Key Points
Lenalidomide remains the maintenance therapy of choice for patients with NDMM.
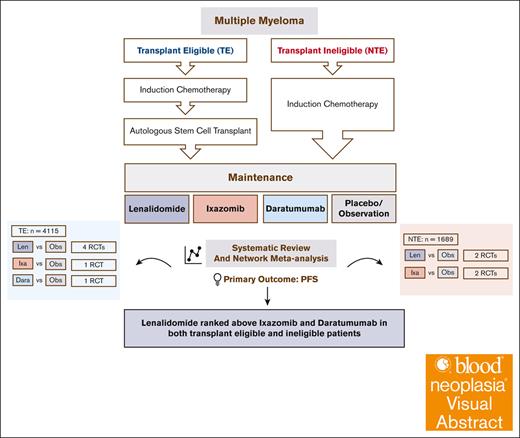
Visual Abstract
Lenalidomide, ixazomib, and daratumumab have been proposed as maintenance therapies for patients with newly diagnosed multiple myeloma (MM; NDMM). There are, however, no randomized controlled trials (RCTs) comparing them. We conducted a network meta-analysis (NMA) of RCTs comparing these agents against placebo in NDMM. A Bayesian NMA model was used to assess the relative effects of competing treatments on progression-free survival (PFS) and overall survival (OS) in 9 studies including 4115 patients with transplant-eligible MM (TEMM) and 1689 patients with non–transplant-eligible MM (NTEMM). Lenalidomide and daratumumab but not ixazomib were associated with improved PFS compared with placebo in patients with TEMM (lenalidomide [hazard ratio (HR), 0.46; 95% credible interval (CrI), 0.36-0.56]; daratumumab [HR, 0.49; 95% Crl, 0.32-0.76]; and ixazomib [HR, 0.72; 95% CrI, 0.46-1.12]) and those with NTEMM (lenalidomide [HR, 0.46; 95% CrI, 0.29-0.75] and ixazomib [HR, 0.69; 95% CrI, 0.43-1.18]). The PFS benefit for daratumumab was present regardless of whether daratumumab-based induction therapy was received. None of the agents showed an OS benefit, and PFS benefits were not seen in patients with high-risk cytogenetics. Lenalidomide was associated with second malignancies, ixazomib with thrombocytopenia, and daratumumab with pneumonia. We propose that lenalidomide remains the maintenance therapy of choice for NDMM.
Introduction
The advent of novel agents has led to significant improvements in outcomes for patients with multiple myeloma (MM).1 Novel agent–based maintenance therapy is a standard of care for both patients with transplant-eligible MM (TEMM) and non–transplant-eligible MM (NTEMM).2-4 The aims of maintenance therapy in newly diagnosed MM (NDMM) are to improve the depth of response achieved during induction, delay relapse, and improve survival.5 These benefits have to be balanced against toxicities and impact on quality of life. Lenalidomide is considered the optimal choice for maintenance4 based on improved progression-free survival (PFS) in both TEMM and NTEMM, with overall survival (OS) benefits seen in the former.6-11
Proteasome inhibitor (PI)–based maintenance therapy has been proposed as an alternative to lenalidomide for patients with high cytogenetic risk (HCR) MM.12 These recommendations are based on the HOVON-65/GMMG-HD4 trial, which compared bortezomib with thalidomide maintenance.13 More recently, the oral PI ixazomib was compared with placebo as maintenance after autologous stem cell transplant (ASCT) in the TOURMALINE MM3 trial.14 This trial suggested that ixazomib confers a PFS benefit (median PFS, 26.5 vs 21.3 months), with benefits also seen in HCR MM. In NTEMM, the TOURMALINE MM4 trial also demonstrated a significant PFS benefit (median PFS, 17.4 vs 9.4 months) when ixazomib was compared with placebo.15
There are no randomized controlled trials (RCTs) comparing lenalidomide and ixazomib maintenance. The maintenance randomization of the CASSIOPEIA trial showed that daratumumab maintenance resulted in a PFS benefit regardless of whether daratumumab was used for induction, adding a further maintenance option for consideration.16,17 Previous network meta-analyses (NMAs) have compared lenalidomide against thalidomide- and bortezomib-based maintenance but not against ixazomib or daratumumab.18 We present a systematic review and an NMA comparing the maintenance options of lenalidomide, ixazomib, and daratumumab in NDMM.
Methods
This study is reported according to the PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) extension statement for NMA.19 The study was registered in the prospective register of systematic reviews (PROSPERO CR 42021226157), and the protocol synopsis is presented under supplemental Methods.
