Key Points
Octogenarians and nonagenarians with AML can be treated successfully with VEN-HMA, and a subset of patients (∼25%) have prolonged survival.
Reduced dosing duration may be appropriate in this population, adjusted on a patient-by-patient basis to minimize myelosuppression.
Visual Abstract
Venetoclax (VEN) plus a hypomethylating agent (HMA) regimen is the standard of care for older adults with acute myeloid leukemia (AML); however, it is associated with significant myelosuppression and complications, potentially limiting its use in those who are very old. We performed a multicenter retrospective analysis of VEN-HMA treatment in octogenarians and nonagenarians to further understand the tolerability, feasibility, dosing considerations, and clinical efficacy in this unique group. Patients with AML aged ≥80 years who received VEN-HMA between March 2015 and April 2022 were reviewed. VEN-HMA dosing was determined by treating physician, accounting for CYP3A4 drug interaction dose adjustments. In total, 154 patients were included, with a median age of 82 years (range, 80-92), who received treatment with VEN-HMA (83% with azacitidine and 17% with decitabine). Most patients (53%) had European LeukemiaNet 2017 adverse risk AML, 33% had intermediate, 8% had favorable, and 6% were unknown. With a median follow-up of 7.7 months, 36 patients (23%) remained in remission, with 31 (20%) still on VEN-HMA. The 30-day and 60-day mortality rates were 8.5% and 17%, respectively. The composite complete remission (CRc) rate for patients with newly diagnosed AML without prior myelodysplastic syndrome was 73% (48 of 66). Median overall survival (OS) was 8.1 months, and in patients who achieved a response (CRc), median OS was 13.2 months. Landmark analysis from the time CRc was first achieved showed that patients receiving VEN for ≤14 days had improved OS; median, 24.0 months. Patients who are very old can be treated safely with combination VEN-HMA with expectations of dose reductions and cycle extensions to ensure tolerability over the long term.
Introduction
The initial treatment for acute myeloid leukemia (AML) depends on patient-specific factors such as age, performance status, comorbidities, and disease characteristics. Historically, intensive cytotoxic therapy was used, but this is prohibitively toxic in older adults.1-4 Consequently, many older patients went untreated or received monotherapy with a hypomethylating agent (HMA), comprising azanucleosides that inhibit DNA methyltransferases as the primary mechanism of action.5-7
Subsequently, the BCL-2 inhibitor venetoclax (VEN) plus HMA combination regimen was shown to be highly synergistic with improved response rates and overall survival (OS) compared with an HMA alone, and it became the new standard of care for older adults with AML or those otherwise “unfit” to receive intensive chemotherapy (7+3) upon its approval in 2018.8-10 Although better tolerated than intensive chemotherapy, VEN-HMA (azacitidine [AZA] or decitabine [DEC]) causes significant myelosuppression and the potential for infectious and other noninfectious complications; the extent to which this may limit its use in a population of extreme advanced age (≥80 years) is not known. In the pivotal phase 3 VIALE-A study, 61% of patients were aged ≥75 years. To mitigate myelosuppression, therapy could be delayed between treatment cycles for up to 14 days and VEN duration reduced from 28 to ≤21 days for patients in complete remission (CR)/CR with incomplete count recovery (CRi) to allow for count recovery, and granulocyte colony-stimulating factor support was used in 50.3% of patients achieving CR/CRi.8,11 In recent VIALE-A long-term follow-up analysis, 76% of patients (111/146) who achieved CR/CRi and were treated for at least 6 cycles required a reduction in VEN dosing duration.10 Other reports11,12,13 have recommended VEN-HMA cycle extensions/treatment interruptions and/or reduced VEN duration before a reduction in VEN dose. A recent study explored a 7-day VEN duration starting in cycle 1, which, on preliminary analysis, demonstrated similar clinical outcomes with less myelosuppression.14
VEN-HMA demonstrates high response rates, but most patients still relapse.15 VEN resistance may occur by innate or acquired mechanisms. Mutations in RAS pathway signaling and FLT3-ITD, and TP53 alterations all convey resistance. Cellular alterations associated with VEN-HMA resistance include monocytic differentiation; acquired BAX mutations; aberrant upregulation of alternative BCL2 family member proteins such as MCL1; and alterations in mitochondrial structure, function, and metabolsim.16-19 The prevalence and impact of classical VEN resistance mechanisms in patients aged ≥80 years is unclear, as is the influence of more favorable features such as NPM1 and IDH1/2 mutations.20,21
There is controversy about whether VEN-HMA truly represents nonintensive therapy, particularly given the attendant cytopenias, dose reductions and delays, and risk of infections.7 Its tolerability and efficacy in patients of very advanced age (eg, aged ≥80 years) is thus worthy of study. To our knowledge, no report has specifically examined VEN-HMA in an exclusively octogenarian and nonagenarian population. Therefore, we performed a multicenter retrospective analysis of VEN-HMA treatment in these patients to further understand its tolerability, feasibility and dosing considerations, and clinical efficacy in this unique population.
Methods
Patients
This was a multicenter retrospective study conducted at 6 institutions in the United States and Italy. All patients with AML who were aged ≥80 year at the time of first treatment with VEN-HMA combination therapy between March 2015 and April 2022 were reviewed and analyzed. Patients must have received at least 1 day of VEN-HMA for the first time to be eligible. No patients meeting these criteria were excluded. Patients were identified using each institution’s electronic medical record to capture every patient with AML in the target age range who received VEN-HMA for the first time. Patients who received prior treatment with an HMA alone or any other therapy that was not VEN-HMA for either myelodysplastic syndrome (MDS), myeloproliferative neoplasm, MDS/myeloproliferative neoplasm overlap syndrome, or AML were included in the study design. Eastern Cooperative Oncology Group (ECOG) performance status (PS) was assessed. Molecular mutations were assessed by next-generation sequencing locally at each institution. This study was approved by the institutional review board of the University of Miami (approval no. 20210521). This study was conducted in accordance with the Declaration of Helsinki.
Treatment regimen
Patients received VEN ramp-up on an inpatient or outpatient basis per treating physician discretion. Starting dose of VEN and HMA was determined by the treating physician. Dose adjustments for drug interactions, most commonly with antifungals, are presented. Specifically, VEN 200 mg combined with moderate CYP3A4 inhibitors (fluconazole or isavuconazole) or VEN 100 mg combined with strong CYP3A4 inhibitors (posaconazole or voriconazole) were categorized as a target dose of 400 mg. Final VEN dose and duration was defined as the dose and duration the patient was receiving at last follow-up. HMA dose reductions were defined as any dose and schedule below the standard azacitidine 75 mg/m2 daily for 7 days every 28 days, or decitabine 20 mg/m2 daily for 5 days every 28 days. No patients received oral decitabine/cedazuridine.
Assessments
OS was the primary end point and defined as the number of days from first dose of VEN and HMA to the date of last follow-up or death. Secondary end points included composite CR (CRc) rate (complete remission or complete remission with partial hematologic recovery or CRi), median VEN dose and duration during cycle 1 and at last follow-up (final VEN dose and duration), VEN-HMA cycle length, and the rate of HMA dose reduction. Safety outcomes included rates of treatment-emergent grade 3 or higher anemia, thrombocytopenia, neutropenia, and febrile neutropenia. All patients were included in the survival, response (CRc) rate, and safety analyses by the intention-to-treat (ITT) principle. In a response-evaluable analysis of CRc, patients who did not have at least 1 bone marrow biopsy assessment after VEN-HMA treatment was started were excluded, unless they had evidence of disease progression in the peripheral blood before first bone marrow biopsy assessment.
