Key Points
The enhanced utilization of native L-Asp markedly improved the treatment outcome of adolescent and young adult patients with non-Ph ALL.
Visual Abstract
The enhanced utilization of native L-asparaginase (L-Asp) aims to improve treatment outcomes for adult patients with non-Philadelphia chromosome (Ph) acute lymphoblastic leukemia (ALL). In this measurable residual disease 2014 (MRD2014) study, we modified our protocol to include an augmented dose of native L-Asp. Compared with former MRD2008, the total dose of L-Asp was raised from 36 000 U/m2 to 232 000 U/m2 in patients aged 16 to 35 and from 36 000 U/m2 to 132 000 U/m2 in patients aged 36 to 65 years. Adult patients with ALL were enrolled between January 2014 and December 2019 based on the following eligibility criteria: non-L3 ALL, age 16 to 65 years, Eastern Cooperative Oncology Group performance status of 0 to 2, and adequate liver and kidney functions (serum bilirubin ≤ 2.0 mg/dL; serum creatinine ≤ 2.0 mg/dL). The median follow-up time was 1128 days (range, 35-2400). A total of 81 patients with non-Ph ALL (40 males and 41 females; median age, 39 years [range, 16-64]) in whom MRD status was assessed were included. Complete remission was achieved in 72 patients (89%). The probability of 3-year event-free survival (EFS) and overall survival (OS) in these patients were 55% and 72%, respectively. The outcomes for patients aged 16 to 35 years demonstrated remarkable improvement. The 3-year EFS of MRD2008 at 45% significantly increased to 71% for MRD2014. Our study unequivocally demonstrated the beneficial effects of augmented use of L-Asp in this adolescent and young adult population. This trial was registered at UMIN Clinical Trials Registry as #UMIN000012382.
Introduction
With contemporary therapies, almost 90% of adult patients with Philadelphia chromosome (Ph)–negative acute lymphoblastic leukemia (ALL) will achieve hematologic or morphologic complete remission (CR), although their 5-year survival is just 40% to 50%. Leukemia relapse is the most common cause of treatment failure in ALL.1-3
Measurable residual disease (MRD) is accepted as the strongest independent prognostic factor in ALL.4-9 We previously reported that MRD monitoring is useful for determining the clinical indications for allogeneic hematopoietic stem cell transplantation (HSCT) in the treatment of ALL in CR1; in patients who are MRD negative after induction therapy, 3-year event-free survival (EFS) of 70% can be expected without HSCT.10,11
Recent studies have shown that a pediatric-inspired ALL chemotherapy protocol significantly improves treatment outcomes in adolescent and young adult (AYA) patients with ALL.12-18
In this study, that is ALL MRD2014, we prospectively monitored the MRD status after CR induction and consolidation chemotherapies in adult patients with non-Ph ALL, to optimize postremission therapies. We have modified our protocol to include an augmented dose of native Escherichia coli L-asparaginase (L-Asp), in contrast to the previous MRD2002 and MRD2008 protocols.
Patients and methods
Patient eligibility criteria
A total of 164 adult patients with ALL were enrolled in this study between January 2014 and December 2019 on the basis of the following eligibility criteria: non-L3 ALL, age 16 to 65 years, Eastern Cooperative Oncology Group performance status of 0 to 2, and adequate liver and kidney functions (serum bilirubin ≤2.0 mg/dL; serum creatinine ≤ 2.0 mg/dL). Cytogenetic studies were performed on pretreated bone marrow and unstimulated blood samples using the standard banding technique. The treatment protocol was approved by the institutional review board of each participating hospital. Written informed consent was obtained from all patients in accordance with the Declaration of Helsinki. This study was conducted by the Fukuoka Blood and Marrow Transplantation Group and registered with the University Hospital Medical Information Network (UMIN) Clinical Trials Registry (number UMIN000012382).
