Key Points
COVID-19 spike antibodies in classic and variant hairy cell leukemia correlate with normal B cells, which are reduced after anti-CD20 mAbs.
Because normal B cells become reduced for several years after anti-CD20 mAbs, vaccines should be used before treatment, if possible.
Visual Abstract
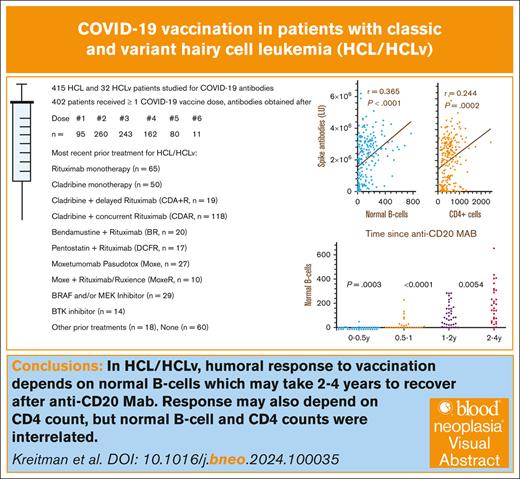
Patients with the B-cell malignancy hairy cell leukemia (HCL) and the poorer-prognosis variant HCLv often receive anti-CD20 monoclonal antibodies (mAbs), which kill normal B cells, impairing humoral immunity. We measured COVID-19 antibodies after doses of COVID-19 vaccine in patients with HCL (n = 415) and HCLv (n = 32). After the second COVID-19 vaccine dose, spike antibody level most strongly correlated with normal B-cell levels (r = 0.365, P < .0001), followed by CD4+ T-cell count (r = 0.244, P = .0002), and was less related to immunoglobulin G level (r = 0.101, P = .14). Spike antibody also correlated with normal B cells after the third to fifth vaccine doses and with CD4+ count after the third dose. Normal B-cells were undetectable in 87% of patients within 6 months after the last dose of anti-CD20 mAb and were lower than in patients at 6 to 12 months (P = .0003), which, in turn, were lower than at 12 to 18 months (P = .0002). Infection with COVID-19 became more common after use of the third vaccine dose; spike antibody levels were higher in patients with prior infection (positive vs negative nucleocapsid antibodies; P < .0001). Spike antibodies decreased faster after ibrutinib or anti-CD20 mAb. We conclude that decreased levels of normal B cells in patients with HCL/HCLv, due to disease and/or anti-CD20 therapy, are associated with lower COVID-19 vaccination efficiency and such patients may not respond well to vaccines. The associated studies were registered at www.ClinicalTrials.gov as #NCT01087333 (HCL/HCLv) and #NCT04362865 (COVID-19).
Introduction
The COVID-19 pandemic began as a serious and deadly public health problem for the general population, with originally 5% of infected people requiring intensive care unit admission. Although this percentage fell with time because of changes in the virus,1 vaccination, and improved treatments, patients with hematologic malignancies remain at high risk for adverse outcomes.2,3 Vaccination against COVID-19 has been studied in patients with hematologic malignancies, particularly using messenger RNA (mRNA) vaccines, and spike antibody concentrations were reported significantly lower in patients with chronic lymphocytic leukemia (CLL) and non-Hodgkin lymphomas (NHL).4 In patients with CLL, relatively low vaccine responses were observed in patients treated with Bruton tyrosine kinase inhibitors (BTKi) or venetoclax targeting B-cell lymphoma 2 protein, and of 20 patients with CLL who were <1 year from anti-CD20 monoclonal antibody (mAb) therapy, none responded to COVID-19 vaccination.5 In patients with B-cell NHL, absolute lymphocyte count (ALC) and time since anti-CD20 mAb correlated with COVID-19 vaccine response.6
Hairy cell leukemia (HCL) is a B-cell malignancy characterized by excellent responses but frequent relapses after immunosuppressive treatments, including purine analogs pentostatin7 and cladribine (CDA),8 anti-CD20 mAb rituximab (R),9 CDA plus R given sequentially (CDA + R)10 or concurrently (CDAR),11 anti-CD22 recombinant immunotoxins BL2212 and moxetumomab pasudotox (Moxe),13 BTKi ibrutinib,14 and B-cell lymphoma 2 protein inhibitor venetoclax.15 The anti–B-cell agents targeting normal B-cells and BTKis can compromise humoral immunity, and purine analogs can result in prolonged reductions in CD4+ T cells.16,17 Venetoclax can also cause neutropenia.18 V-Raf Murine Sarcoma Viral Oncogene Homolog B (BRAF) inhibitors (BRAFi) with or without MEK inhibitors (MEKi) generally spare normal lymphocytes19-21 unless combined with anti-CD20 mAbs.22,23 BRAFi vemurafenib monotherapy can improve cytopenias and allow successful COVID-19 vaccination.24 A more aggressive, poorer prognosis variant of HCL, called HCLv, is inappropriate for BRAFis because of absence of the BRAF V6f00E mutation.25-27 Because of the relative resistance of HCLv to purine analog monotherapy, HCLv is more often treated with purine analogs combined with R.28-30
COVID-19 infection in HCL was reported relatively early in the pandemic, including studies reporting deaths in 9 of 40 patients in 1 series31 and 5 of 48 in another.32 In the latter study, 28 patients with HCL were vaccinated, including 17 with previous COVID-19 infection and 11 without.32 In a study of 83 patients with HCL who were infected with COVID-19 during the Omicron surge, 27 (68%) of 40 vaccinated patients responded serologically.33 In a study in which 503 HCL/HCLv were followed-up during the pandemic, we reported evidence of COVID-19 infection in 253 patients, and only 2 COVID-19–related deaths resulted.34 Even patients without vaccination or with recent treatments recovered, but lower levels of normal B cells were associated with lower spike antibody levels after response to the virus, and longer recovery time. In this study, we focused on efficiency of vaccination against COVID-19 in >400 patients with HCL/HCLv, and studied the effect of normal B cells, CD4+ T-cell and immunoglobulin G (IgG) concentrations, and time from anti-CD20 mAb treatment.