Statistics
Baseline characteristics were analyzed for OS and CRc rate using χ2/Fisher exact test for categorical variables. For CRc analysis, both the ITT and response-evaluable populations were reported. All patients (ITT) were assessed for OS and safety. OS was defined as the time from first dose of VEN and HMA to death, event-free patients were censored at the date of last follow-up. OS was estimated by Kaplan-Meier method and associations with prognostic factors assessed by log-rank test. Cox proportional hazard regression model for OS, and logistic regression analysis for CRc rate, were performed. Multivariate analysis (MVA) was performed with variable selection based on literature review and the univariable analysis results, using entry/stepwise backward selection. For MVA, variables with P < .1 remained in the final model for Cox regression, and P < .2 for logistic regression to assess the association between clinically meaningful prognostic factors and OS and CRc rate, respectively. Variables analyzed in MVA for CRc included: ECOG PS, disease status at diagnosis, VEN duration in the first cycle, and TP53 mutation/complex karyotype. Logistic regression analysis of CRc was performed in patients with a response assessment. Cox models for OS included the following variables: ECOG PS, European LeukemiaNet 2017 (ELN 2017) risk, disease status at diagnosis, VEN dose and duration at last follow-up, and TP53 mutation/complex karyotype. Results are reported as hazard ratios (HRs) or odds ratios (ORs) with 95% confidence intervals (95% CIs). Statistical significance was set at a threshold of P < .05. All analysis was conducted using SAS version 9.4 for Windows (SAS Institute Inc, Cary, NC).
Results
Baseline demographics and disease characteristics
Among 154 patients with AML aged ≥80 years with VEN-HMA (83% with AZA, 17% with DEC), 77% were newly diagnosed, 10% relapsed/refractory (R/R), and 14% unknown. Among newly diagnosed patients, 56% had newly diagnosed AML without prior MDS (or other myeloid neoplasm) and 44% had newly diagnosed AML with a history of prior MDS (or other myeloid neoplasm). Prior single-agent HMA was received in 48 patients (31%), and 7 patients (5%) had prior VEN (not with HMA). Of 48 patients who received prior HMA, 29 received HMA for prior MDS, 13 for prior AML, and 6 were unknown. Median age was 82 years (range, 80-92 years), 69% of patients were male, and 30% of patients had an ECOG PS of ≥2 (Table 1). Most patients (53%) had ELN 2017 adverse risk AML, 33% had intermediate, 8% had favorable, and 6% were unknown. In patients with molecular data available, 31 of 140 (22%) patients had a TP53 mutation, 17 of 142 (12%) had NPM1, 27 of 141 (19%) had IDH1/2, 12 of 144 (8%) FLT3-ITD, and 11 of 144 (7%) FLT3-TKD (Table 1). RAS pathway mutations (KRAS, NRAS, PTPN11, CBL, and NF1) were present in 20 of 126 (16%) patients.
Baseline characteristics in all patients and response-evaluable patients and association with CRc response
Characteristics . | All patients (N = 154)∗ . | Patients with response assessment (n = 138) . | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N . | % . | No CRc . | CRc . | Unknown . | P value† . | n . | % . | No CRc . | CRc . | P value† . | ||||||
| n . | % . | n . | % . | n . | % . | n . | % . | n . | % . | |||||||
| All patients | 154 | 100.0 | 51 | 33.1 | 87 | 56.5 | 16 | 10.4 | 138 | 100.0 | 51 | 37.0 | 87 | 63.0 | ||
| Clinical response | NA | NA | ||||||||||||||
| No CR | 65 | 42.2 | 45 | 69.2 | 20 | 30.8 | — | — | 65 | 47.1 | 45 | 69.2 | 20 | 30.8 | ||
| CR | 63 | 40.9 | — | — | 63 | 100.0 | — | — | 63 | 45.7 | — | — | 63 | 100.0 | ||
| Unknown | 26 | 16.9 | 6 | 23.1 | 4 | 15.4 | 16 | 61.5 | 10 | 7.2 | 6 | 60.0 | 4 | 40.0 | ||
| Sex | .229 | .107 | ||||||||||||||
| Male | 106 | 68.8 | 39 | 36.8 | 55 | 51.9 | 12 | 11.3 | 94 | 68.1 | 39 | 41.5 | 55 | 58.5 | ||
| Female | 48 | 31.2 | 12 | 25.0 | 32 | 66.7 | 4 | 8.3 | 44 | 31.9 | 12 | 27.3 | 32 | 72.7 | ||
| Age at diagnosis, y | .335 | .290 | ||||||||||||||
| Mean (SD) | 83.2 (3.0) | 83.7 (2.9) | 83.0 (3.0) | 83.2 (2.9) | 83.3 (3.0) | 83.7 (2.9) | 83.0 (3.0) | |||||||||
| Median (min, max) | 82 (80, 92) | 83 (80, 91) | 82 (80, 92) | 82.5 (80, 90) | 82 (80, 92) | 83 (80, 91) | 82 (80, 92) | |||||||||
| Race/ethnicity | .911 | .939 | ||||||||||||||
| White | 125 | 81.2 | 41 | 32.8 | 70 | 56.0 | 14 | 11.2 | 111 | 80.4 | 41 | 36.9 | 70 | 63.1 | ||
| Black | 5 | 3.2 | 2 | 40.0 | 3 | 60.0 | — | — | 5 | 3.6 | 2 | 40.0 | 3 | 60.0 | ||
| Hispanic | 19 | 12.3 | 7 | 36.8 | 10 | 52.6 | 2 | 10.5 | 17 | 12.3 | 7 | 41.2 | 10 | 58.8 | ||
| Other/unknown | 5 | 3.2 | 1 | 20.0 | 4 | 80.0 | — | — | 5 | 3.6 | 1 | 20.0 | 4 | 80.0 | ||
