Key Points
Lower C3 levels were associated with a higher likelihood of developing HLH.
Patients with low C3 had a high risk of death in overall survival and dynamic changes in C3 could reflect patients’ prognosis.
Visual Abstract
Hemophagocytic lymphohistiocytosis (HLH) is a life-threatening hyperinflammatory syndrome. Complement component 3 (C3), a central effector molecule in 3 separate complement pathways, has been linked to inflammatory diseases. Hence, we aimed to investigate the clinical significance of C3 in adult HLH. In this retrospective cohort study, patients meeting ≥5 of 8 HLH-2004 criteria were classified as the HLH group (n = 627), whereas those meeting 1 to 4 criteria were the partial HLH group (n = 588). C3 was significantly lower in the HLH group than the partial HLH group (P < .0001), and low C3 was an independent factor predicting progression from partial HLH to HLH (odds ratio, 3.94; P < .001). Low C3 was associated with more severe cytopenia, coagulation abnormalities, and liver dysfunction. Additionally, patients with low C3 had poorer overall survival (P = .00099), and low C3 was an independent risk factor for early death in HLH (hazard ratio, 1.64; P = .019). Most patients with HLH had normal C3 before HLH onset, followed by a decline after HLH development (P < .0001). Moreover, survivors showed an increase in C3 (P = .0003), whereas nonsurvivors exhibited a decrease in C3 (P = .90). In conclusion, our study identified C3 as a valuable predictive and prognostic biomarker in adult HLH. Monitoring the dynamic changes in C3 levels may reflect therapeutic response and guide timely clinical interventions.
Introduction
Hemophagocytic lymphohistiocytosis (HLH), also known as hemophagocytic syndrome (HPS), is a life-threatening hyperinflammatory syndrome characterized by the abnormal activation and proliferation of inflammatory cells. This immune dysregulation leads to the release of excessive cytokines. HLH can be caused by genetic or acquired factors and is divided into primary (genetic) and secondary (reactive) forms, with various triggers including infections, tumors, rheumatic diseases (RHDs), drugs, and other related causes.1,2 In adults, infections, particularly Epstein-Barr virus (EBV) infection, are the most common triggers for HLH. Patients with malignancies, especially hematologic malignancies such as lymphoma, are also susceptible to HLH. Additionally, RHDs play a significant role as triggering factors for adult HLH. Clinical manifestations of HLH include persistent fever, cytopenia, hepatosplenomegaly, liver dysfunction, reduced or absent natural killer (NK)–cell activity, and hemophagocytosis of tissues.3-5
Despite HLH being primarily recognized as a hereditary disease affecting children, recent researches have revealed that it can occur at any age. However, until now, most clinical guidelines, prospective studies, and clinical trials have focused on pediatric patients.6,7 The HLH-2004 diagnostic criteria, originally developed for pediatric patients, are still the most commonly used criteria in adult patients. The diagnostic criteria for HLH-2004 consist of 8 items, and a diagnosis of HLH is made when ≥5 of these criteria are met.8 In clinical practice, it is often observed that adult patients meet some criteria of HLH-2004 due to secondary causes but do not progress to full HLH. This may indicate a partial HLH condition.9 Therefore, we aim to identify the factors that contribute to the progression from partial HLH to HLH. In terms of prognosis, mortality rates in adult HLH range from 20% to 88%, primarily due to refractory HLH, secondary infections, and progression of the underlying triggering disease.10 It is important to note that these evaluations are objective and based on statistical data. Many studies have reported high mortality rates during induction therapy, particularly within the first 8 weeks.11,12 So far, there have been some studies discussing the prognostic indicators of HLH,13-17 but reports on the prognostic significance of complement component 3 (C3) in HLH are still lacking.
In this study, we reviewed the clinical data of adult patients with HLH and partial HLH over an 11-year period at our center. The aim was to identify the clinical characteristics of adult patients with HLH and specific biomarkers for discriminating HLH and partial HLH. Notably, complement C3, a crucial component of the complement system, was found to be a significant biomarker distinguishing HLH from partial HLH. Furthermore, we discovered that C3 levels were associated with disease severity and early mortality in adult patients with HLH. By doing so, we believe these data could provide clinicians with a better understanding of disease prediction and therapeutic efficacy evaluation among patients.
Methods
Patient eligibility and follow-up
We conducted a retrospective analysis of patient data collected between 1 December 2012 and 9 November 2023 at Wuhan Union Hospital in China. The inclusion criteria for patients were as follows: (1) age ≥18 years; (2) diagnosis or suspicion of HLH; (3) patients fulfilling ≥5 of 8 HLH-2004 diagnostic criteria were classified as HLH group, whereas those who met 1 to 4 criteria were assigned to the partial HLH group; and (4) traceable clinical record. The diagnostic criteria of secondary HLH in HLH-2004 are as follows8: (1) fever; (2) splenomegaly; (3) cytopenia affecting ≥2 of 3 lineages in the peripheral blood (hemoglobin [Hb] <90 g/L; platelets [PLTs] <100 × 109/L; neutrophils <1.0 × 109/L); (4) hypertriglyceridemia and/or hypofibrinogenemia (fasting triglycerides [TGs] ≥3.0 mmol/L; fibrinogen [FIB] ≤1.5 g/L); (5) hemophagocytosis in the bone marrow, spleen, or lymph nodes; (6) low or absent NK-cell activity (according to local laboratory reference); (7) serum ferritin ≥500 mg/L; and (8) soluble CD25 (sCD25; ie, soluble interleukin-2 [IL-2] receptor) ≥2400 U/mL or ≥6400 pg/mL. Exclusion criteria included patients aged <18 years and patients with refractory or relapsed HLH. Telephone follow-ups were conducted based on hospitalization information. The follow-up period ended on 10 December 2023 or on the date of death or loss to follow-up.