Treatment
Patients received a 7-day steroid prephase with oral prednisolone (PSL; 60 mg/m2 [maximum, 100 mg]), during which the presence of the BCR::ABL1 transcript was centrally confirmed. During this steroid prephase, the first intrathecal administration of methotrexate (MTX; 15 mg per body) was performed. We used a modified Cancer and Leukemia Group B (CALGB) 1980219,20 treatment protocol that comprised 6 courses of chemotherapy administered in the order of A-B-C-A-B-C regimens, followed by a maintenance phase. Induction chemotherapy (course A) was a 21-day course consisting of cyclophosphamide (1000 mg/m2 on day 1), daunorubicin (50 mg/m2 on days 1, 2, and 3), vincristine (VCR; 1.3 mg/m2 [maximum, 2 mg] on days 1, 8, 15, and 22), L-Asp (6000 U/m2 on days 9, 11, 13, 16, 18, and 20), and PSL (60 mg/m2 [maximum, 100 mg] for 21 days). Granulocyte colony-stimulating factor (filgrastim) was administered starting from day 4 and continued until neutrophil recovery. For patients aged ≥36 years, the dose of L-Asp was reduced to 3000 U/m2. For patients aged ≥56 years, the doses of cyclophosphamide were reduced to 500 mg/m2. Furthermore, PSL therapy was shortened to 7 days in these patients. The first consolidation therapy (course B) consisted of mitoxantrone (10 mg/m2 on days 2 and 3), cytarabine (AraC; 2000 mg/m2 per day on days 1, 2, 3, and 4), L-Asp (10 000 U/m2 on day 5), and intrathecal administration of MTX (15 mg per body on day 1). For patients ≥36 years, the dose of L-Asp was reduced to 6000 U/m2. For patients aged ≥55 years, the doses of mitoxantrone and AraC were reduced to 7 mg/m2 and 1500 mg/m2 per day, respectively. The second consolidation therapy (course C) consisted of VCR (1.3 mg/m2 [maximum, 2 mg] on days 1 and 15) and MTX (1500 mg/m2 on days 1 and 15), with leucovorin rescue, L-Asp (10 000 U/m2 on days 2 and 16), and intrathecal MTX (on days 1 and 15). The patients received the following maintenance chemotherapy regimen: PSL, 60 mg/m2 on days 1 to 5; VCR, 1.3 mg/m2 (maximum, 2 mg) on day 1; L-Asp, 10 000 U/m2 on day 1; oral MTX, 20 mg/m2 weekly; and oral 6-mercaptopurine, 60 mg/m2 daily every 4 weeks for 10 cycles. For patients ≥36 years, the dose of L-Asp was reduced to 6000 U/m2. Subsequently, they underwent another 10 cycles of oral MTX, 20 mg/m2 weekly; and oral 6-mercaptopurine, 60 mg/m2 daily every 4 weeks.
MRD status was assessed in our study at 2 critical points: first, after induction therapy (first course A), denoted as the end of induction (EOI); and second, after the second consolidation therapy (first course C), referred to as the end of consolidation (EOC). EOC MRD-positive patients were considered to be indicated for HSCT as soon as possible.
MRD analysis
Real-time quantitative PCR analysis of chimeric mRNA
Messenger RNA (mRNA) samples from bone marrow cells were analyzed for the presence of major and minor BCR::ABL1, ETV::RUNX1, KMT2A::AFF1, KMT2A::MLLT3, KMT2A::MLLT4, KMT2A::MLLT1, TCF3::PBX1, and SIL::TAL1 chimeric genes. Samples were amplified by real-time quantitative polymerase chain reaction (PCR) and quantified by parallel amplification of serial dilutions of transcript-containing plasmids.21,22
PCR analysis of TCR/Ig rearrangement
For quantitation of MRD, quantitative clone-specific PCR analyses for detection of clonal immunoglobulin or T-cell receptor gene rearrangements were performed as previously described.23-25 MRD quantifications were performed using allele-specific oligonucleotide primers with a sensitivity of <1 × 10–4, and the lower limit of detection of MRD positivity was defined as ≥1 × 10–4.
Statistical analysis
The primary end point of this study was 3-year EFS. Secondary end points included 3-year overall survival (OS), CR rate, protocol completion rate, and treatment-related toxicities.