| ECOG PS score | .109 | .938 | ||||||||||||||
| 0 | 22 | 14.3 | 9 | 40.9 | 13 | 59.1 | — | — | 22 | 15.9 | 9 | 40.9 | 13 | 59.1 | ||
| 1 | 83 | 53.9 | 29 | 34.9 | 47 | 56.6 | 7 | 8.4 | 76 | 55.1 | 29 | 38.2 | 47 | 61.8 | ||
| 2 | 34 | 22.1 | 10 | 29.4 | 20 | 58.8 | 4 | 11.8 | 30 | 21.7 | 10 | 33.3 | 20 | 66.7 | ||
| ≥3 | 12 | 7.8 | 3 | 25.0 | 6 | 50.0 | 3 | 25.0 | 9 | 6.5 | 3 | 33.3 | 6 | 66.7 | ||
| Unknown | 3 | 1.9 | — | — | 1 | 33.3 | 2 | 66.7 | 1 | 0.7 | — | — | 1 | 100.0 | ||
| ELN 2017 risk | .048 | .067 | ||||||||||||||
| Favorable | 12 | 7.8 | 1 | 8.3 | 11 | 91.7 | — | — | 12 | 8.7 | 1 | 8.3 | 11 | 91.7 | ||
| Intermediate | 51 | 33.1 | 17 | 33.3 | 31 | 60.8 | 3 | 5.9 | 48 | 34.8 | 17 | 35.4 | 31 | 64.6 | ||
| Poor | 82 | 53.2 | 31 | 37.8 | 41 | 50.0 | 10 | 12.2 | 72 | 52.2 | 31 | 43.1 | 41 | 56.9 | ||
| Unknown | 9 | 5.8 | 2 | 22.2 | 4 | 44.4 | 3 | 33.3 | 6 | 4.3 | 2 | 33.3 | 4 | 66.7 | ||
| Disease status | .008 | .007 | ||||||||||||||
| Newly diagnosed | 66 | 42.9 | 13 | 19.7 | 48 | 72.7 | 5 | 7.6 | 61 | 44.2 | 13 | 21.3 | 48 | 78.7 | ||
| R/R | 15 | 9.7 | 6 | 40.0 | 5 | 33.3 | 4 | 26.7 | 11 | 8.0 | 6 | 54.5 | 5 | 45.5 | ||
| Prior MDS | 52 | 33.8 | 22 | 42.3 | 25 | 48.1 | 5 | 9.6 | 47 | 34.1 | 22 | 46.8 | 25 | 53.2 | ||
| Unknown | 21 | 13.6 | 10 | 47.6 | 9 | 42.9 | 2 | 9.5 | 19 | 13.8 | 10 | 52.6 | 9 | 47.4 | ||
| Prior treatment HMA | .001 | <.001 | ||||||||||||||
| No | 106 | 68.8 | 26 | 24.5 | 70 | 66.0 | 10 | 9.4 | 96 | 69.6 | 26 | 27.1 | 70 | 72.9 | ||
| Yes | 48 | 31.2 | 25 | 52.1 | 17 | 35.4 | 6 | 12.5 | 42 | 30.4 | 25 | 59.5 | 17 | 40.5 | ||
| VEN dose, first cycle | .058 | .574 | ||||||||||||||
| 100 | 15 | 9.7 | 3 | 20.0 | 11 | 73.3 | 1 | 6.7 | 14 | 10.1 | 3 | 21.4 | 11 | 78.6 | ||
| 200 | 12 | 7.8 | 5 | 41.7 | 6 | 50.0 | 1 | 8.3 | 11 | 8.0 | 5 | 45.5 | 6 | 54.5 | ||
| 400 | 99 | 64.3 | 34 | 34.3 | 58 | 58.6 | 7 | 7.1 | 92 | 66.7 | 34 | 37.0 | 58 | 63.0 | ||
| 600 | 9 | 5.8 | 4 | 44.4 | 5 | 55.6 | — | — | 9 | 6.5 | 4 | 44.4 | 5 | 55.6 | ||
| Unknown | 19 | 12.3 | 5 | 26.3 | 7 | 36.8 | 7 | 36.8 | 12 | 8.7 | 5 | 41.7 | 7 | 58.3 | ||
| VEN duration (d), first cycle | .003 | .347 | ||||||||||||||
| <28 | 25 | 16.2 | 10 | 40.0 | 12 | 48.0 | 3 | 12.0 | 22 | 15.9 | 10 | 45.5 | 12 | 54.5 | ||
| 28 | 116 | 75.3 | 38 | 32.8 | 71 | 61.2 | 7 | 6.0 | 109 | 79.0 | 38 | 34.9 | 71 | 65.1 | ||
| Unknown | 13 | 8.4 | 3 | 23.1 | 4 | 30.8 | 6 | 46.2 | 7 | 5.1 | 3 | 42.9 | 4 | 57.1 | ||
| VEN duration (d), first cycle | <.001 | .071 | ||||||||||||||
| ≤7 | 6 | 3.9 | 4 | 66.7 | — | — | 2 | 33.3 | 4 | 2.9 | 4 | 100.0 | — | — | ||
| 8-14 | 11 | 7.1 | 3 | 27.3 | 7 | 63.6 | 1 | 9.1 | 10 | 7.2 | 3 | 30.0 | 7 | 70.0 | ||
| 15-21 | 8 | 5.2 | 3 | 37.5 | 5 | 62.5 | — | — | 8 | 5.8 | 3 | 37.5 | 5 | 62.5 | ||
| >21 | 116 | 75.3 | 38 | 32.8 | 71 | 61.2 | 7 | 6.0 | 109 | 79.0 | 38 | 34.9 | 71 | 65.1 | ||
| NPM1 | .024 | .089 | ||||||||||||||
| No | 125 | 81.2 | 44 | 35.2 | 69 | 55.2 | 12 | 9.6 | 113 | 81.9 | 44 | 38.9 | 69 | 61.1 | ||
| Yes | 17 | 11.0 | 3 | 17.6 | 14 | 82.4 | — | — | 17 | 12.3 | 3 | 17.6 | 14 | 82.4 | ||
| Unknown | 12 | 7.8 | 4 | 33.3 | 4 | 33.3 | 4 | 33.3 | 8 | 5.8 | 4 | 50.0 | 4 | 50.0 | ||
| IDH1/2 | .160 | .366 | ||||||||||||||
| No | 114 | 74.0 | 41 | 36.0 | 64 | 56.1 | 9 | 7.9 | 105 | 76.1 | 41 | 39.0 | 64 | 61.0 | ||
| Yes | 27 | 17.5 | 7 | 25.9 | 17 | 63.0 | 3 | 11.1 | 24 | 17.4 | 7 | 29.2 | 17 | 70.8 | ||
| Unknown | 13 | 8.4 | 3 | 23.1 | 6 | 46.2 | 4 | 30.8 | 9 | 6.5 | 3 | 33.3 | 6 | 66.7 | ||
| NPM1 or IDH1/2 | .029 | .145 | ||||||||||||||
| No | 105 | 68.2 | 39 | 37.1 | 57 | 54.3 | 9 | 8.6 | 96 | 69.6 | 39 | 40.6 | 57 | 59.4 | ||
| Yes | 40 | 26.0 | 10 | 25.0 | 27 | 67.5 | 3 | 7.5 | 37 | 26.8 | 10 | 27.0 | 27 | 73.0 | ||
| Unknown | 9 | 5.8 | 2 | 22.2 | 3 | 33.3 | 4 | 44.4 | 5 | 3.6 | 2 | 40.0 | 3 | 60.0 | ||
| FLT3-ITD or FLT3-TKD | .007 | .709 | ||||||||||||||
| No | 128 | 83.1 | 44 | 34.4 | 73 | 57.0 | 11 | 8.6 | 117 | 84.8 | 44 | 37.6 | 73 | 62.4 | ||
| Yes | 23 | 14.9 | 7 | 30.4 | 14 | 60.9 | 2 | 8.7 | 21 | 15.2 | 7 | 33.3 | 14 | 66.7 | ||
| TP53 mutation | .038 | .224 | ||||||||||||||
| No | 109 | 70.8 | 34 | 31.2 | 68 | 62.4 | 7 | 6.4 | 102 | 73.9 | 34 | 33.3 | 68 | 66.7 | ||
| Yes‡ | 31 | 20.1 | 12 | 38.7 | 14 | 45.2 | 5 | 16.1 | 26 | 18.8 | 12 | 46.2 | 14 | 53.8 | ||
| Unknown | 14 | 9.1 | 5 | 35.7 | 5 | 35.7 | 4 | 28.6 | 10 | 7.2 | 5 | 50.0 | 5 | 50.0 | ||
| Complex karyotype | .035 | .221 | ||||||||||||||
| No | 99 | 64.3 | 32 | 32.3 | 61 | 61.6 | 6 | 6.1 | 93 | 67.4 | 32 | 34.4 | 61 | 65.6 | ||
| Yes§ | 43 | 27.9 | 17 | 39.5 | 20 | 46.5 | 6 | 14.0 | 37 | 26.8 | 17 | 45.9 | 20 | 54.1 | ||
| Unknown | 12 | 7.8 | 2 | 16.7 | 6 | 50.0 | 4 | 33.3 | 8 | 5.8 | 2 | 25.0 | 6 | 75.0 | ||