All procedures performed in studies involving human participants were in accordance with the Declaration of Helsinki. This study was approved by the local ethics committee. This study used deidentified data and was approved by the local ethics committee and exempted from informed consent.
Clinical and biochemical data acquisition
We retrospectively collected patient information from our center’s electronic medical record database. The following variables were identified based on the electronic medical records: age, gender, underlying diseases (such as malignancy, infection, RHD, other causes, and unknown), and laboratory findings at diagnosis. The following laboratory parameters were measured: C3, C4, white blood cell (WBC), Hb, absolute neutrophil count (ANC), PLT, alanine aminotransferase, aspartate aminotransferase (AST), total bilirubin, albumin (Alb), creatinine, blood urea nitrogen, TGs, lactate dehydrogenase, C-reactive protein, procalcitonin (PCT), FIB, activated partial thromboplastin time (APTT), prothrombin time (PT), D-dimer, carbohydrate antigen 125 (CA125), CA153, neuron-specific enolase (NSE), and EBV DNA copies in plasma and peripheral blood mononuclear cells (PBMCs). Changes in the percentage of lymphocytes in peripheral blood, including total T cell, CD4+ T cell, CD8+ T cell, B cell, and NK cell, as well as cytokine levels (such as IL-2, IL-4, IL-6, IL-10, interferon gamma [IFN-γ], and tumor necrosis factor α), and relevant variables of diagnostic experiment (such as the highest recorded temperature, splenomegaly, hemophagocytosis, serum ferritin, elevated sCD25 levels, and NK-cell activity) were collected. Additionally, we collected data on the dynamic changes of C3 values before, during, and after HLH treatment.
Statistical analysis
Continuous variables with a normal distribution were presented as the mean and standard deviation, whereas those with a nonnormal distribution were described as the median and interquartile range (IQR). Categorical variables were reported as counts (n) and percentages (%). When comparing 2 independent groups, we applied the independent samples t test to continuous variables with a normal distribution and the Mann-Whitney U test to continuous variables with a nonnormal distribution. For categorical variables, we used the χ2 test. To evaluate the diagnostic value of serum C3, we constructed receiver operating characteristic (ROC) curves and calculated the area under the ROC curve. The optimal cutoff points for C3 were determined using the Youden index (sensitivity + specificity – 1), which is calculated based on the sensitivities and specificities produced by ROC curves. The probabilities of overall survival were estimated by the Kaplan-Meier method and compared using the log-rank test. Variables with a P value <.05 in the univariate analysis were included in multiple logistic or Cox regression analyses. C3 was included in the multivariate analysis regardless of its significance in the univariate analysis. After excluding variables with very low prevalence, only variables with clinical relevance and statistical significance were included in the multiple regression analysis to determine the clinical significance of C3. All statistical analyses and graphs were performed using R software version 4.2.2 and GraphPad Prism version 9.0 (GraphPad Software).
Results
Patient selection and clinical characteristics
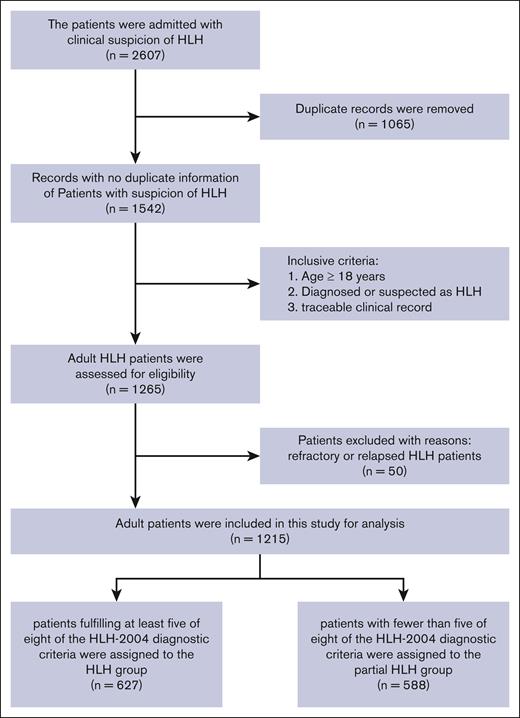
This single-center study enrolled adult patients suspected or diagnosed with HLH at Wuhan Union Hospital between 1 December 2012 and 9 November 2023. The flowchart of the patient selection process is shown in Figure 1. A total of 2607 patients were initially included, and 1065 duplicate patient records were removed according to identifiable information. Then, 1265 patients were included after screening based on inclusion criteria such as age at onset, discharge diagnosis, and traceable clinical record. Due to the higher missing values of C3, we excluded 50 relapsed and refractory patients with HLH, but we still collected their data for reference (supplemental Table 1). Finally, a total of 1215 patients were enrolled for analysis in this study. Subsequently, the complete medical records of the patients were reviewed, and those who met ≥5 of the 8 HLH-2004 diagnostic criteria were assigned to the HLH group (n = 627), whereas those with <5 of the criteria were assigned to the partial HLH group (n = 588).
The flowchart of patient selection process. We retrospectively reviewed the patients admitted to our center from 1 December 2012 to 9 November 2023. Finally, 1215 patients were enrolled in this study, with 627 being assigned to the HLH group and 588 to the partial HLH group.
The flowchart of patient selection process. We retrospectively reviewed the patients admitted to our center from 1 December 2012 to 9 November 2023. Finally, 1215 patients were enrolled in this study, with 627 being assigned to the HLH group and 588 to the partial HLH group.