Eligibility criteria
This study incorporated RCTs including newly diagnosed TEMM or NTEMM. The RCTs needed to have at least 2 arms comparing modalities of maintenance. The maintenance treatments of interest were daratumumab, ixazomib, lenalidomide, and placebo/observation. We excluded studies evaluating thalidomide maintenance as a previous NMA18 demonstrated the superiority of lenalidomide over thalidomide. The only RCT that involved the evaluation of single-agent bortezomib maintenance13 was excluded because patients in the trial were randomly assigned to different induction and maintenance pathways at the start of treatment, and there was no maintenance randomization. This trial, therefore, cannot be considered a study of maintenance therapy. Trials in which patients were treated with standard novel agent–based induction were included. Single-arm studies and those using multiagent maintenance were excluded because there is no evidence to support their superiority over single-agent maintenance in the setting of standard induction therapy.
Information sources, search strategy, and study selection
We identified eligible RCTs by searching MEDLINE, EMBASE, and the Cochrane Central Register of Controlled Trials from the date of inception to 1 July 2024. We searched the proceedings of key hemato-oncology conferences between 2010 and 2024, as well as reference lists of relevant review articles and reports of eligible trials. We used the search strategy that included the medical subject heading of “multiple myeloma,” as well as keywords “daratumumab,” “ixazomib,” and “lenalidomide” (supplemental Table 1). The search was restricted to “randomized clinical trial.” Two reviewers independently screened all titles and abstracts first using the software Covidence (https://app.covidence.org). Articles identified as potentially eligible were retrieved as full text for closer assessment. Disagreements were resolved by a third reviewer.
Data items and collection process
The same reviewers extracted the data independently using standardized data collection forms created in Covidence. We collected information on trial characteristics (publication status, trial registration number, study status, sample size, and design), population characteristics (age, sex, stage, cytogenetic risk, transplant eligibility, and induction treatment), maintenance treatment characteristics (drug, route, dose, duration, and frequency), and outcomes (PFS, OS, and adverse events [AE]s).
Geometry of the network
We grouped the maintenance treatments into common nodes based on the drug and not on dose or duration. We drew the network plot using the plot function in the R package GeMTC, with the edge thickness corresponding to the number of studies we included for that comparison.
Risk of bias within individual studies
We used version 2.0 of the Cochrane risk-of-bias tool to assess the risk of bias for 5 domains for both PFS and OS outcomes. Details are presented under supplemental Methods.
Summary measures
We summarized the direct and indirect effects of various maintenance treatments on time-to-event outcomes using hazard ratios (HRs) and the corresponding 95% credible intervals (95% CrIs). The primary outcome was PFS, defined as the time from randomization to disease progression or death from any cause. The secondary outcome was OS, defined as the time from randomization to death from any cause. We estimated the individual trial log HRs and their variances using published methods when appropriate summary statistics or Kaplan-Meier curves were reported. The individual log HRs and their variances were analyzed using the Bayesian NMA model. An HR of <1 indicates an advantage for that particular treatment.
Planned methods of analysis
We used random effects for both PFS and OS outcomes and conducted the NMA and pairwise meta-analysis using a Bayesian framework with noninformative uniform and normal prior distributions. We estimated the overall rankings of treatments by calculating the surface under the cumulative ranking (SUCRA) curve for each. A treatment is certain to be the best when SUCRA is 1 and is certain to be the worst when SUCRA is 0. Further details are presented under the supplemental Methods.
Certainty of evidence
We used the grading of recommendations, assessment, development, and evaluation approach to assess the certainty of evidence.20 Further details are provided under the supplemental Methods.
Results
Study selection
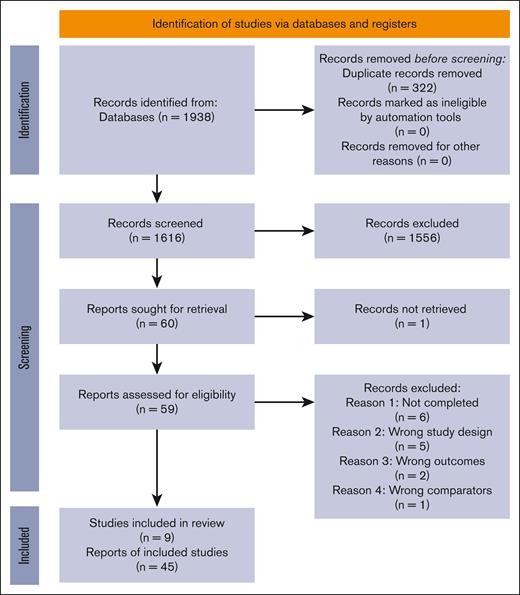
We screened 1616 records and retrieved the full text of 60 articles for assessment of eligibility. We included 9 trials after excluding 14 studies due to study not being completed (n = 6), study design other than RCT (n = 5), wrong outcome measures (n = 2), and wrong comparators (n = 1). The PRISMA chart describing the study selection process is shown in Figure 1, and details of the excluded studies are presented under supplemental Results.
