TO THE EDITOR:
Venetoclax (VEN) is a potent, oral drug that selectively binds the B-cell lymphoma 2 (BCL2) protein to promote apoptosis.1 VEN changed the treatment landscape of various hematologic diseases with its tolerability and combinatorial efficacy with standard-of-care (SOC) therapies. VEN-based regimens are approved for acute myeloid leukemia (AML) and chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL).2-4 Still, novel combinations that safely enhance VEN efficacy are needed to further improve responses. Statins are well-tolerated and relatively inexpensive oral agents widely used to treat high cholesterol. By suppressing mevalonate synthesis, statins deplete biosynthetic intermediates often commandeered by oncogenic processes.5 In preclinical studies, statins sensitize to VEN in a variety of blood cancer cells, including primary CLL and AML cells,6 through the cancer-selective increase of apoptotic sensitizers.7 The clinical significance of these findings is supported by retrospective subgroup analyses of VEN clinical trial data demonstrating improved responses in patients with CLL who were concurrently on a statin.6 Prior clinical trials assessing the chemo-sensitizing effects of a hydrophilic statin, pravastatin, in AML were encouraging even in unfavorable-risk groups but did not meet predetermined efficacy criteria.8 Pitavastatin (PIT) has been identified as an optimal lipophilic statin for oncology trials.7,9
We conducted a phase 1 study adding PIT to VEN-based therapy in VEN-naïve adult patients with AML or CLL/SLL. This trial was registered at www.clinicaltrials.gov as #NCT04512105. The study protocol was approved by the University of California, Irvine Institutional Review Board. Potential toxicities were not likely to be different between the disease states; therefore, this heterogeneous group was selected to help expedite accrual. Newly diagnosed patients with AML were eligible if they received induction therapy with azacitidine (AZA) and VEN per SOC. Patients with newly diagnosed or relapsed/refractory CLL/SLL could receive VEN with either obinutuzumab or rituximab. A 3 + 3 design was used. Dose level 1 (DL1) was PIT 2 mg daily and dose level 2 (DL2) was 4 mg daily. DL minus 1 was included to allow for dose reduction in those not tolerating DL1. Those enrolled at DL2 were required to have a creatinine clearance (CrCl) of 60 mL/min or higher based on the prescribing information for PIT. Patients on other statins for hypercholesterolemia were allowed if the other statin was stopped for 72 hours before the initiation of the assigned dose of PIT. Treatment with PIT was started after patients had completed the VEN ramp-up and had been on a stable dose for at least 5 days. VEN and PIT were planned to continue until progression or intolerance for AML and 1-year fixed duration of treatment for CLL. The primary objectives were 1) to assess the safety and tolerability and 2) to determine the recommended phase 2 dose of PIT in combination with VEN-based therapies. Dose-limiting toxicities (DLTs) are described in the supplemental Information. Secondary objectives included complete response (CR) rates defined by the International Workshop on CLL guidelines10 or International Working Group response criteria for AML11 and minimal residual disease (MRD) assessed per the treating investigator. Exploratory assays included BH3 profiling of pretreatment and posttreatment blood samples to assess whether add-on treatment with PIT increases apoptotic priming, as seen in preclinical models.6 Peripheral blood mononuclear cells (PBMCs) were isolated, cryopreserved, and later thawed for exploratory studies.
The study completed the accrual of 6 patients without the need for additional cohorts. Fourteen patients signed informed consent. Six were deemed ineligible, and 2 withdrew consent before starting PIT therapy. Six patients received PIT on study, and accrual is now closed. Two patients had AML and received AZA + VEN. Two patients had CLL and 2 patients had SLL. All the patients with CLL/SLL received VEN + obinutuzumab. Several patients had disease features associated with an unfavorable prognosis (Table 1).12,13 One patient (enrolled in the 4 mg dose cohort) was on a statin for hypercholesterolemia before enrollment (rosuvastatin 40 mg). The other 5 patients were statin-naive.
All patients achieved the best response of complete response (Table 1). Study therapy was terminated early for 1 patient with CLL due to neutropenia that was thought to be due to liver disease rather than study therapy. Two others with CLL/SLL ended the study early due to personal choice but did not experience significant toxicity. Of the patients with AML, 1 remained on AZA for the treatment of myelodysplastic syndrome without recurrence of AML.
MRD was assessed for most patients either by flow cytometry (University of Washington) or another US Food and Drug Administration–approved test per the treating investigator’s discretion. One patient with AML and 2 with SLL were negative for MRD by flow cytometry (level of detection 10–3 to 10–4). One patient with CLL was MRD-negative by clonoSEQ testing of the peripheral blood (limit of detection 10–6 for the specific sequence). Two patients (1 with AML and 1 with CLL) were positive for MRD by flow cytometry.