The demographic and diagnostic information of the enrolled patients is shown in Table 1, and additional laboratory findings are shown in supplemental Table 2. There were no significant differences in age at onset between the HLH and the partial HLH groups (P = .872). However, there was a higher proportion of females in the partial HLH group (P = .007). Among the underlying triggers, infection was the primary trigger in both groups, whereas malignancy was more prevalent in the HLH group, and RHD was more common in the partial HLH group (Figure 2A; supplemental Figure 1A). In the HLH group, females accounted for 309 cases (49.3%) and had a median age of diagnosis of 51 years (IQR, 34-63). Abnormal diagnostic indicators were more common in the HLH group, with a median number of 5 HLH-2004 criteria fulfilled (IQR, 5-6), whereas the partial HLH group had a median number of 3 criteria fulfilled (IQR, 2-4). In addition, the median C3 level of the HLH group was 0.63 g/L, which was significantly lower than the median C3 level of the partial HLH group (0.84 g/L; P < .001; Table1; Figure 2B). However, there was no significant difference in C4 levels between the 2 groups (P = .582). The HLH group exhibited severe cytopenia compared with the partial HLH group, with significantly lower levels of WBC (P < .001), Hb (P < .001), and PLT (P < .001). Abnormal coagulation function (D-dimer, P = .002; PT, P < .001; and APTT, P < .001) and liver dysfunction (total bilirubin, P < .001; alanine aminotransferase, P = .002; AST, P = .006; and Alb, P < .001) were also more common in the HLH group. The inflammatory marker C-reactive protein showed no significant difference between the 2 groups (P = .625), but PCT was significantly higher in the HLH group (P = .001). Additionally, tumor markers CA125 (P < .001), CA153 (P < .001), and NSE (P < .001) were significantly elevated in the HLH group. The HLH group exhibited a higher ratio of total T cells in peripheral blood lymphocytes (P = .025). Although there were no significant differences, the percentage of B cells (P = .1) and NK cells (P = .105) were decreased in the HLH group. The HLH group also showed significant increases in IL-2 (P = .032), IL-6 (P < .001), IL-10 (P < .001), and IFN-γ (P < .001). Moreover, the levels of EBV DNA copies in both plasma (P = .01) and PBMCs (P < .001) were significantly higher in the HLH group than in the partial HLH group (supplemental Table 2).
Demographic and diagnostic information of patients with HLH and partial HLH
| Variables . | Total (N = 1215) . | HLH (n = 627) . | Partial HLH (n = 588) . | P value† . |
|---|---|---|---|---|
| Age, median (IQR), y | 51 (34-63) | 51 (34-63) | 52 (33-63) | .872 |
| Female, n (%) | 645 (53.1) | 309 (49.3) | 336 (57.1) | .007∗∗ |
| HLH triggers, n (%) | ||||
| Malignancy | 285 (23.5) | 207 (33) | 78 (13.3) | <.001∗∗∗ |
| HM | 228 (18.8) | 169 (27) | 59 (10) | <.001∗∗∗ |
| T-/NK-cell lymphoma | 117 (9.6) | 84 (13.4) | 33 (5.6) | <.001∗∗∗ |
| B-cell lymphoma | 78 (6.4) | 62 (9.9) | 16 (2.7) | <.001∗∗∗ |
| Hodgkin lymphoma | 6 (0.5) | 4 (0.6) | 2 (0.3) | .688 |
| Leukemia | 14 (1.2) | 12 (1.9) | 2 (0.3) | .021∗ |
| MDS | 11 (0.9) | 6 (1) | 5 (0.9) | 1 |
| Multiple myeloma | 1 (0.1) | 1 (0.2) | 0 (0) | 1 |
| POEMS syndrome | 1 (0.1) | 0 (0) | 1 (0.2) | .484 |
| Other malignancy | 57 (4.7) | 38 (6.1) | 19 (3.2) | .028∗ |
| Infection‡ | 580 (47.7) | 271 (43.2) | 309 (52.6) | .001∗∗ |
| EBV infection | 505 (41.6) | 310 (49.4) | 195 (33.2) | <.001∗∗∗ |
| EBV with malignancy | 149 (12.3) | 121 (19.3) | 28 (4.8) | <.001∗∗∗ |
| EBV with RHD | 75 (6.2) | 26 (4.1) | 49 (8.3) | .004∗∗ |
| Only EBV infection | 281 (23.1) | 163 (26) | 118 (20.1) | .017∗ |
| Other infection | 299 (24.6) | 108 (17.2) | 191 (32.5) | <.001∗∗∗ |
| RHD, n (%) | 195 (16) | 71 (11.3) | 124 (21.1) | < .001∗∗∗ |