PRISMA flowchart describing the selection of studies. Literature search yielded a total of 1938 results. After a 2-staged screening (as mentioned in the text), unsuitable reports were excluded, and a total of 9 studies (45 records) were included.
PRISMA flowchart describing the selection of studies. Literature search yielded a total of 1938 results. After a 2-staged screening (as mentioned in the text), unsuitable reports were excluded, and a total of 9 studies (45 records) were included.
Summary of network geometry
Transplant eligible
We identified 4 RCTs comparing lenalidomide with placebo, and 1 RCT each comparing ixazomib with placebo and daratumumab with observation in patients with TEMM. All these trials reported PFS outcomes. Only 4 RCTs comparing lenalidomide with placebo reported OS outcomes. The reference arm was placebo or observation (supplemental Figures 1 and 2).
Transplant ineligible
We identified 2 RCTs comparing lenalidomide with placebo or observation and 2 RCTs comparing ixazomib with placebo in patients with NTEMM. All these trials reported PFS outcomes. Two trials that compared lenalidomide with placebo or observation and 1 trial that compared ixazomib with placebo reported OS outcomes. The reference arm was placebo or observation (supplemental Figures 3 and 4).
Study characteristics
Transplant eligible
The characteristics of the included studies for patients with TEMM are summarized in supplemental Table 2. The median sample size was 635, and the median age of patients ranged from 57 to 60 years. Male patients comprised 59.6%, and 16% had International staging system (ISS) stage III disease. Five trials reported cytogenetic risk profiles, and 16% of patients in these trials had HCR MM. Three-quarters of patients achieved a complete response or very good partial response at the point of maintenance randomization.
Transplant ineligible
The characteristics of the included studies for participants with NTEMM are summarized in supplemental Table 3. The median sample size of the included studies was 444. The median age of the patients ranged from 70 to 73 years. Male patients comprised 59.3%, and 33% had ISS stage III disease. All studies reported cytogenetic risk profiles, and 18% of patients had HCR MM. Seventy-one percent of patients achieved a complete response or very good partial response at the point of maintenance randomization.
Risk of bias within studies
Transplant eligible
The overall risk of bias was judged to be low in 5 trials, with some concerns in 1 trial. Further details are presented in the supplemental Results.
Transplant ineligible
The overall risk of bias was judged to be low in 3 trials, with some concerns in 1 trial. Further details are presented in the supplemental Results. The summary of the risk-of-bias assessment is presented in supplemental Figure 5.
Results of individual studies
Transplant-eligible patients
PFS
Four trials demonstrated that maintenance lenalidomide improved PFS significantly compared with placebo, with HRs as follows: Attal et al HR, 0.37 (95% confidence interval [CI], 0.26-0.53); Palumbo et al HR, 0.47 (95% CI, 0.33-0.65); Jackson et al HR, 0.48 (95% CI, 0.4-0.58); and McCarthy et al HR, 0.5. One trial (Dimopoulos et al) demonstrated that maintenance ixazomib was associated with a 34% reduction in the hazards for progression and death compared with placebo (HR, 0.72; 95% CI, 0.58-0.89). One trial (Moreau et al) demonstrated that daratumumab was associated with a 51% reduction in the hazards for progression and death compared with placebo (HR, 0.49; 95% CI, 0.40-0.59). In HCR patients, lenalidomide (demonstrated in 2 trials) and daratumumab (reported in the updated CASSIOPEIA trial results) demonstrated significantly improved PFS compared with placebo (further details are presented under supplemental Results).
OS
Three trials demonstrated that maintenance lenalidomide was associated with statistically significant improvement in OS; further data are presented in the supplemental Results.