Adverse events deemed related to PIT included myalgia, diarrhea, and elevated liver enzymes and were grade 1 to 2. Serious (grade 3-4) adverse events were primarily hematologic and felt to be likely resulted from the disease and SOC therapy. Two patients (1 with AML and 1 with CLL) had dose reductions of PIT due to severe neutropenia. One patient died from a lung infection that was felt to be related to disease. Overall, the toxicities were similar at DL1 and DL2. No patients required a dose reduction of PIT to DL minus 1.
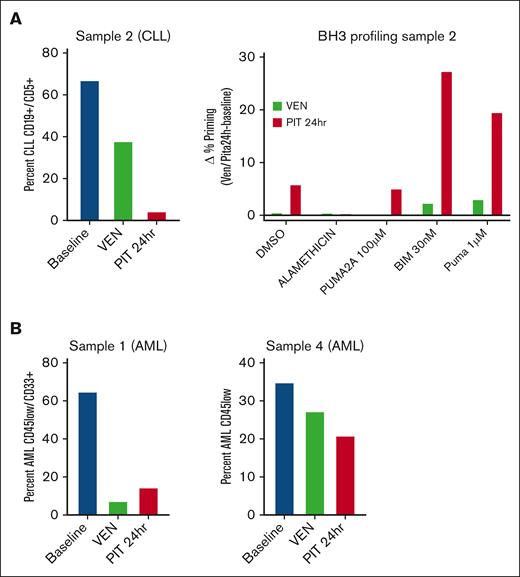
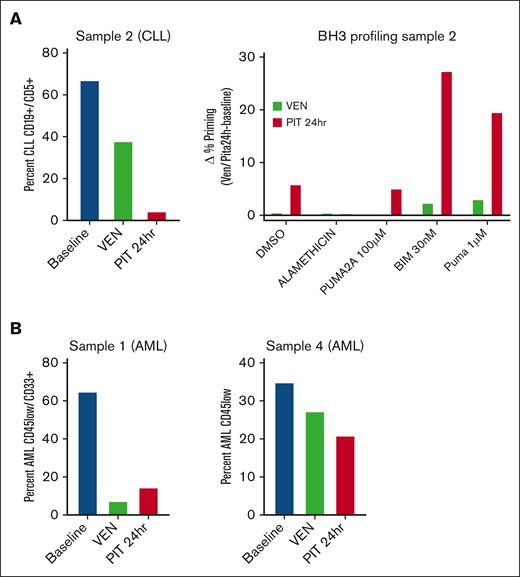
Peripheral blood samples were obtained from 2 patients with AML and 1 patient with CLL pretreatment (baseline), post-VEN ramp-up (post-VEN), and ∼24 hours after the first dose of PIT (post-PIT). We did not conduct studies with samples from the second patient with CLL because no baseline sample was collected, and the low percentage of circulating leukemia cells in the 2 patients with SLL (<1%) precluded correlative studies. We measured changes in leukemia burden and proximity to the mitochondrial apoptotic threshold (priming) by flow cytometry-based BCL2 homology domain 3 (BH3) profiling.14 AML cells were distinguished by immunophenotype with CD45-low/CD33+. CLL cells were identified as CD19+CD5+CD23+. No treatments were performed ex vivo; the cells were analyzed directly by thawing cryopreserved PBMCs.
Analysis of PBMCs from patient no. 2 (CLL) showed reduced leukemia burden post-VEN (38% vs 67% at baseline), with a further decrease after the first PIT dose (4%) (Figure 1A). BH3 profiling of surviving CLL cells showed increased priming in cells post-PIT relative to post-VEN (Figure 1A). There was no increase in the priming of post-VEN cells compared with baseline, which might be due to the death of primed cells during the ramp-up period. These results suggest that in this patient with CLL, a single dose of PIT reduced the leukemia burden by reducing the threshold for apoptosis, even in cells resistant to VEN alone. The 2 AML peripheral blood samples gave mixed results. In patient no. 1, VEN ramp-up eliminated most of the leukemia cells (7% vs 65% at baseline), and there was a slight increase in priming post-PIT (15%) (Figure 1B). In patient no. 4, there was a stepwise reduction in leukemic burden with each treatment (35% at baseline, 27% post-VEN, and 21% post-PIT) (Figure 1B). BH3 profiling applied to both AML samples showed no consistent increase in priming post-VEN or post-PIT (data not shown).