| Other causes, n (%) | 61 (5) | 25 (4) | 36 (6.1) | .116 |
| Unknown, n (%) | 94 (7.7) | 53 (8.5) | 41 (7) | .391 |
| Fever, median (IQR) | 39.2 (38.8-39.9) | 39.4 (39-40) | 39.05 (38.5-39.8) | <.001∗∗∗ |
| Splenomegaly, n (%) | 674 (58.3) | 488 (82) | 186 (33.1) | <.001∗∗∗ |
| Hemophagocytosis, n (%) | 565 (60.2) | 399 (73.5) | 166 (41.9) | <.001∗∗∗ |
| Cytopenia ≥2 lineages, n (%) | 673 (55.4) | 504 (80.4) | 169 (28.7) | <.001∗∗∗ |
| Low NK-cell activity, n (%) | 316 (52.3) | 216 (59) | 100 (42) | <.001∗∗∗ |
| Elevated sCD25§, n (%) | 245 (40.7) | 210 (57.9) | 35 (14.6) | <.001∗∗∗ |
| SF >2000 μg/L, n (%) | 873 (73.8) | 486 (78.1) | 387 (69) | <.001∗∗∗ |
| FIB, median (IQR), g/L | 2.44 (1.39-3.99) | 1.57 (1.18-2.92) | 3.14 (2.2-4.74) | <.001∗∗∗ |
| TG, median (IQR), mmol/L | 2.12 (1.39-3.26) | 2.5 (1.67-3.49) | 1.78 (1.27-2.64) | <.001∗∗∗ |
| C3, median (IQR), g/L | 0.72 (0.52-0.95) | 0.63 (0.48-0.8) | 0.84 (0.61-1.06) | <.001∗∗∗ |
| C4, median (IQR), g/L | 0.21 (0.14-0.28) | 0.21 (0.13-0.29) | 0.21 (0.15-0.28) | .582 |
| No. of HLH-2004 criteria fulfilled, median (IQR) | 5 (3-5) | 5 (5-6) | 3 (2-4) | <.001∗∗∗ |
| Variables . | Total (N = 1215) . | HLH (n = 627) . | Partial HLH (n = 588) . | P value† . |
|---|---|---|---|---|
| Age, median (IQR), y | 51 (34-63) | 51 (34-63) | 52 (33-63) | .872 |
| Female, n (%) | 645 (53.1) | 309 (49.3) | 336 (57.1) | .007∗∗ |
| HLH triggers, n (%) | ||||
| Malignancy | 285 (23.5) | 207 (33) | 78 (13.3) | <.001∗∗∗ |
| HM | 228 (18.8) | 169 (27) | 59 (10) | <.001∗∗∗ |
| T-/NK-cell lymphoma | 117 (9.6) | 84 (13.4) | 33 (5.6) | <.001∗∗∗ |
| B-cell lymphoma | 78 (6.4) | 62 (9.9) | 16 (2.7) | <.001∗∗∗ |
| Hodgkin lymphoma | 6 (0.5) | 4 (0.6) | 2 (0.3) | .688 |
| Leukemia | 14 (1.2) | 12 (1.9) | 2 (0.3) | .021∗ |
| MDS | 11 (0.9) | 6 (1) | 5 (0.9) | 1 |
| Multiple myeloma | 1 (0.1) | 1 (0.2) | 0 (0) | 1 |
| POEMS syndrome | 1 (0.1) | 0 (0) | 1 (0.2) | .484 |
| Other malignancy | 57 (4.7) | 38 (6.1) | 19 (3.2) | .028∗ |
| Infection‡ | 580 (47.7) | 271 (43.2) | 309 (52.6) | .001∗∗ |
| EBV infection | 505 (41.6) | 310 (49.4) | 195 (33.2) | <.001∗∗∗ |
| EBV with malignancy | 149 (12.3) | 121 (19.3) | 28 (4.8) | <.001∗∗∗ |
| EBV with RHD | 75 (6.2) | 26 (4.1) | 49 (8.3) | .004∗∗ |
| Only EBV infection | 281 (23.1) | 163 (26) | 118 (20.1) | .017∗ |
| Other infection | 299 (24.6) | 108 (17.2) | 191 (32.5) | <.001∗∗∗ |
| RHD, n (%) | 195 (16) | 71 (11.3) | 124 (21.1) | < .001∗∗∗ |
| Other causes, n (%) | 61 (5) | 25 (4) | 36 (6.1) | .116 |
| Unknown, n (%) | 94 (7.7) | 53 (8.5) | 41 (7) | .391 |
| Fever, median (IQR) | 39.2 (38.8-39.9) | 39.4 (39-40) | 39.05 (38.5-39.8) | <.001∗∗∗ |
| Splenomegaly, n (%) | 674 (58.3) | 488 (82) | 186 (33.1) | <.001∗∗∗ |
| Hemophagocytosis, n (%) | 565 (60.2) | 399 (73.5) | 166 (41.9) | <.001∗∗∗ |
| Cytopenia ≥2 lineages, n (%) | 673 (55.4) | 504 (80.4) | 169 (28.7) | <.001∗∗∗ |
| Low NK-cell activity, n (%) | 316 (52.3) | 216 (59) | 100 (42) | <.001∗∗∗ |
| Elevated sCD25§, n (%) | 245 (40.7) | 210 (57.9) | 35 (14.6) | <.001∗∗∗ |
| SF >2000 μg/L, n (%) | 873 (73.8) | 486 (78.1) | 387 (69) | <.001∗∗∗ |
| FIB, median (IQR), g/L | 2.44 (1.39-3.99) | 1.57 (1.18-2.92) | 3.14 (2.2-4.74) | <.001∗∗∗ |
| TG, median (IQR), mmol/L | 2.12 (1.39-3.26) | 2.5 (1.67-3.49) | 1.78 (1.27-2.64) | <.001∗∗∗ |
| C3, median (IQR), g/L | 0.72 (0.52-0.95) | 0.63 (0.48-0.8) | 0.84 (0.61-1.06) | <.001∗∗∗ |
| C4, median (IQR), g/L | 0.21 (0.14-0.28) | 0.21 (0.13-0.29) | 0.21 (0.15-0.28) | .582 |
| No. of HLH-2004 criteria fulfilled, median (IQR) | 5 (3-5) | 5 (5-6) | 3 (2-4) | <.001∗∗∗ |
HM, hematologic malignancy; MDS, myelodysplastic syndromes; POEMS, polyneuropathy, organomegaly, endocrinopathy, M-protein, skin changes; SF, serum ferritin.
∗P < .05; ∗∗P < .01; ∗∗∗P < .001.
Infection means only EBV infection and other infection.
Elevated sCD25 means sCD25 ≥2400 U/mL or ≥6400 pg/mL.