Transplant-ineligible patients
PFS
Two trials demonstrated that lenalidomide was associated with statistically significant improvement in PFS: Jackson et al (HR, 0.44; 95% CI, 0.37-0.53) and Palumbo et al (HR, 0.49; CI not reported; P < .001). One trial demonstrated that ixazomib was associated with statistically significant improvement in PFS (Dimopoulos et al; HR, 0.66; 95% CI, 0.54-0.80). One trial (Zweegman et al) did not show a statistically significant PFS benefit for ixazomib (HR, 0.8; 95% CI, 0.48-1.34). One trial (Jackson et al) showed a statistically significant PFS benefit for lenalidomide in HCR patients, with an HR of 0.45 (95% CI, 0.3-0.67) for standard risk and 0.53 (95% CI, 0.35-0.79) for HCR (further details in supplemental Results).
OS
An OS benefit was not shown in any trials involving patients with NTEMM; details are presented under the supplemental Results.
AEs (TRANSPLANT-ELIGIBLE AND -INELIGIBLE PATIENTS)
Six trials used the common terminology criteria for AEs grading system to define and grade AEs, whereas 1 trial each used the World Health Organization toxicity criteria and Medical Dictionary for Regulatory Activities systems. The most common grade 3 or 4 AEs in the trials evaluating lenalidomide and ixazomib were neutropenia and thrombocytopenia, respectively, whereas neutropenia and pneumonia were reported with daratumumab. Second malignancies were also reported with lenalidomide (further details under supplemental Results; supplemental Tables 3-8).
Synthesis of results
Transplant eligible
PFS
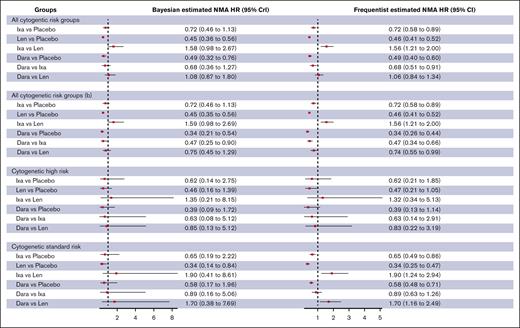
The Bayesian random effects NMA model showed that lenalidomide and daratumumab but not ixazomib were associated with a statistically significant reduction in the hazards for progression or death compared with placebo in unselected patients with TEMM (lenalidomide [HR, 0.46; 95% CrI, 0.36-0.56; high certainty]; daratumumab [HR, 0.49; 95% CrI, 0.32-0.76; high certainty]; ixazomib [HR, 0.72; 95% CrI, 0.46-1.12; high certainty]; Table 1; Figure 2; supplemental Table 9). The SUCRA values for superior PFS using lenalidomide, ixazomib, and daratumumab were 88%, 35%, and 75%, respectively.
Comparison of PFS between maintenance strategies for transplant-eligible patients. “b” indicates sensitivity analysis using data from the CASSIOPEIA trial focusing on patients who did not have Dara as induction therapy. Dara, daratumumab; Ixa, ixazomib; Len, lenalidomide.
Comparison of PFS between maintenance strategies for transplant-eligible patients. “b” indicates sensitivity analysis using data from the CASSIOPEIA trial focusing on patients who did not have Dara as induction therapy. Dara, daratumumab; Ixa, ixazomib; Len, lenalidomide.
Sensitivity analysis with frequentist random effects NMA model showed similar pooled effect estimates and P scores. The pooled effect estimates for lenalidomide vs placebo were similar for the network or pairwise frequentist and Bayesian meta-analysis models. The I-squared statistic for the frequentist random effects NMA model was 0% (supplemental Table 15). We evaluated the impact of daratumumab induction on the efficacy of daratumumab maintenance. When we used HRs from patients in the CASSIOPEIA trial who received bortezomib, thalidomide, and dexamethasone induction, the SUCRA values for superior PFS favored maintenance daratumumab (lenalidomide, 69%; ixazomib, 33%; daratumumab, 96%). There were no effect modifications based on subgroups defined for the duration of maintenance (fixed vs continuous) or the use of PI-based induction (supplemental Table 10). None of the agents studied resulted in an improvement in PFS compared with placebo in HCR patients with TEMM. The synthesis of data by cytogenetic subgroups is presented in the supplemental Results and supplemental Tables 9-11.
OS
The Bayesian pairwise Meta analysis (MA) model showed that maintenance lenalidomide was not associated with statistically significant reduction in the hazards for death compared with placebo in unselected patients with TEMM (HR, 0.76; 95% CrI, 0.41-1.36; low certainty). Sensitivity analysis with frequentist random effects pairwise MA model showed a similar pooled effect estimate (HR, 0.77; 95% CI, 0.54-1.09).