Correlative studies suggest that pitavastatin reduces leukemia burden after a single dose in some patients. (A) Analysis of PBMCs from a patient with CLL. Left: the percentage of leukemia cells was determined by flow cytometry before treatment (baseline), after VEN ramp-up (VEN), and 24 hours after the first PIT dose (PIT 24 hours). Right: BH3 profiling was performed on gated CLL blasts by measuring cytochrome C release after exposure to DMSO alone, alamethicin (positive control apoptotic stimulus), PUMA2A (negative control peptide), or active BIM or PUMA peptides. The change in the percentage of cytochrome C–low cells between the VEN or PIT conditions and baseline was graphed as delta percentage priming. (B) Analysis of PBMCs from 2 patients with AML. The percentage of AML blasts was determined using flow cytometry. Each experiment in panels A and B was done with a single sample, without technical replicates. DMSO, dimethyl sulfoxide; PUMA, p53 upregulated modulator of apoptosis; BIM, BCL2 interacting mediator of cell dealth.
Correlative studies suggest that pitavastatin reduces leukemia burden after a single dose in some patients. (A) Analysis of PBMCs from a patient with CLL. Left: the percentage of leukemia cells was determined by flow cytometry before treatment (baseline), after VEN ramp-up (VEN), and 24 hours after the first PIT dose (PIT 24 hours). Right: BH3 profiling was performed on gated CLL blasts by measuring cytochrome C release after exposure to DMSO alone, alamethicin (positive control apoptotic stimulus), PUMA2A (negative control peptide), or active BIM or PUMA peptides. The change in the percentage of cytochrome C–low cells between the VEN or PIT conditions and baseline was graphed as delta percentage priming. (B) Analysis of PBMCs from 2 patients with AML. The percentage of AML blasts was determined using flow cytometry. Each experiment in panels A and B was done with a single sample, without technical replicates. DMSO, dimethyl sulfoxide; PUMA, p53 upregulated modulator of apoptosis; BIM, BCL2 interacting mediator of cell dealth.
Overall, PIT was well-tolerated to 4 mg once daily when added to standard VEN-based therapies. Toxicities were deemed similar to those of VEN-based therapy in standard clinical practice. Grade 3 to 4 toxicities were primarily hematologic and consistent with SOC treatment and the patients’ underlying disease.
Based on the tolerability of adding PIT to VEN-based therapies and encouraging clinical outcomes, a phase 2 study is planned to better assess the efficacy of this strategy in TP53-mutant AML. This cohort was chosen given the high clinical need; the median OS after VEN-based regimens is 6 months.15 Preclinical data demonstrate that the apoptosis-sensitizing effects of statins in blood cancers are independent of TP53 function.6,7 Additionally, there is reason to suspect an additional dependency on the mevalonate pathway in TP53-mutated AML cells.16
We encountered an unexpected barrier to enrollment in the DL2 cohort given a CrCl eligibility of 60 mL/min or higher based on the prescribing information for PIT. All but 1 of the screen fails were due to CrCl <60 despite adequate hydration, and nearly all were patients with AML. This will be taken into consideration for phase 2 planning, and this was the primary driver for the determination of 2 mg as the recommended phase 2 dose. We hope that the strategy of adding PIT to BH3 mimetic-based therapy will have a widespread effect in improving patient outcomes without added toxicity.
Acknowledgment: This work was funded by the Impact Award W81XWH-20-1-0867 from the Department of Defense, and by University of California, Irvine (UCI) Anti-Cancer Challenge Pilot Grant.
Contribution: E.A.B., D.A.F., K.S., D. Juarez, and R.B. wrote the manuscript; D. Juarez and D.A.F. performed the preclinical studies that led to the trial; E.A.B. wrote the protocol and was the principal investigator for the study with guidance from D. Jeyakumar and S.O.; and T.H.T. helped with the statistical design of the protocol.
Conflict-of-interest disclosure: E.A.B. reports speaking for BeiGene, AstraZeneca, Seagen, Incyte/MorphoSys, and AbbVie/Genmab; and consultancy for BeiGene, ADC Therapeutics, AstraZeneca, Caribou Biosciences, Genentech, and MorphoSys. D. Jeyakumar reports research funding from Jazz Pharmaceuticals and Pfizer. S.O. reports consultancy for AbbVie, AstraZeneca, Autolus, BeiGene, Bristol Myers Squibb, Eli Lilly, Glaxosmithkline, Janssen, Johnson & Johnson, Loxo, Merck, Pfizer, Pharmacyclics, and TG Therapeutics; and research support from AstraZeneca, Caribou Biosciences, Gilead, Kite, Mustang Bio, Nurix, Pfizer, Pharmacyclics, and TG Therapeutics. The remaining authors declare no competing financial interests.
Correspondence: Elizabeth A. Brem, Department of Medicine, University of California, Irvine, 101 The City Dr South, Orange, CA 92868; email: ebrem@hs.uci.edu.
References
Author notes
Data are available on request from the corresponding author, Elizabeth A. Brem (ebrem@hs.uci.edu).
The full-text version of this article contains a data supplement.