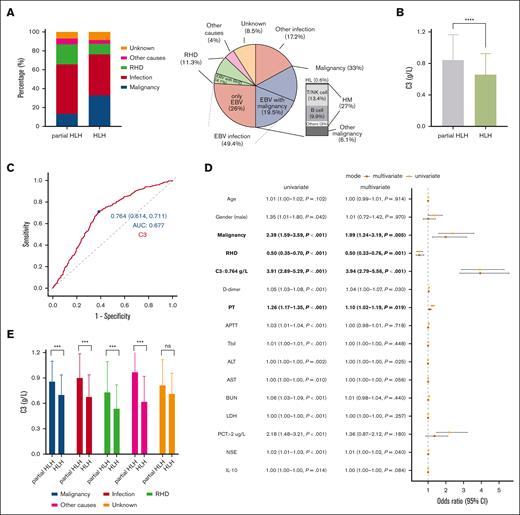
The predictive value of C3 in the progression from partial HLH to HLH. (A) Distribution of different triggers in partial HLH and HLH groups. The HLH group mainly triggered by infection and malignancy. (B) C3 was significantly lower in the HLH group than the partial HLH group. (C) The ROC curve of C3 for discriminating partial HLH and HLH. (D) The forest plot of univariate and multivariate logistic regression analysis. (E) In different triggers, C3 was significantly lower in the HLH group than the partial HLH group. ∗∗∗P < .001; ∗∗∗∗P < .0001; ns, nonsignificance. AUC, area under the ROC curve; 95% CI, 95% confidence interval; ALT, alanine aminotransferase; BUN, blood urea nitrogen; HM, hematologic malignancy; LDH, lactate dehydrogenase; Tbil, total bilirubin.
The predictive value of C3 in the progression from partial HLH to HLH. (A) Distribution of different triggers in partial HLH and HLH groups. The HLH group mainly triggered by infection and malignancy. (B) C3 was significantly lower in the HLH group than the partial HLH group. (C) The ROC curve of C3 for discriminating partial HLH and HLH. (D) The forest plot of univariate and multivariate logistic regression analysis. (E) In different triggers, C3 was significantly lower in the HLH group than the partial HLH group. ∗∗∗P < .001; ∗∗∗∗P < .0001; ns, nonsignificance. AUC, area under the ROC curve; 95% CI, 95% confidence interval; ALT, alanine aminotransferase; BUN, blood urea nitrogen; HM, hematologic malignancy; LDH, lactate dehydrogenase; Tbil, total bilirubin.
Predictive value of C3 in the progression from partial HLH to HLH
We conducted ROC analysis to determine the optimal cutoff point of C3 concentration in the serum for distinguishing between patients with HLH and partial HLH. Based on this curve and the highest Youden index, the cutoff point for C3 was determined to be 0.764 g/L (Figure 2C). The area under the ROC curve for C3 was superior to that of the classical diagnostic indicators, such as ANC, TGs, and AST (supplemental Figure 1B). C3 values below the cutoff point were defined as low C3, whereas values above the cutoff were defined as high C3. To clarify the predictive role of low C3 in the progression from partial HLH to HLH, a multivariate logistic regression analysis was performed on indicators with a significance level of P value <.05 in the univariate analysis. The results verified that low C3 (C3 <0.764 g/L) was an independent factor for predicting the progression from partial HLH to HLH (odds ratio, 3.94; P < .001). Lower C3 levels were associated with a higher likelihood of developing HLH. Malignancy and PT were also identified as independent factors for predicting progression from partial HLH to HLH, whereas RHD was identified as a protective factor (Figure 2D; supplemental Table 3).
Among the triggers of malignancy, infection, RHD, and other causes, C3 levels were significantly lower in the HLH group than the partial HLH group, except for the trigger of unknown (P = .185; Figure 2E). Furthermore, among patients with HLH with different underlying diseases, the RHD subgroup had the lowest C3 levels compared with other triggers (supplemental Figure 1C).
The severity of HLH was associated with serum C3 levels
To further investigate the relationship between low C3 and the severity of HLH, we conducted a series of analyses. The results showed no significant differences in age (P = .591) and gender (P = .066) between the high C3 and low C3 groups. However, there was a significant difference in mortality rate between the 2 groups, with the low C3 group having a higher mortality rate, accounting for 51% (P = .009). Patients with low C3 exhibited more severe decreases in WBC (P = .034), ANC (P = .022), and PLT count (P < .001). Regarding coagulation function, the low C3 group showed significantly increased D-dimer levels (P = .002), prolonged PT (P < .001), and APTT (P = .001), as well as decreased FIB (P < .001). Additionally, elevated AST (P = .029), CA153 (P = .022), EBV DNA copies in PBMCs (P = .019), and decreased Alb (P = .013) were more common in the low C3 group (Table 2).
The relationship between C3 levels and other variables
| Variables . | Total (N = 381) . | High C3 (n = 110) . | Low C3 (n = 271) . | P value . |
|---|---|---|---|---|
| Age, median (IQR), y | 50 (33-62) | 50.5 (36-62) | 50 (32-60.5) | .591 |
| Female, n (%) | 203 (53) | 50 (45) | 153 (56) | .066 |
| Follow-up time, median (IQR) | 46 (16-556) | 97 (19-589) | 41 (14-539.5) | .245 |