Patients and methods
Patients
Patients with HCL and HCLv were followed-up on sample collection protocols for HCL/HCLv (ClinicalTrials.gov identifier: NCT01087333) and for COVID-19 (ClinicalTrials.gov identifier: NCT04362865). All patients signed consents approved by the National Cancer Instituter institutional review board. Most patients were from the United States and had samples sent by overnight shipping. Some international patients including those from Canada and Europe required international shipping. Some patients were on HCL/HCLv treatment protocols at the National Institutes of Health (NIH), and had samples drawn at the NIH during patient visits or sent overnight when not at the NIH. Other patients who were not on NIH treatment protocols sent samples to characterize their HCL cells and consented to both the HCL/HCLv and COVID-19 protocols. Some patients were from the local area and had samples at the NIH. For safety, patients who were sending samples were sent empty tubes with appropriate containers, with directions to draw a complete blood count differential at home at the time tubes were filled. A complete blood count differential could be run at the NIH for patients who did not complete this at home. Assessment dates ranged between September 2020 and December 2023. For patients on NIH treatment protocols, assessments were often made every 12 weeks, and for many patients who alerted us to their receiving a COVID-19 vaccination, samples were often sent in before, and 3 to 4 weeks after, the vaccine dose.
Assessments
Patients were assessed by (1) quantifying HCL/HCLv cells in the peripheral blood by flow cytometry; (2) quantifying lymphocyte subsets by flow cytometry; (3) measuring antibody levels to spike and nucleocapsid regions of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2); and (4) quantifying IgG, IgA, and IgM levels. As previously described,34,35 flow cytometry involved detailed characterization and quantitation of HCL/HCLv cells by multiple antigens including CD19, CD22, CD20, CD11c, CD25, CD45, CD103, CD11c, CD123, and CD200. Non-HCL/HCLv populations including CLL and monoclonal B-cell lymphocytosis were quantified to determine the number of normal B cells. As previously reported,34 spike and nucleocapsid antibodies were assessed by 2 assays. From April 2020 to February 2022, the luciferase immunoprecipitation system (LIPS) was used to quantify antibodies against the SARS-CoV-2 spike and nucleocapsid proteins.36 The Elecsys Anti-SARS-CoV-2 S and Elecsys Anti-SARS-CoV-2 N double-sandwich enzyme-linked immunosorbent assays (ELISAs) were subsequently used.37 Nucleocapsid and spike antibodies were detected beginning December 2020 and February 2021, respectively, and spike antibodies quantified beginning in July 2021. Statistical analysis included the Wilcoxon rank-order test (Mann-Whitney U test) and simple linear regression, with r and P values determined by GraphPad Prism version 9.3. All P values were 2-sided. The same program calculated half-lives of decreases in spike antibody levels over time, using 1-phase exponential decay curves fitted using a weighting factor of 1/y2.
Results
Patient characteristics
As shown in Table 1, 447 patients with HCL/HCLv were evaluable for COVID-19 serology, including 415 with HCL and 32 with HCLv. The male-to-female ratio of ∼4 was typical for HCL. Of 447 patients with COVID-19 antibody levels, 45 patients had antibody levels but were never vaccinated. A total of 402 patients had COVID-19 serology after vaccination, including 95 patients after the first vaccine dose; 260 after the second dose; 243 after the third dose; and 162, 80, and 11 after the fourth, fifth, and sixth doses, respectively. Sixty patients were untreated whereas 387 had a variety of different treatments. A total of 334 patients had prior treatment with an anti-CD20 mAb, which was R or a biosimilar in 332 cases and obinutuzumab in 2 cases. The most common prior treatment was with CDA combined with R begun on day 1 of CDA (CDAR),11 and 19 patients had CDA with R begun only after the CDA was completed (CDA + R).10,28 Many patients received other agents combined with R, including Moxe-R/Ruxience (n = 10), Moxe-obinutuzumab (n = 1), CHOP-R (cyclophosphamide, doxorubicin, Oncovin [vincristine], and prednisone plus R; n = 1), pentostatin-R (n = 17), ibrutinib-R (n = 1), and vemurafenib-obinutuzumab (n = 1).