| TP53 mutation and complex karyotype | .006 | .421 | ||||||||||||||
| No-no | 87 | 56.5 | 28 | 32.2 | 55 | 63.2 | 4 | 4.6 | 83 | 60.1 | 28 | 33.7 | 55 | 66.3 | ||
| Yes-yes | 22 | 14.3 | 9 | 40.9 | 9 | 40.9 | 4 | 18.2 | 18 | 13.0 | 9 | 50.0 | 9 | 50.0 | ||
| Other | 41 | 26.6 | 14 | 34.1 | 22 | 53.7 | 5 | 12.2 | 36 | 26.1 | 14 | 38.9 | 22 | 61.1 | ||
| Unknown | 4 | 2.6 | — | — | 1 | 25.0 | 3 | 75.0 | 1 | 0.7 | — | — | 1 | 100.0 | ||
| Date start VEN-HMA | .964 | .830 | ||||||||||||||
| 2015-2019 | 74 | 48.1 | 25 | 33.8 | 41 | 55.4 | 8 | 10.8 | 66 | 47.8 | 25 | 37.9 | 41 | 62.1 | ||
| 2020-2023 | 80 | 51.9 | 26 | 32.5 | 46 | 57.5 | 8 | 10.0 | 72 | 52.2 | 26 | 36.1 | 46 | 63.9 | ||
Characteristics . | All patients (N = 154)∗ . | Patients with response assessment (n = 138) . | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N . | % . | No CRc . | CRc . | Unknown . | P value† . | n . | % . | No CRc . | CRc . | P value† . | ||||||
| n . | % . | n . | % . | n . | % . | n . | % . | n . | % . | |||||||
| All patients | 154 | 100.0 | 51 | 33.1 | 87 | 56.5 | 16 | 10.4 | 138 | 100.0 | 51 | 37.0 | 87 | 63.0 | ||
| Clinical response | NA | NA | ||||||||||||||
| No CR | 65 | 42.2 | 45 | 69.2 | 20 | 30.8 | — | — | 65 | 47.1 | 45 | 69.2 | 20 | 30.8 | ||
| CR | 63 | 40.9 | — | — | 63 | 100.0 | — | — | 63 | 45.7 | — | — | 63 | 100.0 | ||
| Unknown | 26 | 16.9 | 6 | 23.1 | 4 | 15.4 | 16 | 61.5 | 10 | 7.2 | 6 | 60.0 | 4 | 40.0 | ||
| Sex | .229 | .107 | ||||||||||||||
| Male | 106 | 68.8 | 39 | 36.8 | 55 | 51.9 | 12 | 11.3 | 94 | 68.1 | 39 | 41.5 | 55 | 58.5 | ||
| Female | 48 | 31.2 | 12 | 25.0 | 32 | 66.7 | 4 | 8.3 | 44 | 31.9 | 12 | 27.3 | 32 | 72.7 | ||
| Age at diagnosis, y | .335 | .290 | ||||||||||||||
| Mean (SD) | 83.2 (3.0) | 83.7 (2.9) | 83.0 (3.0) | 83.2 (2.9) | 83.3 (3.0) | 83.7 (2.9) | 83.0 (3.0) | |||||||||
| Median (min, max) | 82 (80, 92) | 83 (80, 91) | 82 (80, 92) | 82.5 (80, 90) | 82 (80, 92) | 83 (80, 91) | 82 (80, 92) | |||||||||
| Race/ethnicity | .911 | .939 | ||||||||||||||
| White | 125 | 81.2 | 41 | 32.8 | 70 | 56.0 | 14 | 11.2 | 111 | 80.4 | 41 | 36.9 | 70 | 63.1 | ||
| Black | 5 | 3.2 | 2 | 40.0 | 3 | 60.0 | — | — | 5 | 3.6 | 2 | 40.0 | 3 | 60.0 | ||
| Hispanic | 19 | 12.3 | 7 | 36.8 | 10 | 52.6 | 2 | 10.5 | 17 | 12.3 | 7 | 41.2 | 10 | 58.8 | ||
| Other/unknown | 5 | 3.2 | 1 | 20.0 | 4 | 80.0 | — | — | 5 | 3.6 | 1 | 20.0 | 4 | 80.0 | ||
| ECOG PS score | .109 | .938 | ||||||||||||||
| 0 | 22 | 14.3 | 9 | 40.9 | 13 | 59.1 | — | — | 22 | 15.9 | 9 | 40.9 | 13 | 59.1 | ||
| 1 | 83 | 53.9 | 29 | 34.9 | 47 | 56.6 | 7 | 8.4 | 76 | 55.1 | 29 | 38.2 | 47 | 61.8 | ||
| 2 | 34 | 22.1 | 10 | 29.4 | 20 | 58.8 | 4 | 11.8 | 30 | 21.7 | 10 | 33.3 | 20 | 66.7 | ||
| ≥3 | 12 | 7.8 | 3 | 25.0 | 6 | 50.0 | 3 | 25.0 | 9 | 6.5 | 3 | 33.3 | 6 | 66.7 | ||
| Unknown | 3 | 1.9 | — | — | 1 | 33.3 | 2 | 66.7 | 1 | 0.7 | — | — | 1 | 100.0 | ||
| ELN 2017 risk | .048 | .067 | ||||||||||||||
| Favorable | 12 | 7.8 | 1 | 8.3 | 11 | 91.7 | — | — | 12 | 8.7 | 1 | 8.3 | 11 | 91.7 | ||
| Intermediate | 51 | 33.1 | 17 | 33.3 | 31 | 60.8 | 3 | 5.9 | 48 | 34.8 | 17 | 35.4 | 31 | 64.6 | ||
| Poor | 82 | 53.2 | 31 | 37.8 | 41 | 50.0 | 10 | 12.2 | 72 | 52.2 | 31 | 43.1 | 41 | 56.9 | ||
| Unknown | 9 | 5.8 | 2 | 22.2 | 4 | 44.4 | 3 | 33.3 | 6 | 4.3 | 2 | 33.3 | 4 | 66.7 | ||
| Disease status | .008 | .007 | ||||||||||||||
| Newly diagnosed | 66 | 42.9 | 13 | 19.7 | 48 | 72.7 | 5 | 7.6 | 61 | 44.2 | 13 | 21.3 | 48 | 78.7 | ||
| R/R | 15 | 9.7 | 6 | 40.0 | 5 | 33.3 | 4 | 26.7 | 11 | 8.0 | 6 | 54.5 | 5 | 45.5 | ||
| Prior MDS | 52 | 33.8 | 22 | 42.3 | 25 | 48.1 | 5 | 9.6 | 47 | 34.1 | 22 | 46.8 | 25 | 53.2 | ||
| Unknown | 21 | 13.6 | 10 | 47.6 | 9 | 42.9 | 2 | 9.5 | 19 | 13.8 | 10 | 52.6 | 9 | 47.4 | ||
| Prior treatment HMA | .001 | <.001 | ||||||||||||||
| No | 106 | 68.8 | 26 | 24.5 | 70 | 66.0 | 10 | 9.4 | 96 | 69.6 | 26 | 27.1 | 70 | 72.9 | ||
| Yes | 48 | 31.2 | 25 | 52.1 | 17 | 35.4 | 6 | 12.5 | 42 | 30.4 | 25 | 59.5 | 17 | 40.5 | ||
| VEN dose, first cycle | .058 | .574 | ||||||||||||||
| 100 | 15 | 9.7 | 3 | 20.0 | 11 | 73.3 | 1 | 6.7 | 14 | 10.1 | 3 | 21.4 | 11 | 78.6 | ||
| 200 | 12 | 7.8 | 5 | 41.7 | 6 | 50.0 | 1 | 8.3 | 11 | 8.0 | 5 | 45.5 | 6 | 54.5 | ||
| 400 | 99 | 64.3 | 34 | 34.3 | 58 | 58.6 | 7 | 7.1 | 92 | 66.7 | 34 | 37.0 | 58 | 63.0 | ||
| 600 | 9 | 5.8 | 4 | 44.4 | 5 | 55.6 | — | — | 9 | 6.5 | 4 | 44.4 | 5 | 55.6 | ||
| Unknown | 19 | 12.3 | 5 | 26.3 | 7 | 36.8 | 7 | 36.8 | 12 | 8.7 | 5 | 41.7 | 7 | 58.3 | ||
| VEN duration (d), first cycle | .003 | .347 | ||||||||||||||
| <28 | 25 | 16.2 | 10 | 40.0 | 12 | 48.0 | 3 | 12.0 | 22 | 15.9 | 10 | 45.5 | 12 | 54.5 | ||
| 28 | 116 | 75.3 | 38 | 32.8 | 71 | 61.2 | 7 | 6.0 | 109 | 79.0 | 38 | 34.9 | 71 | 65.1 | ||