| Death, n (%) | 194 (51) | 39 (35) | 155 (57) | <.001∗∗∗ |
| FIB, median (IQR), g/L | 1.49 (1.14-2.82) | 2.2 (1.36-3.83) | 1.39 (1.06-2.42) | <.001∗∗∗ |
| C4, median (IQR), g/L | 0.21 (0.13-0.29) | 0.28 (0.21-0.33) | 0.18 (0.11-0.25) | <.001∗∗∗ |
| WBC, median (IQR), ×109/L | 3.26 (1.82-6.14) | 3.81 (2.29-6.52) | 3.04 (1.69-6.06) | .034∗ |
| ANC, median (IQR), ×109/L | 2.03 (0.94-4.11) | 2.6 (1.35-4.57) | 1.76 (0.9-3.97) | .022∗ |
| PLT, median (IQR), ×109/L | 53 (31-82) | 65 (42.25-96) | 47 (27-75) | <.001∗∗∗ |
| D-dimer, median (IQR), mg/L | 3.52 (1.96-8.59) | 2.57 (1.47-7.32) | 4.14 (2.16-9.1) | .002∗∗ |
| PT, median (IQR), sec | 15.1 (13.6-16.5) | 14.25 (13.1-15.4) | 15.4 (13.85-17) | <.001∗∗∗ |
| APTT, median (IQR), sec | 41.8 (36.4-49.3) | 39.8 (35.1-45.27) | 42.7 (37.4-50.45) | .001∗∗ |
| Alb, median (IQR), g/L | 29 (25.6-35) | 30.8 (26.35-38.65) | 28.3 (25.1-33.5) | .013∗ |
| AST, median (IQR), U/L | 57 (30.1-165) | 44.5 (29.75-109) | 69 (30.15-191.5) | .029∗ |
| CRP, median (IQR), mg/L | 32 (13.05-70.22) | 50.3 (15.47-80.48) | 28 (10.1-64.16) | .016∗ |
| CA153, median (IQR), U/mL | 16.5 (10.8-25.8) | 14.8 (8.67-21.9) | 17.2 (11.4-27.6) | .022∗ |
| EBV DNA in PBMC, median (IQR), copies per mL, ×104 | 2.5 (0.28-98.4) | 0.95 (0.22-9.98) | 4.07 (0.32-134) | .019∗ |
| Variables . | Total (N = 381) . | High C3 (n = 110) . | Low C3 (n = 271) . | P value . |
|---|---|---|---|---|
| Age, median (IQR), y | 50 (33-62) | 50.5 (36-62) | 50 (32-60.5) | .591 |
| Female, n (%) | 203 (53) | 50 (45) | 153 (56) | .066 |
| Follow-up time, median (IQR) | 46 (16-556) | 97 (19-589) | 41 (14-539.5) | .245 |
| Death, n (%) | 194 (51) | 39 (35) | 155 (57) | <.001∗∗∗ |
| FIB, median (IQR), g/L | 1.49 (1.14-2.82) | 2.2 (1.36-3.83) | 1.39 (1.06-2.42) | <.001∗∗∗ |
| C4, median (IQR), g/L | 0.21 (0.13-0.29) | 0.28 (0.21-0.33) | 0.18 (0.11-0.25) | <.001∗∗∗ |
| WBC, median (IQR), ×109/L | 3.26 (1.82-6.14) | 3.81 (2.29-6.52) | 3.04 (1.69-6.06) | .034∗ |
| ANC, median (IQR), ×109/L | 2.03 (0.94-4.11) | 2.6 (1.35-4.57) | 1.76 (0.9-3.97) | .022∗ |
| PLT, median (IQR), ×109/L | 53 (31-82) | 65 (42.25-96) | 47 (27-75) | <.001∗∗∗ |
| D-dimer, median (IQR), mg/L | 3.52 (1.96-8.59) | 2.57 (1.47-7.32) | 4.14 (2.16-9.1) | .002∗∗ |
| PT, median (IQR), sec | 15.1 (13.6-16.5) | 14.25 (13.1-15.4) | 15.4 (13.85-17) | <.001∗∗∗ |
| APTT, median (IQR), sec | 41.8 (36.4-49.3) | 39.8 (35.1-45.27) | 42.7 (37.4-50.45) | .001∗∗ |
| Alb, median (IQR), g/L | 29 (25.6-35) | 30.8 (26.35-38.65) | 28.3 (25.1-33.5) | .013∗ |
| AST, median (IQR), U/L | 57 (30.1-165) | 44.5 (29.75-109) | 69 (30.15-191.5) | .029∗ |
| CRP, median (IQR), mg/L | 32 (13.05-70.22) | 50.3 (15.47-80.48) | 28 (10.1-64.16) | .016∗ |
| CA153, median (IQR), U/mL | 16.5 (10.8-25.8) | 14.8 (8.67-21.9) | 17.2 (11.4-27.6) | .022∗ |
| EBV DNA in PBMC, median (IQR), copies per mL, ×104 | 2.5 (0.28-98.4) | 0.95 (0.22-9.98) | 4.07 (0.32-134) | .019∗ |
∗P < .05; ∗∗P < .01; ∗∗∗P < .001.
CRP, C-reactive protein.
Prognostic and survival significance of C3 in patients with HLH
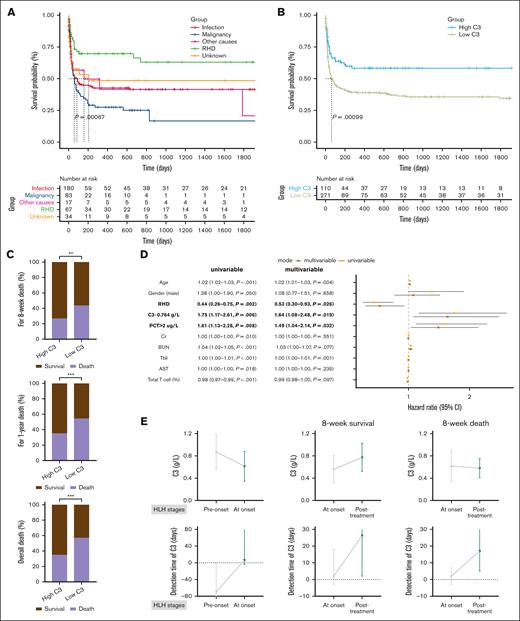
Survival analysis was conducted to assess the prognostic and survival significance of C3 in patients with HLH. Of the total number of patients, 57 patients (14.96%) were lost to follow-up. The overall median follow-up time was 46 days, with a median follow-up time of 97 days for the high C3 group and 41 days for the low C3 group. There was no significant difference in the follow-up time between the 2 groups (P = .245; Table 2). The high C3 group did not reach the median survival time, whereas the low C3 group had a median survival time of 63 days. The overall mortality rate was significantly different between the high C3 group and the low C3 group (35% vs 57%; P < .001; Table 2). Survival analysis revealed that malignancy-associated HLH was associated with the highest mortality risk, whereas RHD-associated HLH exhibited the lowest mortality risk. HLH triggered by other factors demonstrated an intermediate mortality risk (P = .00067; Figure 3A). Additionally, survival analysis indicated that the low C3 group had a higher risk of death in overall survival (P = .00099; Figure 3B). Considering that most patients with HLH died during the induction period, we analyzed the survival rates at 4 weeks, 8 weeks, 1 year, 2 years, and 3 years. The results showed that most patients in the low C3 group died within 1 year, with most deaths occurring within 8 weeks. Similarly, for the high C3 group, most deaths occurred within 8 weeks, and there were no additional deaths after 1 year (Figure 3C; supplemental Table 4).