COVID-19 antibodies in HCL/HCLv after the second dose of vaccine
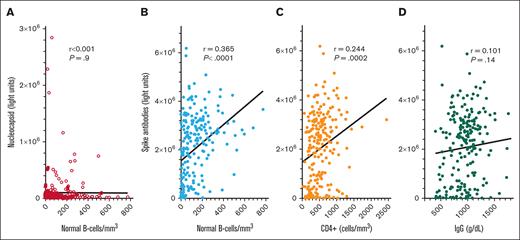
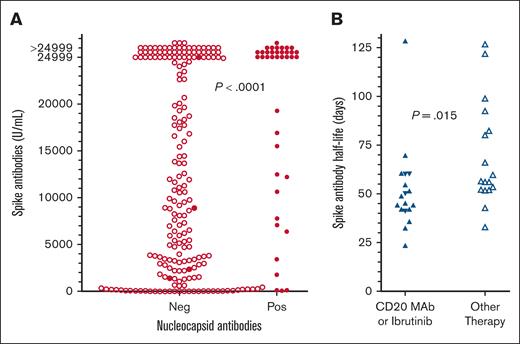
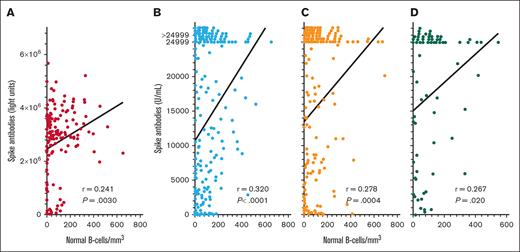
To determine COVID-19 antibody levels after vaccination and the relationship between COVID-19 antibodies and immune parameters including normal B-cell numbers, CD4+ T-cell count, and IgG levels, these values were measured after the second dose of vaccine in patients with HCL/HCLv. Nearly all patients had 1 of the 2 mRNA vaccines, from Moderna or Pfizer. Normal B cells were determined using flow cytometry, by subtracting malignant cells from total B cells. In many cases, patients with treated or untreated HCL/HCLv had CLL or monoclonal B-cell lymphocytosis cells with or without HCL/HCLv cells, and quantitation of normal B cells required subtracting all types of monoclonal B cells from the total number of B cells. As shown in Figure 1A-B, spike but not nucleocapsid antibody level correlated with normal B-cell level, with an r value of 0.365 (P < .0001). Only 31 (14%) of 223 patients evaluated after 2 vaccine doses had high (>125 000 light units) levels of nucleocapsid antibody, indicating prior infection with COVID-19, consistent with the relatively low rate of COVID-19 infection after COVID-19 mRNA vaccines were first introduced. In contrast, spike antibody levels were high (>45 000 light units) in 202 (91%) of 223 patients with HCL/HCLv. Spike antibody levels after 2 vaccine doses also correlated with the level of CD4+ T cells per mm3 (Figure 1C; r = 0.244, P = .0002), although not as strongly as with normal B cells. Spike antibody levels correlated poorly with IgG level (Figure 1D; r = 0.101, P = .14). Thus, in patients with HCL/HCLv after 2 doses of COVID-19 vaccine, spike antibody levels correlated with normal B cells and CD4+ T-cell counts but not with IgG level.
COVID-19 antibody levels after the second vaccine dose by LIPS assay. Levels of nucleocapsid (○) and spike (•) antibodies in 223 patients with HCL/HCLv. COVID-19 antibodies vs levels of circulating normal B cells (A-B), CD4+ T-cell counts (C), and plasma IgG levels (D). Linear regression lines are shown. Upper limits of negative are 125 000 and 45 000 light units for nucleocapsid and spike antibodies, respectively.
COVID-19 antibody levels after the second vaccine dose by LIPS assay. Levels of nucleocapsid (○) and spike (•) antibodies in 223 patients with HCL/HCLv. COVID-19 antibodies vs levels of circulating normal B cells (A-B), CD4+ T-cell counts (C), and plasma IgG levels (D). Linear regression lines are shown. Upper limits of negative are 125 000 and 45 000 light units for nucleocapsid and spike antibodies, respectively.
