressed anti-RhD mAb. Rhoclone is widely used in India and other low-income countries for the prevention of HDFN. Although a clinical trial showed that Rhoclone prevented alloimmune sensitization,48-51 data on parity, fetal-maternal ABO compatibility, and the outcomes for subsequent pregnancies have not yet been reported for this study. Additionally, recruitment criteria may have led to the exclusion of potential nonresponders, limiting its generalizability.52,53
Ab engineering, such as glycoengineering and Fc mutagenesis, have emerged as powerful strategies to enhance ADCC. Mogamulizumab (anti-CCR4 mAb for the treatment of mycosis fungoides),54 benralizumab (anti–interleukin-5 receptor mAb for severe eosinophilic asthma),55 and inebilizumab (anti-CD19 mAb for the treatment of neuromyelitis optica)56 are AFUC mAbs that demonstrate enhanced ADCC. Other strategies, such as Fc mutagenesis, have also proven effective. For instance, Monjuvi, featuring the S239D, I332E Fc mutations, is approved by the US Food and Drug Administration for B-cell lymphoma.57 BI 836826, a mAb targeting CD37, features the S239D/I332E mutations and is currently in phase 2 clinical trials for chronic lymphocytic leukemia.58 Botensilimab is also a multifunctional, Fc-enhanced anti-CTLA-4 mAb, incorporating the S239D, A330L, and I332E Fc mutations,59 and is currently in phase 2 clinical trials for various cancers.
Previous studies have shown that reducing anti-RhD mAb core fucosylation enables rapid RBC clearance by enhancing ADCC efficacy.18,42 However, these studies usually relied on papain- or bromelain-treated RBCs to increase assay sensitivity by partial removal of the glycocalyx from the RBC cell surface. This approach risks introducing false-positive results and misinterpreting Ab efficacy. In our ADCC studies using bromelain-treated RBCs, all engineered mAbs demonstrated efficacy comparable with RhD-pIgG. In contrast, when ADCC assays were conducted with native untreated RBCs, both GASDALIE and AFUC GASDALIE mAbs exhibited significantly higher activity than both AFUC mAbs and RhD-pIgG. These findings suggest that Fc mutagenesis may be a more effective strategy than glycoengineering for developing ADCC-enhanced anti-RhD mAbs and highlight the importance of assessing ADCC in the more physiological context of intact RBC.
In summary, we demonstrate, to our knowledge, for the first time that Fc mutations increase ADCC (the most predictive of clinical efficacy) of anti-RhD mAbs. Fc-mutated mAbs, developed against other diseases, have demonstrated not only enhanced efficacy but also a favorable safety profile, with minimal stimulation of anti-drug Abs in clinical trials.57-59 Furthermore, Fc mutagenesis offers a simpler and more cost-effective alternative to glycoengineering because it can be readily integrated into existing mAb manufacturing processes, avoiding batch-to-batch variation and reducing production costs. We suggest that Fc mutagenesis of anti-RhD mAbs provides a practical advancement in the pursuit of global prevention of HDFN.
Acknowledgments
The visual abstract was created with BioRender.com.
This work was supported by funding from the Norman Beischer Medical Research Foundation, the Walter and Eliza Hall Institute (WEHI) Innovation Fund (IF23-07), and seed funding kindly provided by John Silke. E.D.G.-B. was supported by the Rebecca L. Cooper Foundation; I.P.W. was supported by Australian National Health and Medical Research Council (1154325) and the John T. Reid Charitable Trusts.
Authorship
Contribution: B.H. and I.P.W. contributed to conceptualization and oversight of the experiment; B.H. contributed to antibody engineering and expression and functional assays; D.B.D. and H.W. contributed to western blot; E.D.G.-B. contributed for vital reagents and advice on antibody glycoengineering; B.H., D.B.D., and H.W. contributed to analysis, figures, and original draft of the manuscript; B.H., D.B.D., E.D.G., and I.P.W. contributed to reviewing and editing of the manuscript; and B.H. and I.P.W. contributed to funding acquisition.
Conflict-of-interest disclosure: B.H. and I.P.W. are inventors on a corresponding patent from Walter and Eliza Hall Institute: Anti-Rhesus D human monoclonal antibodies (AU2023902692). The remaining authors declare no competing financial interests.
Correspondence: Ian P. Wicks, Walter and Eliza Hall Institute of Medical Research, 1G Royal Parade, Parkville, VIC 3052, Australia; email: wicks@wehi.edu.au; and Behnaz Heydarchi, Walter and Eliza Hall Institute of Medical Research, 1G Royal Parade, Parkville, VIC 3052, Australia; email: heydarchi.b@wehi.edu.au.
References
Author notes
Further information and any requests for protocols, resources, and reagents should be directed to the corresponding authors, Behnaz Heydarchi (heydarchi.b@wehi.edu.au) and Ian P. Wicks (wicks@wehi.edu.au).