The prognostic and survival significance of C3 in patients with HLH. (A) Overall survival (OS) analysis of HLH associated with various triggers. (B) OS analysis indicated that the low C3 group had a high risk of death. (C) The mortality rates between the low C3 group and the high C3 group at 8 weeks, 1 year, and overall death. (D) The forest plot of the univariate and multivariate Cox regression analysis. (E) The dynamic changes in C3 levels were analyzed from pre-onset to HLH onset (N = 66) and from HLH onset to post-treatment in patients (N = 47) who survived for 8 weeks (n = 36) and those who died within 8 weeks (n = 11). Day 0 represented the day of diagnosis, negative values represented days before diagnosis, and positive values represented days after diagnosis. ∗∗P < .01; ∗∗∗P < .001. 95% CI, 95% confidence interval; BUN, blood urea nitrogen; Cr, creatinine; Tbil, total bilirubin.
The prognostic and survival significance of C3 in patients with HLH. (A) Overall survival (OS) analysis of HLH associated with various triggers. (B) OS analysis indicated that the low C3 group had a high risk of death. (C) The mortality rates between the low C3 group and the high C3 group at 8 weeks, 1 year, and overall death. (D) The forest plot of the univariate and multivariate Cox regression analysis. (E) The dynamic changes in C3 levels were analyzed from pre-onset to HLH onset (N = 66) and from HLH onset to post-treatment in patients (N = 47) who survived for 8 weeks (n = 36) and those who died within 8 weeks (n = 11). Day 0 represented the day of diagnosis, negative values represented days before diagnosis, and positive values represented days after diagnosis. ∗∗P < .01; ∗∗∗P < .001. 95% CI, 95% confidence interval; BUN, blood urea nitrogen; Cr, creatinine; Tbil, total bilirubin.
The multivariate Cox regression analysis included variables with P value <.05 and low C3. Given that most patients died within 8 weeks and long-term mortality of HLH was mainly linked to secondary factors, we analyzed the risk factors for early death within the first 8 weeks in patients with HLH. Our results indicated that low C3 (C3 <0.764 g/L) was an independent risk factor for early death in patients with HLH within 8 weeks (hazard ratio, 1.64; P = .019). Lower C3 values were associated with an increased risk of mortality. According to the results, PCT >2 μg/L was also identified as a risk factor associated with early death, whereas RHD was identified as a protective factor (Figure 3D; supplemental Table 5). C3 demonstrated greater predictive capability for early death in HLH than other prognostic indicators, such as PLT, PT, and IL-10 (supplemental Figure 1D).
Dynamic changes of C3 in patients with HLH
The dynamic changes in C3 levels in patients with HLH were also collected. A total of 66 patients had C3 values measured both before the onset and at the onset of HLH. The median time for C3 detection before diagnosis was 69.5 days, whereas during the onset of HLH, it was 6.7 days. These patients had a median C3 level of 0.87 g/L before the onset of HLH, whereas at the onset of HLH, the median C3 level declined to 0.62 g/L. The results showed that most patients had normal C3 values before HLH onset, followed by a decline in C3 values at HLH onset (P < .0001). Furthermore, 47 patients with HLH had their C3 levels tested both at onset and after treatment. Among these patients, 11 died, and 36 survived within 8 weeks. For patients who survived within 8 weeks, the median time for C3 detection at onset was 2 days after diagnosis and 26.3 days after diagnosis for posttreatment status. After HLH treatment, the median C3 level increased from 0.56 g/L at onset to 0.77 g/L (P = .0003). For patients who died within 8 weeks, the median time for C3 detection at onset was also 2 days after diagnosis and 17 days after diagnosis for the posttreatment status. The median C3 level was 0.62 g/L at onset and decreased to 0.58 g/L after HLH treatment (P = .90). These findings suggested that C3 levels declined as HLH developed and that changes in C3 values could predict the therapeutic response and subsequent changes in the patients’ condition (Figure 3E).
Discussion
In this study, we have made significant progress in understanding the role of the complement system in HLH. Our research, which is, to our knowledge, the largest retrospective study of HLH in 1 center, sheds light on the clinical significance of C3 in HLH. This is, to our knowledge, the first cohort study to explore the clinical role of C3 in HLH, providing valuable insights into the pathogenesis of the disease. The study focused on adult patients with HLH, with an average age at diagnosis of 51 years and a nearly equal distribution between males and females. Undoubtedly, infection was the most common cause of HLH in adult patients, followed by malignancy. Most patients had coagulation disorders and liver dysfunction. Elevated levels of IL-6, IL-10, and IFN-γ were typically observed. In the proportion of peripheral blood lymphocytes, CD8+ T cells were increased, whereas the proportion of B cells and NK cells were decreased, with all these results mainly consistent with other publications.18,19 Consistent with previous reports,10-12,20 a total of 194 of 381 patients (51%) with HLH died, including 112 patients (29%) who died within 4 weeks, 149 (39%) who died within 8 weeks, and 187 (49%) who died within 1 year. Regarding tumor markers, the levels of CA125, CA153, and NSE were significantly increased. Although 1 study suggested that NSE might be a potential indicator of macrophage activation,21 the differences might be caused by the heterogeneity of secondary diseases.