Transplant ineligible
PFS
The Bayesian random effects NMA model showed that lenalidomide but not ixazomib was associated with a statistically significant reduction in the hazards for progression or death compared with placebo in unselected patients with NTEMM (lenalidomide [HR, 0.46; 95% CrI, 0.29-0.75; high certainty]; ixazomib [HR, 0.69; 95% CrI, 0.43-1.18; low certainty]; Figure 3; supplemental Tables 12-14). The SUCRA values for superior PFS using lenalidomide and ixazomib were 96% and 50%, respectively.
Comparison of PFS and OS between maintenance strategies for non–transplant-eligible patients. “b” indicates sensitivity analysis by excluding Zweegman et al trial.21
Comparison of PFS and OS between maintenance strategies for non–transplant-eligible patients. “b” indicates sensitivity analysis by excluding Zweegman et al trial.21
Sensitivity analysis with frequentist random effects NMA model showed similar pooled effect estimates and P scores. The pooled effect estimates for lenalidomide vs placebo and ixazomib vs placebo were similar for the network, pairwise frequentist, and Bayesian meta-analysis models. The I-squared statistic for the frequentist random effects NMA model was 0% (supplemental Table 15). The HOVON trial used ixazomib-based induction, which may have an effect modification on the efficacy of ixazomib maintenance.21 When the meta-analysis excluded the HOVON trial, the SUCRA values for superior PFS using lenalidomide and ixazomib were similar to that of the primary analysis (lenalidomide, 92% vs 96% [primary analysis]; ixazomib, 53% vs 50% [primary analysis]). There was no effect modification based on subgroups defined for the duration of maintenance (fixed vs continuous) or the use of PI-based induction (supplemental Table 13). Neither lenalidomide nor ixazomib showed a PFS benefit in HCR patients with MM; the synthesis of data by cytogenetic subgroups are presented in the supplemental Results, Table 2, and supplemental Table 11.
OS
No trials demonstrated an OS benefit for any of the maintenance agents (Figure 3).
AEs OF GRADE ≥3 IN TRANSPLANT-ELIGIBLE AND -INELIGIBLE PATIENTS
The direction of effect for neutropenia and thrombocytopenia was greater with lenalidomide and ixazomib, respectively. Second primary malignancies showed a greater effect with lenalidomide, and peripheral neuropathy did not show a direction of effect toward any agent (supplemental Table 8).
Discussion
We present, to our knowledge, the first NMA comparing ixazomib, lenalidomide, and daratumumab maintenance in NDMM. Our findings suggest that lenalidomide maybe superior to ixazomib, and daratumumab maintenance is most beneficial when it is not used for induction. Gay et al previously reported an NMA evaluating a variety of maintenance therapies against placebo in NDMM and found that lenalidomide was the optimal choice.18 The superiority of lenalidomide over thalidomide- and bortezomib-based maintenance was confirmed in 2 further NMAs.22,23
A MA of 3 RCTs evaluating ixazomib maintenance compared with placebo confirmed the superiority of ixazomib with regard to PFS and depth of response.24 It is noteworthy that the PFS benefit was reduced in HCR patients, and there was no comparison with other maintenance agents. Bortezomib or ixazomib were proposed to be superior to thalidomide maintenance based on pooled PFS estimates in a separate study.25 This analysis is, however, difficult to interpret as one of the included trials used bortezomib in combination with thalidomide, and there was no comparison of PI maintenance with lenalidomide. Previous MAs did not compare lenalidomide with ixazomib or daratumumab maintenance, neither did they consider the effect of induction therapy on the efficacy of maintenance. In addition to comparing these agents for the first time, we demonstrate that the type of induction therapy (PI vs non-PI) and the duration of maintenance did not result in an effect modification when comparing lenalidomide and ixazomib. Other strengths of our study include the use of Bayesian and frequentist analyses (showing consistent results) along with comprehensive assessment of AEs.
Vij et al compared lenalidomide and ixazomib maintenance after standard induction therapy, ASCT, and ixazomib/lenalidomide/dexamethasone consolidation.26 In keeping with our findings, this phase 2 RCT was unable to demonstrate noninferiority of ixazomib to lenalidomide. Multiagent ixazomib-based maintenance after ASCT has also been evaluated, with the lenalidomide-ixazomib doublet in a single-arm study 27 and the ixazomib-lenalidomide-dexamethasone triplet in a randomized trial.28 It is notable that the triplet maintenance regimen did not show a significant PFS benefit over lenalidomide dexamethasone, supporting our findings that ixazomib is not superior to lenalidomide as a maintenance therapy.