| Unknown | 13 | 8.4 | 3 | 23.1 | 4 | 30.8 | 6 | 46.2 | 7 | 5.1 | 3 | 42.9 | 4 | 57.1 | ||
| VEN duration (d), first cycle | <.001 | .071 | ||||||||||||||
| ≤7 | 6 | 3.9 | 4 | 66.7 | — | — | 2 | 33.3 | 4 | 2.9 | 4 | 100.0 | — | — | ||
| 8-14 | 11 | 7.1 | 3 | 27.3 | 7 | 63.6 | 1 | 9.1 | 10 | 7.2 | 3 | 30.0 | 7 | 70.0 | ||
| 15-21 | 8 | 5.2 | 3 | 37.5 | 5 | 62.5 | — | — | 8 | 5.8 | 3 | 37.5 | 5 | 62.5 | ||
| >21 | 116 | 75.3 | 38 | 32.8 | 71 | 61.2 | 7 | 6.0 | 109 | 79.0 | 38 | 34.9 | 71 | 65.1 | ||
| NPM1 | .024 | .089 | ||||||||||||||
| No | 125 | 81.2 | 44 | 35.2 | 69 | 55.2 | 12 | 9.6 | 113 | 81.9 | 44 | 38.9 | 69 | 61.1 | ||
| Yes | 17 | 11.0 | 3 | 17.6 | 14 | 82.4 | — | — | 17 | 12.3 | 3 | 17.6 | 14 | 82.4 | ||
| Unknown | 12 | 7.8 | 4 | 33.3 | 4 | 33.3 | 4 | 33.3 | 8 | 5.8 | 4 | 50.0 | 4 | 50.0 | ||
| IDH1/2 | .160 | .366 | ||||||||||||||
| No | 114 | 74.0 | 41 | 36.0 | 64 | 56.1 | 9 | 7.9 | 105 | 76.1 | 41 | 39.0 | 64 | 61.0 | ||
| Yes | 27 | 17.5 | 7 | 25.9 | 17 | 63.0 | 3 | 11.1 | 24 | 17.4 | 7 | 29.2 | 17 | 70.8 | ||
| Unknown | 13 | 8.4 | 3 | 23.1 | 6 | 46.2 | 4 | 30.8 | 9 | 6.5 | 3 | 33.3 | 6 | 66.7 | ||
| NPM1 or IDH1/2 | .029 | .145 | ||||||||||||||
| No | 105 | 68.2 | 39 | 37.1 | 57 | 54.3 | 9 | 8.6 | 96 | 69.6 | 39 | 40.6 | 57 | 59.4 | ||
| Yes | 40 | 26.0 | 10 | 25.0 | 27 | 67.5 | 3 | 7.5 | 37 | 26.8 | 10 | 27.0 | 27 | 73.0 | ||
| Unknown | 9 | 5.8 | 2 | 22.2 | 3 | 33.3 | 4 | 44.4 | 5 | 3.6 | 2 | 40.0 | 3 | 60.0 | ||
| FLT3-ITD or FLT3-TKD | .007 | .709 | ||||||||||||||
| No | 128 | 83.1 | 44 | 34.4 | 73 | 57.0 | 11 | 8.6 | 117 | 84.8 | 44 | 37.6 | 73 | 62.4 | ||
| Yes | 23 | 14.9 | 7 | 30.4 | 14 | 60.9 | 2 | 8.7 | 21 | 15.2 | 7 | 33.3 | 14 | 66.7 | ||
| TP53 mutation | .038 | .224 | ||||||||||||||
| No | 109 | 70.8 | 34 | 31.2 | 68 | 62.4 | 7 | 6.4 | 102 | 73.9 | 34 | 33.3 | 68 | 66.7 | ||
| Yes‡ | 31 | 20.1 | 12 | 38.7 | 14 | 45.2 | 5 | 16.1 | 26 | 18.8 | 12 | 46.2 | 14 | 53.8 | ||
| Unknown | 14 | 9.1 | 5 | 35.7 | 5 | 35.7 | 4 | 28.6 | 10 | 7.2 | 5 | 50.0 | 5 | 50.0 | ||
| Complex karyotype | .035 | .221 | ||||||||||||||
| No | 99 | 64.3 | 32 | 32.3 | 61 | 61.6 | 6 | 6.1 | 93 | 67.4 | 32 | 34.4 | 61 | 65.6 | ||
| Yes§ | 43 | 27.9 | 17 | 39.5 | 20 | 46.5 | 6 | 14.0 | 37 | 26.8 | 17 | 45.9 | 20 | 54.1 | ||
| Unknown | 12 | 7.8 | 2 | 16.7 | 6 | 50.0 | 4 | 33.3 | 8 | 5.8 | 2 | 25.0 | 6 | 75.0 | ||
| TP53 mutation and complex karyotype | .006 | .421 | ||||||||||||||
| No-no | 87 | 56.5 | 28 | 32.2 | 55 | 63.2 | 4 | 4.6 | 83 | 60.1 | 28 | 33.7 | 55 | 66.3 | ||
| Yes-yes | 22 | 14.3 | 9 | 40.9 | 9 | 40.9 | 4 | 18.2 | 18 | 13.0 | 9 | 50.0 | 9 | 50.0 | ||
| Other | 41 | 26.6 | 14 | 34.1 | 22 | 53.7 | 5 | 12.2 | 36 | 26.1 | 14 | 38.9 | 22 | 61.1 | ||
| Unknown | 4 | 2.6 | — | — | 1 | 25.0 | 3 | 75.0 | 1 | 0.7 | — | — | 1 | 100.0 | ||
| Date start VEN-HMA | .964 | .830 | ||||||||||||||
| 2015-2019 | 74 | 48.1 | 25 | 33.8 | 41 | 55.4 | 8 | 10.8 | 66 | 47.8 | 25 | 37.9 | 41 | 62.1 | ||
| 2020-2023 | 80 | 51.9 | 26 | 32.5 | 46 | 57.5 | 8 | 10.0 | 72 | 52.2 | 26 | 36.1 | 46 | 63.9 | ||
max, maximum; min, minimum; NA, not applicable; SD, standard deviation.
Primary analysis performed in the ITT population; response-evaluable patients are shown for reference.
P values are calculated with either χ2 test or Fisher exact test for association categorical variables, and Student independent 2-sample t test for continuous variables excluding unknown.
Patients with TP53 mutation by disease status: R/R = 13% (2/15), prior MDS = 23% (12/52), and newly diagnosed = 24% (16/66).
Complex karyotype by disease status: R/R = 27% (4 /15), prior MDS = 35% (18/52), and newly diagnosed = 26% (17/66)
Overall, 67% of patients received the standard dose and schedule of VEN (400 mg [78%] for 28 days [77%]) and HMA (AZA 75 mg/m2 for 7 days [95%], DEC 20 mg/m2 for 5 days [100%]) for the first cycle; 72% of patients subsequently underwent a VEN dose reduction, duration reduction, and/or cycle extension. Specifically, 42% of patients had a reduction in VEN duration after the first cycle (in addition to the 23% of patients who started cycle 1 with reduced VEN duration: range, 7-21 days [median, 14 days]). The median final VEN dose and schedule in all patients was 400 mg (range, 20-400) for 21 days (range, 4-28) with a median cycle length of 35 days (range, 28-84), and in patients achieving CRc it was 200 mg (range, 50-400) for 21 days (range, 4-28) with a median cycle length of 35 days (range, 28-84). In patients achieving CRc with duration of >6 months, median final VEN duration was 14 days. Of all patients, 54% had an HMA dose reduction, most commonly from 7 to 5 days of AZA (83% of patients had an AZA dose reduction).