COVID-19 spike antibody and normal B-cell levels after >2 vaccine doses
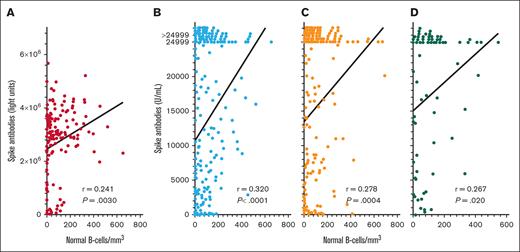
To quantify spike antibodies in patients with HCL/HCLv and determine relationship to normal B cells after >2 vaccine doses, spike antibody levels were measured by 2 different assays after 3, 4, and 5 vaccine doses. As shown in Figure 2, spike antibody levels after the “booster” (third) vaccine dose correlated significantly with levels of normal B cells, by either the LIPS assay (Figure 2A; n = 149, r = 0.241, P = .0030) or the Elecsys Anti-SARS-CoV-2 S double-sandwich ELISA (Figure 2B; n = 224, r = 0.320, P < .0001). We measured spike antibodies by the latter assay and found significant correlations with normal B cells after both the fourth (Figure 2C; n = 161, r = 0.278, P = .0004) and fifth (Figure 2D; n = 76, r = 0.267, P = .020) COVID-19 doses. As shown in Figure 2B-D, the Elecsys Anti-SARS-CoV-2 S double-sandwich ELISA could not quantify spike antibody levels of >24 999 units per mL, and although such levels were assigned a value of 24 999, the correlations with normal B-cell levels were significant. Thus, in patients with HCL/HCLv, spike antibody level correlated significantly with normal B-cell levels after the second, third, fourth, and fifth COVID-19 vaccine doses. We investigated whether the levels of spike antibodies after the third dose by either assay were different in patients with HCL vs HCLv, and found no significant differences (P = .41 to P = .52). We also observed no significant differences in spike antibodies after the second or third vaccine doses with respect to whether patients had received concurrent or sequential CDA and R (P = .55 to P = .94).
COVID-19 spike antibody levels vs normal B cells. Spike antibodies by the LIPS (A) and Elecsys (B-D) assays after the third (n = 149 in panel A; n = 224 in panel B), fourth (n = 161 in panel C), and fifth (n = 76 in panel D) vaccine doses. Points including spike antibody levels of >24 999 U/mL are shown above the 24 999 level.
COVID-19 spike antibody levels vs normal B cells. Spike antibodies by the LIPS (A) and Elecsys (B-D) assays after the third (n = 149 in panel A; n = 224 in panel B), fourth (n = 161 in panel C), and fifth (n = 76 in panel D) vaccine doses. Points including spike antibody levels of >24 999 U/mL are shown above the 24 999 level.
Levels of normal B cells and last dose of anti-CD20 mAb
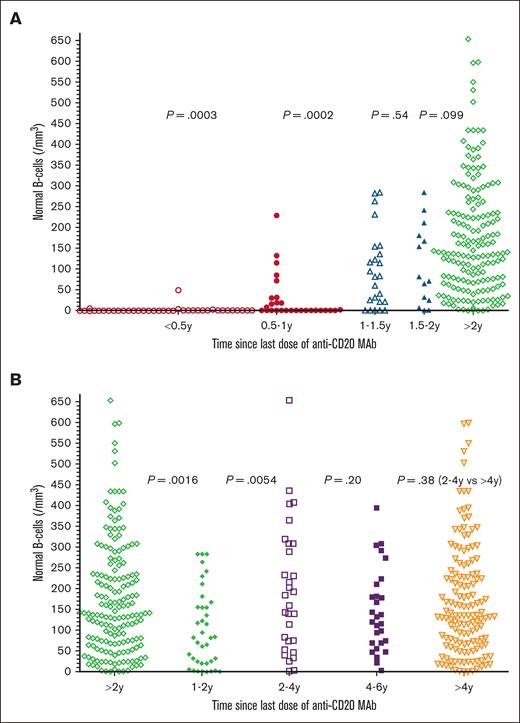
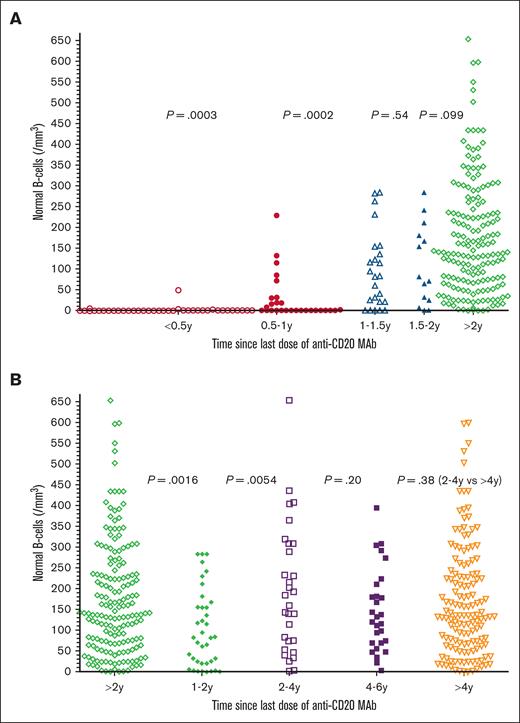
To determine the relationship between time since the last dose of anti-CD20 mAb and the level of normal B cells, 276 of 334 patients with prior anti-CD20 mAb were tested by flow cytometry to determine normal B-cell levels, and time since last dose of anti-CD20 mAb noted. As shown in Figure 3A, only 5 (13%) of 38 patients had detectable normal B-cell levels within 6 months of the last dose of anti-CD20 mAb, with normal B-cell counts of 1, 2, 3, 5, and 49 cells per mm3, respectively. In comparison, at 0.5 to 1 year after last dose of anti-CD20 mAb, 14 (50%) of 28 patients had undetectable normal B-cell counts (P = .0003, 2-sided Mann-Whitney U test). The 14 patients at 0.5 to 1 years had normal B-cell counts, significantly lower than the 24 patients at 1 to 1.5 years (P = .0002). As shown in Figure 3B, normal B-cell counts in the 38 patients at 1 to 2 years were significantly lower than the 172 patients at >2 years (P = .0016) and were also lower than just the 30 at 2 to 4 years (P = .0054). Each of the groups in Figure 3 was analyzed with respect to whether the patients had received anti-CD20 mAb with or without