TO THE EDITOR:
Acute myeloid leukemia (AML) results from excessive growth of transformed progenitor cells at different hematopoietic stages. Identifying the closest normal counterpart of these cells has traditionally aided AML diagnosis and subclassification.1,2 However, there is a progressive displacement of morphologically defined AML subtypes by those with recurrent genetic abnormalities in the World Health Organization classification of myeloid neoplasms.2 Furthermore, the International Consensus Classification of AML and the recommendations of the European Leukemia Net no longer consider the extent of morphologically defined blast differentiation in the subclassification of AML subtypes without recurrent genetic abnormalities.3,4
Besides morphology, multiparameter flow cytometry (MFC) immunophenotyping is important for AML diagnosis.3,4 There is recent evidence that a phenotypic subclassification of AML may be prognostic, particularly in younger patients eligible for intensive chemotherapy.5,6 However, antigen-based blast differentiation assessment is complex and subjective.7,8 Because of this and also due to the scarce MFC data in older AML, here we aimed at a data-driven phenotypic subclassification of patients ineligible to receive intensive chemotherapy.
This study included 252 newly diagnosed patients with AML aged >65 years who were enrolled in the phase 3 FLUGAZA clinical trial (NCT02319135).9 Patients were randomized 1:1 to receive open-label treatment with either azacitidine or low-dose arabinosylcytosine (Ara-C) plus fludarabine. A complete description of the study protocol is available in the supplemental Methods. All patients provided written informed consent, and the trial was approved by appropriate institutional review boards or ethics committees at all sites. The study was conducted according to the Declaration of Helsinki. External validation was performed in a cohort of 307 patients with AML aged >65 years, treated at the Toulouse University Hospital (TUH), where a similar data-driven phenotypic subclassification of AML was previously performed (supplemental Methods).6
The phase 3 FLUGAZA clinical trial included centralized MFC immunophenotyping using the EuroFlow AML panel in ethylenediaminetetraacetic acid (EDTA)-anticoagulated bone marrow aspirates (supplemental Methods).10 Afterward, blasts were isolated according to patient-specific aberrant phenotypes using fluorescence-activated cell sorting. DNA and RNA were coisolated for next-generation sequencing using a custom panel of 41 genes (n = 179/252),11 and for a massively parallel RNA single-cell sequencing framework adapted for bulk RNA sequencing (RNA-seq) (n = 190/252)12,13 (supplemental Methods).
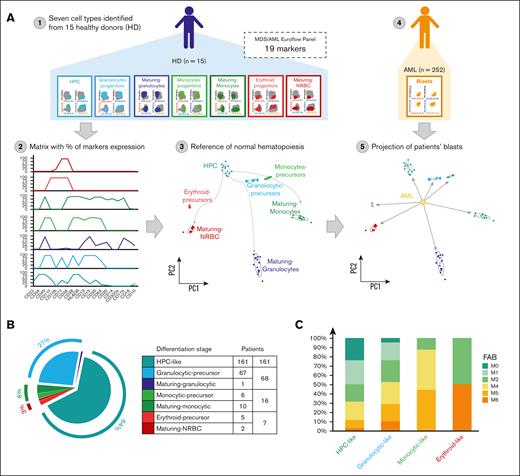
To perform a data-driven and holistic phenotypic subclassification of patients with AML, we first stained bone marrow aspirates of 15 healthy adults with the same EuroFlow AML panel (Figure 1A). After determining the percentage of expression of each marker in hematopoietic progenitor cells (HPCs), granulocytic, monocytic and erythroid precursors, as well as in maturing granulocytes, monocytes, and nucleated red blood cells, the data were merged, and a matrix was obtained for each subject (supplemental Figure 1; supplemental Table 1; supplemental Methods). The centroid for each cell type was calculated using the mean values of principal components 1 and 2. Subsequently, the percentage of expression of each marker was determined in blasts of the 252 patients with AML. After projecting each case onto the matrix generated from healthy adults, the distance between blasts and centroids of normal cell types was calculated, and the shortest distance identified the corresponding differentiation stage (supplemental Figure 1; supplemental Methods).