Patient disposition and safety
The median duration of follow-up was 7.7 months in all patients and 18.6 months (range, 0.5-49.5) in patients still alive at final data cutoff (Table 2). At the time of analysis, 36 patients (23%) remained in remission, with 31 (20%) still on VEN-HMA. The 30-day and 60-day mortality rates were 8.5% and 17%, respectively, with most early deaths due to sepsis or disease progression. Treatment-emergent grade 3-4 anemia occurred in 50%, thrombocytopenia in 48%, neutropenia in 53%, and neutropenic fever in 46%. Cause of death was attributed to progression/relapse (60%), sepsis (8%), and non-AML causes (8%), with the rest of causes unknown.
Follow-up time (in months) by event
Follow-up time (mo) . | All patients . | In CRc . | ||||
|---|---|---|---|---|---|---|
| Total . | Vital status . | Total . | Vital status . | |||
| Alive . | Dead . | Alive . | Dead . | |||
| n | 154 | 36 | 118 | 85 | 27 | 58 |
| Mean | 11.24 | 20.63 | 8.38 | 13.56 | 20.98 | 10.11 |
| Standard deviation | 11.47 | 13.36 | 9.12 | 12.05 | 12.41 | 10.27 |
| Median | 7.69 | 18.61 | 6.24 | 9.13 | 20.24 | 7.34 |
| Minimum | 0.13 | 0.49 | 0.13 | 0.26 | 0.26 | 0.76 |
| Maximum | 49.51 | 49.51 | 46.00 | 46.95 | 46.95 | 44.25 |
Follow-up time (mo) . | All patients . | In CRc . | ||||
|---|---|---|---|---|---|---|
| Total . | Vital status . | Total . | Vital status . | |||
| Alive . | Dead . | Alive . | Dead . | |||
| n | 154 | 36 | 118 | 85 | 27 | 58 |
| Mean | 11.24 | 20.63 | 8.38 | 13.56 | 20.98 | 10.11 |
| Standard deviation | 11.47 | 13.36 | 9.12 | 12.05 | 12.41 | 10.27 |
| Median | 7.69 | 18.61 | 6.24 | 9.13 | 20.24 | 7.34 |
| Minimum | 0.13 | 0.49 | 0.13 | 0.26 | 0.26 | 0.76 |
| Maximum | 49.51 | 49.51 | 46.00 | 46.95 | 46.95 | 44.25 |
Response outcomes (univariate and multivariate analysis)
In all patients (ITT population), the CRc rate was 57% (87/154) and CR rate 41% (63/154; Table 1). In patients who had a response assessment, the CRc rate was 63% (87/138) and CR rate 46% (63/138). In the ITT population, the CRc rate for newly diagnosed AML without prior MDS was 73% (48/66; Table 1; supplemental Table 1). Patients with R/R AML or newly diagnosed AML with prior MDS had lower response rates (CRc rate 33% and 48% vs 73% in newly diagnosed AML without prior MDS, P = .008). Patients with prior treatment with an HMA for MDS or AML had lower response rates (CRc 35% vs 66% without prior HMA; P = .001). Of the 12 patients with favorable risk, 11 (92%) achieved CRc (P = .048, compared with all other risk groups; Table 1); patients with adverse risk had the lowest chance of achieving CRc compared with those with favorable risk (OR, 0.17; P = .059; supplemental Table 2). All of the 12 favorable risk patients had mutated NPM1 (of the total 17 patients with an NPM1 mutation, and without either FLT3-ITD-high [n = 2], complex karyotype [wild-type TP53, n = 2], or TP53 mutation [noncomplex, n = 1]). Gene mutations associated with response included: NPM1 mutation (CRc rate, 82%; P = .024) and FLT3-ITD/TKD (CRc, 61%; P = .007). TP53 mutations and complex karyotype alone or together were associated with a lack of response (TP53 CRc rate, 45%; P = .038; complex karyotype CRc rate, 47%; P = .035; TP53 + complex CRc rate, 41%; P = .006; Table 1). A VEN duration of 28 days during cycle 1 was associated with response when compared with <28 days (CRc rate, 61% vs 48%; P = .003). However, the number of patients receiving <28 days in cycle 1 was small (n = 25), and this association was driven by patients receiving ≤7 days (P < .001; Table 1). Patients receiving 14 to 21 days had similar response outcomes compared with those receiving 28 days. The VEN dose during cycle 1 was not associated with response (n = 27 patients received <400 mg). The year VEN HMA was started was not associated with response. In MVA for CRc, only disease status at diagnosis (R/R AML or prior MDS) was associated with lack of response (OR, 0.20 [95% CI, 0.05-0.81], P = .024; and OR, 0.31 [95% CI, 0.13-0.73], P = .008) (supplemental Table 2).
Survival outcomes (univariate analysis)
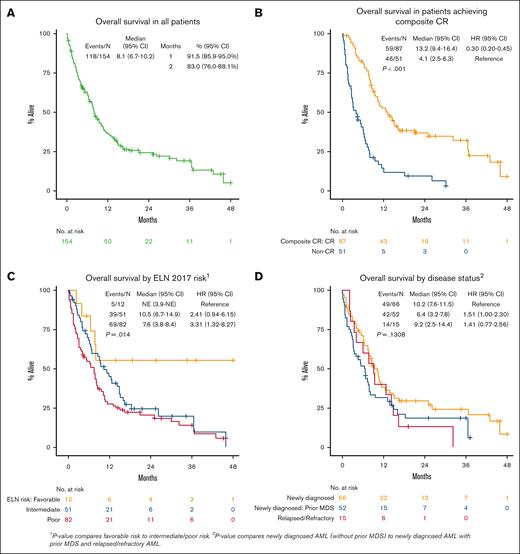
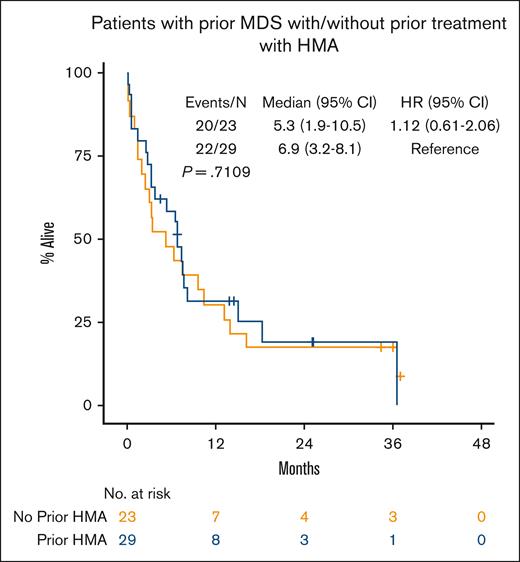
Median OS for all patients was 8.1 months (95% CI, 6.7-10.2; Figure 1A). In patients who achieved a response (CRc), median OS was 13.2 months (95% CI, 9.4-16.4) vs 4.1 months (95% CI, 2.5-6.3) in nonresponders (P < .001; Figure 1B). Patients who achieved a CR had median OS of 13.8 months (95% CI, 9.4-21.1, P < .001; supplemental Figure 1A). In patients who achieved CRc, OS from time of achieving CRc was 10.3 months (95% CI, 8.0-14.5; supplemental Figure 2). There was no difference in OS in patients who started treatment in 2015 through 2019 vs 2020 through 2022 (P = .844; supplemental Table 3). Median OS by ELN 2017 risk was not reached in favorable risk (95% CI, 3.9-NE, not estimable), 10.5 months in intermediate risk (95% CI, 6.7-14.9), and 7.6 months in adverse risk (95% CI, 3.8-8.4; favorable vs intermediate/adverse risk, P = .014; Figure 1C). Although disease status at time of initial VEN-HMA treatment favored patients with newly diagnosed AML without prior MDS, it did not have a significant effect on OS: median, 10.2 months in newly diagnosed AML without prior MDS, 6.4 months in newly diagnosed AML with prior MDS, and 9.2 months in R/R AML (P = .13; Figure 1D; supplemental Figure 1B). Patients who received a prior HMA for MDS had similar OS compared with those who were HMA naïve (median, 6.9 vs 5.3 months; P = .71; Figure 2). VEN dosing duration during cycle 1 was not associated with OS (supplemental Figure 3A), and it was numerically equivalent or better with shorter VEN duration, particularly ≤14 days (supplemental Figure 3B) and 8 to 14 days (supplemental Figure 3C), although the number of patients in these groups was relatively small.