chemotherapy. Within each group, significant differences in normal B cells with or without chemotherapy were noted at 0.5 to 1 years (medians, 0 vs 58 cells per mm3; n = 20 vs 8; P = .0022), at >2 years (median, 158 vs 113 cells per mm3; n = 106 vs 66, P = .012), and at >4 years (median, 159 vs 107 cells per mm3; n = 92 vs 50, P = .0016), respectively. It was expected that normal B cells would be lower at 0.5 to 1 years after anti-CD20 mAb with vs without chemotherapy. The reason that normal B cells were higher at >2 and >4 years after anti-CD20 mAb with vs without chemotherapy is probably because of the high rates of long-term complete remission without minimal residual disease after CDA-R11 and other chemotherapy-R combinations. In 4 of the groups, 1 to 2, 2 to 4, >2, and >4 years after anti-CD20 mAb, there were 4 to 12 patients with HCLv each to compare with patients with HCL with respect to normal B cells, and no significant differences were observed (P = .19 to P = .89). Thus, normal B cells were low or undetectable in patients with HCL/HCLv with recent anti-CD20 mAb treatment, particularly within 1 year, particularly when chemotherapy was used, but even patients at 1 to 2 years had normal B-cell counts lower than patients at 2 to 4 years.
Normal B-cell recovery after last anti-CD20 mAb. In panel A, times after the last mAb treatments were <0.5 years (n = 38, ○), 0.5 to 1 year (n = 28; •), 1 to 1.5 years (n = 24; ▵), 1.5 to 2 years (n = 14; ▲), and >2 years (n = 172; ⋄). In panel B, times after the last mAb treatments were >2 years (n = 172; ◊), 1 to 2 years (n = 38; ○), 2 to 4 years (n = 30; □), 4 to 6 years (n = 29; ▪), and >4 years (n = 142; ▿). Two-sided P values calculated by the Mann-Whitney U test are shown between compared groups. Normal B cells were lower at 0.5 to 1 year than 1 to 2 years (P < .0001).
Normal B-cell recovery after last anti-CD20 mAb. In panel A, times after the last mAb treatments were <0.5 years (n = 38, ○), 0.5 to 1 year (n = 28; •), 1 to 1.5 years (n = 24; ▵), 1.5 to 2 years (n = 14; ▲), and >2 years (n = 172; ⋄). In panel B, times after the last mAb treatments were >2 years (n = 172; ◊), 1 to 2 years (n = 38; ○), 2 to 4 years (n = 30; □), 4 to 6 years (n = 29; ▪), and >4 years (n = 142; ▿). Two-sided P values calculated by the Mann-Whitney U test are shown between compared groups. Normal B cells were lower at 0.5 to 1 year than 1 to 2 years (P < .0001).
COVID-19 spike antibody and CD4+ T-cell counts
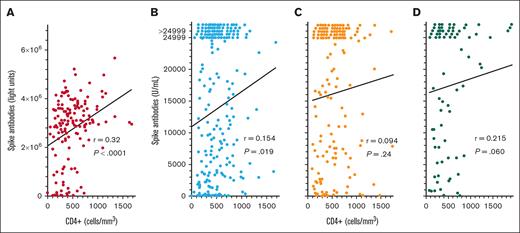
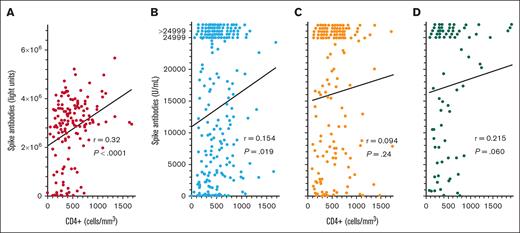
To determine whether COVID-19 spike antibody levels in patients with HCL/HCLv after the third, fourth, and fifth COVID-19 vaccine doses would correlate with CD4+ T-cell counts like they did after the second dose (Figure 1C), CD4+ T-cell levels were compared with spike antibody levels after COVID-19 vaccine doses 3 to 5. As shown in Figure 4, after the third COVID-19 vaccine dose, spike antibody levels did correlate significantly with CD4+ T-cell levels by the original LIPS assay (Figure 4A; n = 155, r = 0.320, P < .0001) and by the Elecsys assay (Figure 4B; n = 231, r = 0.154, P = .019). A significant correlation was not observed after the fourth COVID-19 vaccine dose (Figure 4C; n = 159, r = 0.094, P = .24) and only a trend was observed after the fifth COVID-19 vaccine dose (Figure 4D; n = 77, r = 0.215, P = .060. Thus, for some of the COVID-19 vaccine doses examined in patients with HCL/HCLv, spike antibody levels did correlate with CD4+ T-cell counts, but correlations were not as strong as when spike antibody levels were compared with normal B-cell levels (Figures 1 and 2).