Of the 252 patients, nearly two-thirds (n = 161 [64%]) were classified with an HPC-like AML (Figure 1B). Smaller patient subgroups were classified as having AML with a granulocytic-precursor (n = 67 [27%]), monocytic-precursor (n = 6 [2%]), and erythroid-precursor phenotype (n = 5 [2%]), as well as a maturing-granulocytic (n = 1 [<1%]), maturing-monocytic (n = 10 [4%]), and maturing–nucleated red blood cell phenotype (n = 2 [<1%]). Because of the small numbers in some patient subgroups, precursor and maturing cell types from each lineage were regrouped into granulocytic-, monocytic-, and erythroid-like AML (Figure 1B). The most informative marker to define HPC-like AML was CD34 positivity. In erythroid- and monocytic-like AML, it was the coexpression of CD36 and CD71 as well as of CD64 and HLADR, respectively. Granulocytic-like AML was mainly defined by the expression of CD15 in the absence of HLADR.
Overall, there was a 46% concordance between the phenotypic and the French-American-British (FAB) classification. Although 19%, 20%, and 15% of cases with HPC-like AML were respectively classified as M0, M1, and M2, 16%, 7%, and 2% were respectively classified as M4, M5, and M6 on morphological grounds (Figure 1C). Patients with granulocytic-like AML were heterogeneously distributed according to FAB criteria, whereas the concordance between MFC and morphology was generally higher in monocytic- and erythroid-like AML (Figure 1C). Although the reasons for the higher discordance are observed in patients with granulocytic-like AML, it could be speculated that underlying molecular alterations may contribute to a dissociation between morphology and phenotype within this broad subgroup.
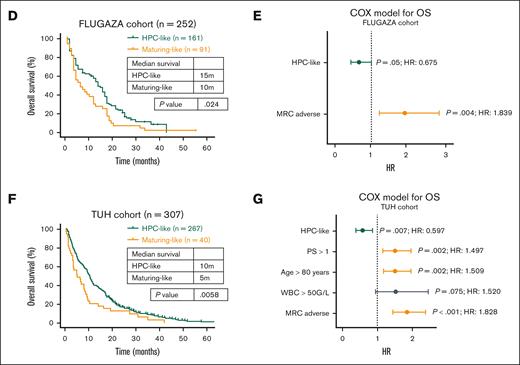
Patients classified as HPC-like AML treated with azacitidine showed longer overall survival (OS) than those with maturing AML subtypes (median, 15 vs 10 months, respectively; P = .024; Figure 1D). In a multivariate analysis of OS, the data-driven phenotypic classification, alongside the 2010 Medical Research Council (MRC) genetic risk stratification, exhibited borderline independent prognostic value (hazard ratio, 0.675; P = .05; Figure 1F). By contrast, no differences in OS were observed in patients receiving semi-intensive chemotherapy (data not shown).
Among 307 patients treated with azacitidine at the TUH, 267 (87%) were classified with an HPC-like AML. These included the hematopoietic stem cell–like (HSC-L), multipotent progenitors–like (MPP-L), and common myeloid progenitor–like (CMP-L) subgroups originally described by Vergez et al6. The median OS of the HPC-like AML vs patients with maturing AML subtypes was 10 vs 5 months, respectively (P = .006; Figure 1F). In a multivariate analysis of OS including the data-driven phenotypic classification and other risk factors, the former was an independent prognostic factor (hazard ratio, 0.6; P = .007; Figure 1G). In accordance with the findings in the FLUGAZA trial, the phenotypic classification had no impact on OS of patients treated with low-dose Ara-C (data not shown). Although these findings should be interpreted cautiously due to minimal differences in OS between groups, as well as differences in OS between the 2 series that could be associated with singular patient characteristics (eg, Eastern Cooperative Oncology Group Performance status [ECOG PS]), there may be a potential link between more differentiated AML and resistance to azacitidine monotherapy.14,15 This association could be treatment specific and should not be generalized to other therapeutic scenarios. Future molecular studies are warranted to identify the mechanistic basis of this possible association.