OS of patients with newly diagnosed AML with prior MDS by prior treatment with HMA.
OS of patients with newly diagnosed AML with prior MDS by prior treatment with HMA.
Patients with wild-type TP53 with a signaling pathway mutation (n = 19 with K/NRAS and/or FLT3-ITD without TP53) had similar OS compared with patients with wild-type TP53 without a K/NRAS/FLT3-ITD mutation (n = 79, P = .84; supplemental Figure 4), whereas patients with any TP53 mutation (n = 31) had inferior survival compared with all other groups (median, 6.4 months; P = .0048; Figure 3A,D). Patients with any RAS pathway mutation (n = 20 with KRAS, NRAS, PTPN11, CBL, or NF1) had similar survival when compared with patients without a RAS pathway mutation (median, 9.1 vs 7.8 months; P = .96; Figure 3B). Patients with mutated FLT3-ITD (n = 12) had numerically improved OS compared with those with wild-type FLT3-ITD (n = 132; median OS, 12.2 vs 7.8 months; P = .44; Figure 3C). In patients with mutated FLT3-ITD without a TP53 mutation (n = 10), median OS was 19.5 months (P = .55; supplemental Figure 5). A high percentage of patients in our study had a complex karyotype (n = 43 [28%], compared with 99 noncomplex [64%] and 12 unknown [8%]) karyotypes. Patients with complex karyotype had inferior OS (median, 6.3 months; P = .0004; supplemental Figure 6A). Patients with both a complex karyotype and TP53 mutation had similarly poor OS (n = 22; median, 6.4 months; P < .001). Patients with a TP53 mutation and no complex karyotype had slightly better outcomes (n = 7; median OS, 7.9 months; supplemental Figure 6B-C). Overall, 52 total patients had a complex karyotype and/or TP53 mutation (35% of our population, 52/150).
Predictors of survival (multivariate analysis)
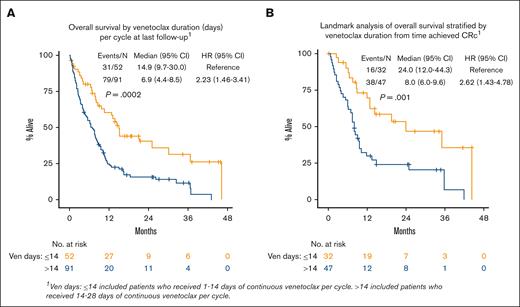
In MVA, ECOG PS, ELN 2017 risk, TP53 mutation, and disease status were associated with inferior OS: ECOG PS score of ≥3 (HR, 3.57 [95% CI, 1.35-9.45]; P = .010), intermediate risk (HR, 3.56 [95% CI, 1.07-11.87]; P = .039), adverse risk (HR, 3.31 [95% CI, 0.99-11.05]; P = .052), TP53 mutation (HR, 1.96 [95% CI, 1.14-3.36]; P = .015), and prior MDS (HR, 1.72 [95% CI, 1.08-2.73]; P = .022]; supplemental Table 3). When examining the effect of VEN exposure (dose and duration) in the multivariate model, patients receiving a shorter final VEN duration (≤14 vs >14 days) had improved OS (VEN >14 days: HR, 2.47 [95% CI, 1.52-4.02]; P < .001; Figure 4A; supplemental Table 3). Lower final VEN dose was not associated with OS, but lower doses numerically favored improved OS. Landmark analysis from the time CRc was achieved showed that patients receiving VEN ≤14 days had improved OS compared with those on VEN >14 days (median, 24.0 months [95% CI, 12.0-44.3] vs median 8.0 months [95% CI, 6.0-9.6]; P = .001; Figure 4B; supplemental Table 4).
Kaplan-Meier plots of OS by molecular subgroup, RAS pathway, FLT3-ITD, and TP53.
Kaplan-Meier plots of OS by molecular subgroup, RAS pathway, FLT3-ITD, and TP53.
Discussion
Patients of very advanced age who are diagnosed with AML represent one of our most vulnerable patient populations. Historically, these patients were often not offered any therapy, and a palliative approach was taken at diagnosis.22 The widespread use of HMAs led to a modest improvement in outcomes, and VEN-HMA combinations may represent an even better opportunity for these patients to extend survival, if they can tolerate it.
In this study, we explored the tolerability and efficacy of VEN-HMA in patients aged ≥80 years. We found that these patients have high response rates consistent with what is seen in a younger patient population. Favorable risk AML, NPM1, and FLT3 mutations were associated with improved response rates, whereas patients with R/R AML, prior MDS, prior HMA, TP53 mutations, and complex karyotype had lower response rates. Patients receiving 28 days of VEN during cycle 1 had improved response rates; however, this improvement may reflect healthier patients able to tolerate the full dose, because the benefit lost significance when examining only response-evaluable patients. Patients with a favorable risk NPM1 mutation had markedly improved survival, whereas those with intermediate or adverse risk, complex karyotype, TP53 mutation, prior MDS, and ECOG PS score of ≥3 all had worse survival. We did not observe survival differences in patients treated before or after 2020 (year of regular VEN approval). The 30-day mortality rate was similar to that observed in the VIALE-A study, and the most common cause of early death (30-day) was sepsis. OS in octogenarians and nonagenarians on our study (median, 8.1 months) was shorter than OS in all ages on VIALE-A (median, 14.7 months) but similar to a recent meta-analysis of real-world VEN-HMA studies including all age groups (median OS, 9.4 months).8,15 Importantly, 31% of patients on our study received prior HMA (excluded from VIALE-A); and these patients had inferior outcomes. Interestingly, patients with newly diagnosed AML with prior MDS had poor OS (median, 6.4 months), not affected by prior HMA exposure, likely reflecting the high proportion of patients with complex cytogenetics and/or TP53 mutation in this group and perhaps also increased frailty in older patients with prior MDS. Newly diagnosed patients without prior MDS had longer OS (median, 10.2 months), closer to but still under the VIALE-A benchmark of 14.7 months, possibly because of the advanced age of these patients, frailty, disease-specific factors, death due to non-AML causes, or other factors such as COVID-19. Patients with R/R AML had similar OS compared with those with newly diagnosed AML with prior MDS (median, 9.2 vs 6.7 months), likely reflecting the high prevalence of TP53 mutations in the prior-MDS population, extensive prior HMA exposure in both groups, and similar disease biology between the 2 groups of older patients. We acknowledge the median follow-up duration of 7.7 months as a limitation of this report. However, median follow-up was 18.6 months in surviving patients at final data cutoff, which underscores the notable response durability in a subset of patients.