COVID-19 spike antibody levels vs CD4+ T cells. Spike antibodies by the LIPS (A) and Elecsys (B-D) assays after the third (n = 155 in panel A; n = 231 in panel B), fourth (n = 159 in panel C), and fifth (n = 77 in panel D) vaccine doses. Points including spike antibody levels of >24 999 U/mL are shown above the 24 999 level.
COVID-19 spike antibody levels vs CD4+ T cells. Spike antibodies by the LIPS (A) and Elecsys (B-D) assays after the third (n = 155 in panel A; n = 231 in panel B), fourth (n = 159 in panel C), and fifth (n = 77 in panel D) vaccine doses. Points including spike antibody levels of >24 999 U/mL are shown above the 24 999 level.
Correlation between CD4+ T-cell counts and normal B cells
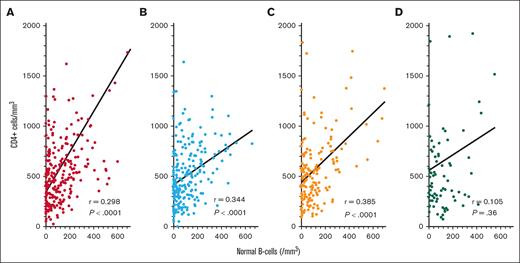
To explore whether COVID-19 spike antibody levels were independently related to both normal B-cell and CD4+ T-cell counts, or whether the correlations observed in Figure 4 could have resulted simply because CD4+ T-cell and normal B-cell counts were interrelated, we compared CD4+ T-cell and normal B-cell counts in patients with HCL/HCLv. As shown in Figure 5, levels of circulating normal B cells and CD4+ T-cell counts were significantly correlated after the second to fourth vaccine doses (P < .0001). The lack of this correlation after the fifth vaccine dose could be because of the much lower number of evaluable samples (n = 79) at that time. Nevertheless, it is possible that COVID-19 spike antibody level appeared related to CD4+ T-cell counts only because normal B-cell and CD4+ T-cell counts were interrelated because of treatments and/or disease, which depressed both cell types.
Correlation between CD4+ T-cell and normal B-cell counts. Correlations are shown for samples drawn after the second (n = 256, A), third (n = 240, B), fourth (n = 161, C), and fifth (n = 79, D) COVID-19 vaccine doses.
Correlation between CD4+ T-cell and normal B-cell counts. Correlations are shown for samples drawn after the second (n = 256, A), third (n = 240, B), fourth (n = 161, C), and fifth (n = 79, D) COVID-19 vaccine doses.
Spike antibodies after vaccination related to COVID-19 infection
To determine whether prior COVID-19 infection would increase the level of spike antibodies after vaccination, patients after the third COVID-19 vaccine dose were compared for spike antibody with respect to positive vs negative nucleocapsid antibody. As shown in Figure 6A, 39 patients with HCL/HCLv after the third COVID-19 vaccine dose with positive nucleocapsid antibodies had higher spike antibodies than 195 patients with negative nucleocapsid antibodies (median, >24 999 vs 9738 U/mL; P < .0001). However, among the 195 patients negative for nucleocapsid antibodies were 4 patients who had tested positive for COVID-19. These 4 patients included 1 on ibrutinib with spike antibody of >24 999 U/mL, 1 with multiply relapsed HCL with 51 normal B cells per mm3 and spike antibody of 1393 U/mL, 1 after CDA+R with absent normal B cells and spike antibody of 2315 U/mL, and 1 after delayed R with normal B cells of 19 cells per mm3 and spike antibody of 8900 U/mL. If these 4 patients had positive nucleocapsid antibodies, spike antibody would still have been higher in the 43 patients with positive nucleocapsid antibodies vs the 191 patients negative for nucleocapsid antibodies (median, >24 999 vs 9868 U/mL; P = .0003). Thus, prior infection with COVID-19 enhanced spike antibody level in patients with HCL/HCLv vaccinated against COVID-19.