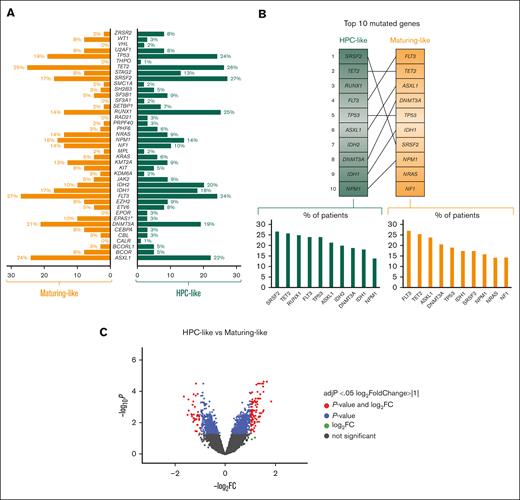
Next, we examined the connection between phenotypically defined stages of blast arrest and underlying mutations. Except for EPAS1 that was less frequently mutated in HPC-like vs maturing AML subtypes (3% vs 10%; P = .04), no significant differences were observed in other genes (Figure 2A; supplemental Figure 2). Although the differentiation stage of blasts was not associated to a specific mutational profile, it was noted that the order of the top 10 mutated genes differed between HPC-like vs maturing AML subtypes (Figure 2B). The most frequently mutated gene in patients having HPC-like AML was SRSF2 (27%), whereas, in maturing AML, it was FLT3 (27%). Another example is RUNX1, which was the third most frequently mutated gene in HPC-like AML and was not among the top 10 mutated genes in maturing AML subtypes (Figures 2A-B). This finding is consistent with a previously described association between RUNX1 mutations and a more immature morphology.16 There were no significant differences regarding the frequency of TP53 mutations in patients with HPC-like vs maturing AML subtypes (24% vs 23%; P = .95).
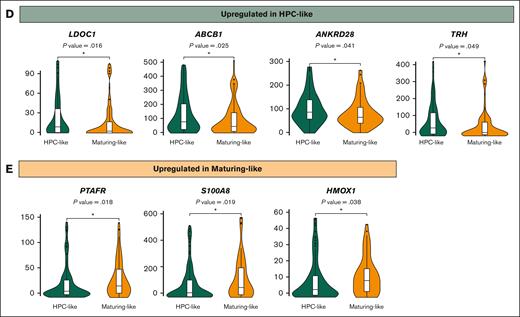
We then investigated the relationship between phenotypically defined stages of blast arrest and gene expression. The comparison between HPC-like vs maturing AML subtypes identified a total of 134 deregulated genes. The 98 upregulated genes in HPC-like included LDOC1, ABCB1, ANKRD28, and TRH, whereas the 36 downregulated genes included S100A8, PTAFR, and HMOX1 (Figure 2D-E), which have been previously associated with myeloid differentiaton.17-20 Next, we compared each AML subtype pairwise. Patients with granulocytic-like AML showed deregulation of 200 genes, of which 44 were upregulated and enriched in granulocytic-related pathways (supplemental Figure 3A). Only 23 genes were deregulated upon comparing the transcriptional profile of HPC- vs monocytic-like blasts (supplemental Figure 3B). A total of 415 genes were deregulated between HPC- and erythroid-like blasts, of which 269 were overexpressed and associated with pathways such as transferrin transport, erythrocyte development, and differentiation (supplemental Figure 3C).