Cytokine storm syndromes, in addition to HLH, also spanned a spectrum from clearly infection-related conditions (sepsis or COVID-19) to iatrogenic causes such as cytokine release syndrome due to immunotherapies.22-25 In critical patients with COVID-19, the level of C3 was decreased and was associated with poor prognosis, which was consistent with our results,26 and the effect product of the complement system sC5b-9 was increased, confirming that activation of the complement system leads to excessive consumption of C3, resulting in decreased C3 levels.27,28 In sepsis, C3 levels were significantly reduced and played an important role in the progression of disease.29,30
The complement system is an important part of innate and adaptive humoral immunity, and C3 is the central effector molecule of 3 separate complement pathways, mediating its multiple functions through different binding sites and their corresponding receptors.31 Most of the cell types, such as macrophages, in the human body express some amount of C3.32,33 Indeed, some studies have demonstrated the relationship between C3 and damage to immune defenses in immune complex–related diseases, bacterial sepsis, and fungal infections.34-36 Decreased C3 level in HLH have been reported in some case reports or case series, particularly regarding their association with rheumatic immune disease, lymphoma, and infections.37-40 Our study demonstrated the relationship between C3 and HLH in a larger retrospective cohort. Because there was very little literature on the relationship between C3 and HLH, we further searched other components of the complement system in HLH. One study suggested that the expression of complement C1q components (C1QA, C1QB, and C1QC) gene was upregulated in single-cell sequencing results of adult patients with HLH, both in active HLH and in remission, compared with controls.41 Additionally, studies42,43 showed that patients with macrophage activation syndrome with thrombotic microangiopathy who received the complement 5 inhibitor eculizumab and the IFN-γ inhibitor emapalumab had complete remission and survival. In addition, V-set and immunoglobulin domain–containing protein 4, a macrophage receptor of C3b and iC3b, was also elevated in patients with HLH and could be used as an alternative marker for diagnosis and prognosis.9 In pediatric patients with HLH, complement C1q subcomponent subunit B was correlated with disease severity.44
In this study, we reported for the first time, to our knowledge, for the first time that serum C3 expression was lower in patients with HLH than patients with partial HLH in a cohort study. A study reported that MATR3 gene expression in peripheral blood was lower in patients with HLH than healthy volunteers, and lower expression of MATR3 gene in bone marrow was associated with a higher risk of mortality.45 CD163 and CD107a have been reported to be highly expressed in patients with HLH and could be a diagnostic biomarker.46,47 Besides the above factors, serum levels of soluble Fas and soluble Fas ligand could be used for early diagnosis and were factors associated with poor prognosis in secondary HLH.48,49 In addition to its diagnostic significance, C3 was also a risk factor for prognosis in secondary HLH. Other factors such as soluble fms-like tyrosine kinase 1, sCD25, PT, IL-10, PLT, and hyponatremia were found to be risk factors associated with poor outcome in adult patients with HLH.13-17 Additionally, some combined indexes, such as optimized HLH inflammatory (OHI) index (sCD25 >3900 U/mL and ferritin >1000 ng/mL) and improved HLH (IH) index (combined index including sCD25, PCT, and estimated glomerular filtration rate), were identified as risk factors influencing patient prognosis.50,51
Undoubtedly, this study cannot escape the limitations inherent to a retrospective clinical study. First, retrospective analysis relies on existing and recorded hospitalization information, which may have recording inconsistencies, errors, or omissions, potentially introducing information bias. Second, due to the large time span, NK-cell activity and sCD25 were relatively missing more in the early stage than other HLH-2004 indicators, which may result in an underestimation of the number of HLH cases in our cohort. Finally, the significance of C3 was validated by a retrospective study in this study, but further prospective studies are needed to verify the role of C3 in HLH. In addition to C3 and C4, other complement activation biomarkers, such as sC5b-9, were not detected in our center. These issues will be addressed in future studies.
To our assumption, the dynamic changes in C3 during HLH progression offer insights into the role of the complement system in the pathogenesis of HLH. The initial normal C3 levels, followed by a decline after HLH onset, may indicate that the complement system is activated and consumed in the hyperinflammatory state. The sustained decrease in C3 levels among nonsurvivors further highlights the deleterious impact of uncontrolled complement activation in HLH. In the future, we will collect samples from patients with HLH for further analysis and dissect the comprehensive mechanisms of C3 in adult HLH. The complement system may be a new target for HLH treatment, which will be the future direction of our efforts.
Conclusion
In conclusion, our study identified C3 as a valuable predictive and prognostic biomarker in adult HLH. Low C3 levels were associated with increased disease severity and poor prognosis. Monitoring the dynamic changes in C3 may help clinicians better understand disease progression and therapeutic response and guide timely therapeutic interventions in adult patients with HLH.
Acknowledgments
The authors thank Wenze Zhong at Shanghai Jiao Tong University School of Medicine for his insightful discussion.
This study was supported by the National Natural Science Foundation of China (numbers 81974008 and 82450107), the Fundamental Research Funds for the Central Universities (numbers YG2022QN013 and YG2024QNB02), the National Key Research and Development Program of China (number 2024YFC2510503), and the Key-Research Fund from State Key Laboratory of Medical Genomics (number 2022-13-2).
Authorship
Contribution: D.W. and T.G. contributed to study concept and design; X.S., X.G., and Y.W. acquired data; X.S., X.G., Y.D., Y.L., and D.W. contributed to analysis and interpretation of data; and X.S., D.W., and T.G. drafted the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dawei Wang, Shanghai Institute of Hematology, State Key Laboratory of Medical Genomics, National Research Center for Translational Medicine at Shanghai, Ruijin Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, No.197 Ruijin Second Road, Shanghai 200025, China; email: wangdawei@shsmu.edu.cn; and Tao Guo, Institute of Hematology, Union Hospital Affiliated to Tongji Medical College, Huazhong University of Science and Technology, Jiefang Dadao 1277, Wuhan 430022, China; email: guotao1968@163.com.
References
Author notes
X.S., X.G., and Y.D. contributed equally to this study.
All data generated during this study are included in the manuscript and supporting files. Deidentified data are available upon reasonable request from the corresponding author, Tao Guo (guotao1968@163.com).
The full-text version of this article contains a data supplement.