Spike antibody levels with or without prior exposure to COVID-19, and spike antibody decrease over time. (A) Spike antibody levels without (n = 191, ○) vs with (n = 43, •) evidence of prior COVID-19 exposure. Included in the 43 patients with evidence of prior COVID-19 exposure were 4 (•) who tested positive by polymerase chain reaction or rapid-antigen tests but had negative nucleocapsid antibodies for COVID-19. (B) Half-lives of decreases in COVID-19 antibody levels over time compared for patients on ibrutinib (n = 4, ▼) or anti-CD20 mAbs (n = 14, ▲) vs other treatments (n = 17, ▵).
Spike antibody levels with or without prior exposure to COVID-19, and spike antibody decrease over time. (A) Spike antibody levels without (n = 191, ○) vs with (n = 43, •) evidence of prior COVID-19 exposure. Included in the 43 patients with evidence of prior COVID-19 exposure were 4 (•) who tested positive by polymerase chain reaction or rapid-antigen tests but had negative nucleocapsid antibodies for COVID-19. (B) Half-lives of decreases in COVID-19 antibody levels over time compared for patients on ibrutinib (n = 4, ▼) or anti-CD20 mAbs (n = 14, ▲) vs other treatments (n = 17, ▵).
Decrease in spike antibody level over time
In several patients, there were enough spike antibody levels over time after a vaccine dose to determine the half-life of the 1-phase exponential decay. A total of 35 patients were evaluable using spike antibody levels from the LIPS (n = 2) or the Elecsys (n = 33) assay, with half-lives of 24 to 129 (median 53) days, and 1-phase r2 values of 0.892 to 1 (median ,0.994). A trend was observed when determining whether half-life was proportional to median normal B-cell counts for each patient (r = 0.288, P = .094). However, as shown in Figure 6B, spike antibody half-lives were shorter in patients treated either with anti-CD20 mAb or with BTKi ibrutinib (median, 47 days; n = 18) vs other treatments (median, 56 days; n = 17; P = .015). Thus, treatments that decrease B-cell numbers or impair B-cell function could be associated with more rapid decrease in spike antibody level over time, but the difference was small and the number of patients evaluable for multiple spike antibody levels after vaccine doses was limited.
Discussion
To determine the efficiency of COVID-19 vaccination in patients with HCL/HCLv with regard to humoral immunity, we studied COVID-19 antibody levels in 447 patients with HCL/HCLv, 402 of them after COVID-19 vaccine doses. This study is, to our knowledge, the largest of its type reported for this disease and, to our knowledge, is for the first time large enough to study relationship of spike antibody levels to immune status including levels of normal B cells and CD4+ T-cell counts. We found that spike antibody levels were related most closely to levels of normal B cells (Figures 1 and 2), after not only the second vaccine dose but also after each of multiple subsequent vaccine doses. Levels of normal B-cells were closely related to time since the last dose of anti-CD20 mAb, being undetectable in 87% of patients at 0 to 0.5 year and 50% at 0.5 to 1 year after last anti-CD20 mAb dose. We found significant differences in normal B cells between 0 to 0.5 and 0.5 to 1 year, 0.5 to 1 and 1 to 1.5 years, and between 1 to 2 and 2 to 4 years after last dose (Figure 3). Spike antibody levels were also related to CD4+ T-cell counts (Figure 4) but less closely than they were to normal B cells, and this apparent relationship was possibly because of the close relationship between CD4+ T-cell and normal B-cell counts (Figure 5). After the third dose of vaccine, COVID-19 infection, which became more common at that time, was associated with higher spike antibody levels than in patients who received the third vaccine dose without evidence of prior COVID-19 infection (Figure 6A). Finally, we found that spike antibody levels decreased in our patients with a median half-life of 53 days and half-life was shorter in patients who received anti-CD20 mAb or were on ibrutinib (Figure 6B).