The partial correlations between phenotypically defined stages of blast arrest and morphology, mutations, or transcriptional features suggest their collective influence on the data-driven phenotypic classification of patients with AML. Thus, this study advances our understanding of the interplay between genetic and cytological drivers of AML heterogeneity and outcome,5-8 applying these concepts to older patients. Our data-driven approach, with the potential for integration into flow cytometry software, offers an objective AML subtype classification based on blast differentiation, potentially providing consistent prognostic information alongside routine diagnostics. Additional data in other series and therapeutic scenarios are needed to confirm its utility for treatment individualization.
Acknowledgments: The authors acknowledge patients, caregivers, and the Biobank of the University of Navarra.
This study was supported by the Instituto de Salud Carlos III/Subdirección General de Investigación Sanitaria and cofinanced by Fondo Europeo de Desarrollo Regional, Spain (PI16/01661, PI16/00517, PI19/01518 and PI23/01331) and the Instituto de Salud Carlos III cofinanced by The Recovery, Transformation and Resilience Plan and the European Next Generation EU recovery funds (PMP22/00069).
Contribution: C.S., C.G., P.M., and B.P. were responsible for study conception and design; C.S., C.G., F.V., A.S., S.B., B.A., and B.P. performed analysis and data interpretation; C.S. and C.G. performed statistical analysis; D.M.-C., J.-M.B., S.V., L.A., M.T., P.M., J.S., P.H., F.R., O.S., E.L., C.Gil., J.-L.L.-L., M.-B.V., C.C., J.L., J.-F.F., M.-J.S., R.A., J.M.-L., S.V., M.-J.C., F.P., J.F.S.-M., M.Á.S., C.R., and P.M. were responsible for provision of study material and/or patients; C.S., C.G., P.M., and B.P. wrote the manuscript; and all authors reviewed and approved the manuscript.
Conflict-of-interest disclosure: F.P. reports honoraria and research funding from Oryzon, Janssen, and Bristol Myers Squibb (BMS)-Celgene. R.A. reports membership on an entity´s board of directors advisory committees for Incyte Corporation, and Astellas; and honoraria from Novartis, Celgene, and Incyte. J.F.S.-M. reports consultancy, membership on an entity´s board of directors advisory committees for AbbVie, Amgen, BMS, Celgene, GlaxoSmithKline, Janssen, Karyopharm, Merck Sharpe & Dohme, Novartis, Regeneron, Roche, Sanofi, SecuraBio, and Takeda. P.M. reports consultancy, membership on an entity´s board of directors advisory committees for, research funding from, speaker’s bureau for Celgene, Sanofi, Incyte, Karyopharm, Novartis, Stemline/Menarini, Agios, Astellas Pharma, and Daiichi Sankyo; membership on an entity´s board of directors advisory committees for Pfizer, Teva, and AbbVie; research funding and speakers bureau fees from Janssen; and consultancy for Tolero Pharmaceutical, Forma Therapeutics, and Glycomimetics. B.P. served as a consultant for and received honoraria from Adaptive, Amgen, Becton Dickinson, BMS/Celgene, GSK, Janssen, Roche, Sanofi, and Takeda; and received research support from BMS/Celgene, GSK, Roche, Sanofi, and Takeda. The remaining authors declare no competing financial interests.
A complete list of the members of the PETHEMA Cooperative Study Group appears in the supplemental Appendix.
Correspondence: Bruno Paiva, Clínica Universidad de Navarra, CIMA Universidad de Navarra, Av. Pío XII 55, 31008 Pamplona, Spain; email: bpaiva@unav.es.
References
Author notes
C.S. and C.G. contributed equally to this work and should be considered joint authors.
B.P. and P.M. contributed equally to this work and should be considered joint senior authors.
The European Genome-Phenome Archive public accession number for our data set is EGAD50000000490 (https://ega-archive.org/datasets/EGAD50000000490).
Any further questions should be directed to the corresponding author, Bruno Paiva (bpaiva@unav.es).
The full-text version of this article contains a data supplement.