Our work demonstrates that patients with AML at the extremes of older age can be treated successfully with VEN-HMA, and a subset of these patients have prolonged survival (∼20% to 25% of all patients, and ∼40% of responders). Older patients may benefit from significant dose reductions, particularly after achieving response. In patients who achieved CRc, the median final dose and duration of VEN was 200 mg daily for 21 days every 35 days. A shorter VEN treatment duration as soon as cycle 1 did not impact OS, although it did decrease the chance of achieving CRc in the minority of patients who received ≤7, which was not significant on MVA. Cycle 1 VEN duration should be determined by patient fitness with consideration of the mutational profile and markers of VEN sensitivity vs resistance, an area that needs to be further explored. A shorter final VEN duration (≤14 days vs >14 days) was associated with improved OS, suggesting that dose reductions do not negatively impact outcomes and may, in fact, improve survival. Considering the known association between achieving remission and improved OS, responding patients are a priori more likely to undergo VEN dose reductions over time, which may confound the conclusion that lower VEN dosing is associated with improved OS. However, on landmark analysis of OS from the time that CRc was achieved, a shorter final VEN duration (≤14 days) remained associated with improved OS. Therefore, for patients in remission, we propose VEN 400 mg daily for 14 days every 35 days or VEN 200 mg daily for 21 days every 35 days as potential optimal dosing regimens for octogenarians and nonagenarians, adjusted on a patient-by-patient basis to minimize myelosuppression.23 In patients with risk factors for poor OS or early mortality, earlier and more significant reductions in VEN duration (7-14 days) may be appropriate. Notably, 60-day mortality was 17% on our study, similar to the 60-day mortality on VIALE-A (15% with VEN-AZA, 17% with AZA-placebo [correspondence from AbbVie, data on file]), perhaps reflecting the reduced total VEN exposure in early cycles in our older patient population. Measurable residual disease (MRD)–driven treatment-free remission may be the ultimate step toward minimizing toxicity in very old patients or those with poor PS, because early reports suggest that subsets of patients with MRD negativity can maintain long-term remission after treatment discontinuation.15
Mutations known to be associated with VEN resistance (FLT3-ITD, RAS pathway, and TP53)16-19 have been associated with inferior clinical outcomes, with patients with mutated TP53 having the shortest OS (median, 5.5 months) on VIALE-A.24 Although TP53 mutations and complex cytogenetics were common and associated with inferior OS in our study (median, 6.4 and 6.3 months, respectively), signaling pathway mutations were not. The reason for this is unclear but could be because of the lower frequency of K/NRAS/FLT3-ITD mutations in our cohort, VEN dosing patterns, or unique disease-specific factors in patients of very advanced age (such as comutational profile and clonal hierarchy, cellular phenotype, mitochondrial dynamics, or metabolism changes). Most notably, patients with mutated FLT3-ITD had numerically improved OS compared with patients with wild-type FLT3-ITD, possibly because of the relative scarcity of FLT3-ITD in these patients (8.3%), disease-specific factors (comutational profile, etc), or the subsequent use of FLT3 inhibitors. Alternative treatment approaches are urgently needed in patients with TP53 mutation, particularly if there is also a complex karyotype (ie, likely “multihit”), which may include clinical trials, HMA alone, early reduction in VEN duration to allow for count recovery and prevent the delay of subsequent cycles, or alternative schedules of VEN-HMA such as metronomic dosing.25
Further studies on the optimal dose and schedule of VEN-HMA that incorporate MRD-guided methods are needed in this older age group, particularly when considering future triplet combinations or the sequencing of targeted therapies. Defining subsets of patients more likely to durably respond, such as those with NPM1, IDH1/2 and RUNX1 mutation, vs those that might benefit from less VEN exposure (those with TP53 mutation or prior MDS), would be beneficial in determining how intensively to treat octogenarians and nonagenarians with VEN-HMA8,21,24,26 In conclusion, those who are very old can be treated safely and effectively with combination VEN-HMA. We recommend truncated VEN dosing of 21 days at the outset, granulocyte colony-stimulating factor support for patients in remission,12 and expectations of further dose reductions and cycle extensions to ensure tolerability over the long term.
Acknowledgment
The visual abstract was created with BioRender.com.
Authorship
Contribution: J.W. and E.M. designed the research and wrote the manuscript; W.Z. and T.K.-S. analyzed the data and performed statistical analysis; E.M., J.L., M.A.S, T.B., N.S.C, J.T., S.G.I, J.G., R.M.M, J.N, I.Z., A.M., N.AA., A.K., G.M., D.A.S, D.A.P, M.R.S., C.P, R.K., and J.W. performed the research and analyzed the data; and all authors edited and approved the manuscript.
Conflict-of-interest disclosure: M.A.S. reports role on advisory boards of Bristol Myers Squibb, Kurome, and Novartis. T.B. reports role on advisory boards of MorphoSys, Sobi, Servier, and Novartis; and reports consulting role with Novartis. N.S.C. reports salary support funded by Gilead Biosciences as a part of Winn Diversity in Clinical Trials Award 2023-2025. S.G.I. served on the advisory board of MorphoSys. A.K. reports consultancy with Geron, Sobi, Servier, MorphoSys, and GM Pharma; served as consultant/speaker bureau member of AbbVie, Astellas, AstraZeneca, Immunogen, Janssen, Menarini/Stemline, Pfizer, Ryvu, Servier, Syros, and Takeda; and reports research support from AbbVie, Astellas, AstraZeneca, Daiichi Sankyo, Pfizer, and Syros. D.A.S. reports consultancy with AbbVie, Affimed Gmbh, Gilead, Incyte, Intellisphere LLC, Molecular Partners AG, PGEN Therapeutics, Inc, Takeda, and Zentalis; served on the advisory board of AvenCell, bluebird bio, Bristol Myers Squibb, Intellia, Jasper Therapeutics, Kite, Magenta Therapeutics, NKARTA, Novartis, Orbital Therapeutics, Shattuck Labs, Servier, Syndax, and Syros; and reports research funding from Aprea and Jazz. D.A.P. served as an advisory board member of, and/or consultant for, Aptevo, Rigel, Novartis, Sumitomo, Adicet, AbbVie, Syros, Qihan, Seres, Gilead, OncoVerity, Bristol Myers Squibb, Boheringer Ingelheim, Sanofi, Karyopharm, MEI, Rigel, Syndax, and Treadwell; and reports research funding from AbbVie, Teva, Karyopharm, and Bristol Myers Squibb. M.R.S. received research funding from ALX Oncology, Astex, Incyte, Takeda, and TG Therapeutics; has equity in Empath Bio, Karyopharm, and Ryvu; reports advisory board/consulting role for AbbVie, Bristol Myers Squibb, Forma, Geron, GlaxoSmithKline, Karyopharm, Ryvu, and Taiho. C.P. reports advisory board role with Pfizer, Astellas, Janssen, GlaxoSmithKline, Blueprint, Jazz Pharmaceuticals, AbbVie, Novartis, and Delbert Laboratoires; and received honoraria from AbbVie, Astellas, Servier, Menarini/Stemline, Bristol Myers Squibb, Pfizer, Amgen, Janssen, Incyte, and Novartis. R.K. reports advisory board role with Taiho, Rigel, and DS InPharmatics; reports speaker’s bureau/advisory board role with AbbVie, CTI biopharma, Jazz, Pharma Essentia, and Servio; served as consultant for Geron; and received a research grant from Bristol Myers Squibb. J.W. reports advisory board/consultancy role with Servier, Rigel, Bristol Myers Squibb, Aptose, Daiichi Sankyo, and Ativarre; and reports research support from Takeda, Rigel, and Immune Systems Key Ltd. The remaining authors declare no competing financial interests.
Correspondence: Justin Watts, University of Miami Sylvester Comprehensive Cancer Center, Division of Hematology 1475 NW 12th Ave, Miami, FL 33136; email: jxw401@miami.edu.
References
Author notes
Data are available on request from the corresponding author, Justin Watts (jxw401@miami.edu).
The online version of this article contains a data supplement.