Comparison to antibody data obtained in CLL and other B-cell disorders
Chang et al studied 122 patients with CLL or NHL and reported that BTKi, venetoclax or anti-CD20 mAb within 1 year was associated with lack of response to COVID-19 vaccine.4 In that study, circulating B cells of at least 4.31 cells per mm3 predicted response to vaccine even at 3 months from last anti-CD20 mAb. Although that study may not have differentiated malignant from normal circulating B cells in CLL as was in our study in HCL/HCLv, it is likely that detectable B cells in CLL and particularly in NHL are associated with circulating normal B cells. The finding reported by Perry et al showed that time since anti-CD20 mAb and ALC correlated with COVID-19 vaccine response in NHL and is consistent with our data in HCL/HCLv, even if ALC reflected T cells, because we found relationships between either normal B-cell (Figures 1 and 2) or T-cell (Figure 4) levels and COVID-19 antibody response, and also found that normal B-cell and CD4+ T-cell levels are interrelated after most vaccine doses (Figure 5). A study of 71 patients with CLL/NHL found that active treatment and low IgA and IgM levels were associated with lack of COVID-19 vaccine antibody response.38Figure 1C only shows spike antibody vs total IgG levels with a relatively poor correlation, but a limited correlation was suggested between spike antibody and either IgA (r = 0.142, P = .040) or IgM levels (r = 0.134, P = .052) in the same data set. From a meta-analysis of 170 studies, vaccine seroconversion was lower in lymphoid malignancies than in myeloid malignancies, with the lowest found in CLL/NHL, because of both treatment and disease.39 From a recent review, strategies to improve response in CLL/NHL include vaccination while the disease process is early, allowing >2 weeks after vaccination before starting treatment, giving up to 6 sequential COVID-19 vaccine doses, and delaying vaccination until >6 months after anti-CD20 mAb.40 The latter recommendation may deprive some patients of a T-cell response, which most studies have not addressed. We have reported a patient with NHL lacking normal B-cells after CHOP-R therapy who had a significant T-cell response to the Omicron variant.41
Vaccine efficiency in HCL/HCLv
Most studies of COVID-19 and HCL/HCLv have focused on infection with COVID-19 in HCL/HCLv and have often reported vaccinations in patients with infection.31,34,42-45 Tiacci et al reported 11 patients with HCL without infection who were vaccinated for COVID-19.32 Eight (73%) developed an antibody response, which did not correlate with time since last treatment, although the 3 without antibody response were only 2 and 3 months after anti-CD20 mAb treatment or 20 days after venetoclax. In the 17 patients from this study who had COVID-19 infection before being vaccinated, there was no association observed between number of vaccine doses and antibody levels. Annunzio et al reported 14 patients with COVID-19 of whom 5 patients were vaccinated before, and 6 after, COVID-19 diagnosis.42 Four of these patients were documented to have positive spike antibodies at different times relative to vaccine and COVID-19 diagnosis.42 Tadmor et al reported that of 85 patients with HCL and COVID-19 infection, 40 were tested for antibodies after vaccination, of whom 27 (68%) had an antibody response.33 The only factor associated with seroconversion was the number of vaccine doses. Our study is, to our knowledge, by far the largest on vaccination in HCL/HCLv and because of its size it was possible to determine relationships between postvaccination antibody levels and clinical factors such as normal B-cell numbers (Figure 1). We did find that prior exposure to COVID-19, assessed mainly by nucleocapsid antibodies, was associated with a higher response to vaccination (Figure 6A).
Strategies for effective vaccination in HCL/HCLv
The high morbidity and mortality early on from COVID-19 necessitated strategies for effective vaccination in patients with HCL/HCLv. Although COVID-19 has become much less deadly and the focus on vaccination has waned, strategies are still relevant, particularly for patients at risk because of immunosuppression and comorbidities. Anti-CD20 mAbs, which are often used in HCL/HCLv treatment and are increasingly used in first line,10,11,23,28,29,46 are known to prevent effective vaccination for 6 to 12 months.5 However, our study shows that normal B-cell counts are still significantly lower 1 to 2 years later than 2 to 4 years later (Figure 3B). Vaccination at least several weeks before beginning immunosuppressive treatment is important, particularly before normal B cells become depleted. However, many patients with HCL/HCLv lack normal B cells before treatment because of disease. For these patients, treatment with a BRAFi before vaccination may be useful, and definitive treatment can be resumed thereafter when needed.24 As recommended by the HCL COVID-19 consensus guidelines,47 COVID-19 antibody levels in patients with HCL may be helpful to confirm that patients are in fact effectively vaccinated, and assessment of normal B-cell counts can be a useful predictor of vaccination efficiency. These strategies may be useful in patients with HCL/HCLv and possibly other B-cell disorders for preventing not only COVID-19 but also other viral and bacterial infections.
Acknowledgments
The authors thank Barbara Debrah for managing clinical and research data, and Alexia Torres and Sonya Duke for coordinating patient testing and sample submission. Ann McCoy and Monica Epstein served as research nurses early in the study. Andy Gillespie and Helen Owens served as research nursing supervisors.
This study was supported by the intramural research programs of the National Cancer Institute, National Institute of Allergy and Infectious Diseases, and the National Institute of Dental and Craniofacial Research.
Authorship
Contribution: R.J.K., L.J., J.F., H.E., O.S.O., M.G., I.S., H.Z., P.D.B., J.I.C., H.-W.W., C.M.Y., and E.A. performed research; R.J.K. and J.I.C. wrote the paper; P.D.B., J.I.C., and E.A. contributed vital new reagents or analytical tools; R.J.K., P.D.B., J.I.C., and E.A. analyzed data; and R.J.K. designed the research.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Robert J. Kreitman, Laboratory of Molecular Biology, National Cancer Institute, National Institutes of Health, Bldg 10, Room 13N248A, 9000 Rockville Pike, Bethesda, MD 20892; email: kreitmar@mail.nih.gov.
References
Author notes
Data are available on request from the corresponding author, Robert J. Kreitman (kreitmar@mail.nih.gov).