Carfilzomib-based maintenance after carfilzomib-based induction has been evaluated in the FORTE29 (carfilzomib-lenalidomide vs lenalidomide) trial, which showed a benefit for the doublet arm over lenalidomide. The ATLAS trial30 explored minimal residual disease status–guided maintenance therapy with carfilzomib-lenalidomide-dexamethasone (KRD) vs lenalidomide alone in the post-ASCT setting. We excluded the FORTE trial from our analysis because carfilzomib-based induction therapy is currently not a standard of care.31 The ATLAS trial was excluded because it evaluated multiagent maintenance including treatment-dose lenalidomide compared with standard-dose lenalidomide maintenance. Furthermore, both FORTE and ATLAS trials evaluated multiagent maintenance regimens that are not the focus of our study.
Although anti-CD38 monoclonal antibody (CD38 Mab)–based induction therapy followed by CD38 Mab–based maintenance/continuous treatment has been used in several key RCTs,32-37only the CASSIOPEIA trial17 conducted a maintenance randomization, allowing for a definitive assessment of maintenance therapy. The latest update of this maintenance randomization demonstrates a PFS benefit regardless of whether daratumumab was used for induction therapy. However, the PFS benefit in daratumumab-naïve patients is arguably of greater clinical significance than that of those who received daratumumab-based induction. It is noteworthy that we excluded RCTs evaluating CD38 Mabs without a maintenance randomization from our analysis. The GMMG HD7 trial, however, included a maintenance randomization (isatuximab-lenalidomide vs lenalidomide), the results of which are awaited.38 These data will provide crucial information on the value of CD38 Mab–based doublet maintenance.
The role of lenalidomide maintenance in HCR patients was challenged by an MA demonstrating limited PFS benefit in this group.39 This analysis was limited by incomplete cytogenetic data in some studies; furthermore, the Myeloma XI trial showed that lenalidomide maintenance also conferred a PFS benefit in HCR patients.7,40 Our study showed that neither ixazomib nor daratumumab was superior to lenalidomide in the HCR subgroup.
A limitation of our analysis is that we do not have patient-level data from each of the RCTs, which would have enabled a more in-depth study of subgroup-specific effects. Although we examined the impact of PI- or daratumumab-based induction on outcomes, we did not evaluate the same for lenalidomide because this information was not available for all the trials. We did not include thalidomide-based maintenance in our study because it is not considered a standard of care, and previous analyses have shown that lenalidomide is superior to thalidomide.3,12,18 We also did not include bortezomib maintenance because the study often quoted in support of this practice was limited by the lack of a maintenance randomization and the arms having different induction therapies.13 Furthermore, this trial compared bortezomib against thalidomide maintenance and hence does not have a common treatment that could be linked into our NMA.
Conclusion
Our study suggests that lenalidomide remains the maintenance therapy of choice for NDMM, regardless of the modality of induction therapy received or the duration of maintenance. Given the marked PFS benefit associated with daratumumab-based induction,41 we propose that it be used for induction rather than maintenance. It is also noteworthy that we did not detect a significant OS benefit for any agent. Future RCTs should be designed to compare maintenance agents against each other, rather than placebo. Evaluation of continued vs limited duration maintenance and health economic analyses would also be valuable in defining the optimal maintenance strategy for NDMM.
Authorship
Contribution: E.L., Y.Y.S., and S.d.M. conceptualized the study and screened for and selected the included articles; A.R.Y.B.L. and S.Y.W. extracted the data; Y.Y.S. performed the analysis; E.L., Y.Y.S., A.R.Y.B.L., S.Y.W., and S.d.M. wrote the original draft of the manuscript; E.L., Y.Y.S., A.R.Y.B.L., S.Y.W., C.Y.S., M.O., W.J.C., and S.d.M. reviewed the manuscript; all authors agreed with the submitted work; and Y.Y.S. and S.d.M. had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Sanjay de Mel, Department of Haematology-Oncology, National University Cancer Institute Singapore, 1, Kent Ridge Rd, Singapore, Singapore 119082; email: sanjay_widanalage@nuhs.edu.sg.
References
Author notes
E.L. and Y.Y.S. contributed equally to this study and are joint first authors.
Data may be shared upon reasonable request from the corresponding author, Sanjay de Mel (sanjay_widanalage@nuhs.edu.sg).
The full-text version of this article contains a data supplement.