Statistical analyses were performed on the data accumulated throughout August 2023. EFS was defined as the time from registration in this study to the first event (induction failure, induction death, relapse, second malignancy, and remission death) or the date of last follow-up, and OS was defined as the time from registration to the end of the trial or death. Survival curves were estimated using the Kaplan-Meier method, and the statistical significance of differences in survival was determined using the log-rank test.
The influence of prognostic factors, including age, white blood cell (WBC) count, EOI MRD, and EOC MRD on EFS, was estimated with univariable and multivariable Cox regression analysis. For multivariable analysis, variable selection was performed using the stepwise method (excluding variables with P value >.2), considering age, WBC, EOI MRD, and EOC MRD. The level of statistical significance was set at .05.
The comparison between the 2 groups was performed using Fisher exact test for categorical variables and the Mann-Whitney U test for continuous variables. All statistical analyses were performed with Easy R (EZR) (Saitama Medical Center, Jichi Medical University, Saitama, Japan) version 1.61 software.26
Results
Treatment outcome
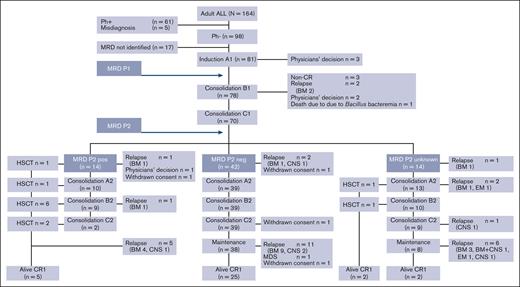
Of the 164 patients enrolled, 61 were excluded from the study because of Ph positivity and 5 because of misdiagnosis. The remaining 98 patients were Ph–; among them, MRD could be evaluated in 81 patients (Figure 1). The median follow-up time was 1128 days (range, 35-2400). A total of 81 patients (40 males and 41 females; median age, 39 years [range, 16-64]) in whom MRD status was assessed were included in the final analysis. The median WBC count at presentation was 9.9 × 109/L (range 1.0 × 109/L to 925.3 × 109/L). There were 6 cases of central nervous system (CNS) involvement on initial cerebrospinal fluid examination. CR was achieved in 72 patients (89%; Table 1).
Flow description of the results of the treatment program (ALL MRD2014 study). A total of 164 adult patients with ALL were enrolled in this study between January 2014 and December 2019. Of the 164 patients enrolled, 61 were excluded from the study because of Ph positivity and 5 due to misdiagnosis. The remaining 98 patients were Ph–; among them, MRD could be evaluated in 81 patients. We used a modified CALGB 19802 treatment protocol that comprised 6 courses of chemotherapy administered in the order of A-B-C-A-B-C regimens, followed by a maintenance phase. MRD status was assessed in our study at 2 critical points: first, after the induction therapy (first course A1), denoted as the EOI; and second, after the second consolidation therapy (first course C), referred to as the EOC. EOC MRD-positive patients were considered to be indicated for allogeneic hematopoietic stem cell transplantation (HSCT) as soon as possible. BM, bone marrow; CNS, central nervous system; EM, extramedullary.
Flow description of the results of the treatment program (ALL MRD2014 study). A total of 164 adult patients with ALL were enrolled in this study between January 2014 and December 2019. Of the 164 patients enrolled, 61 were excluded from the study because of Ph positivity and 5 due to misdiagnosis. The remaining 98 patients were Ph–; among them, MRD could be evaluated in 81 patients. We used a modified CALGB 19802 treatment protocol that comprised 6 courses of chemotherapy administered in the order of A-B-C-A-B-C regimens, followed by a maintenance phase. MRD status was assessed in our study at 2 critical points: first, after the induction therapy (first course A1), denoted as the EOI; and second, after the second consolidation therapy (first course C), referred to as the EOC. EOC MRD-positive patients were considered to be indicated for allogeneic hematopoietic stem cell transplantation (HSCT) as soon as possible. BM, bone marrow; CNS, central nervous system; EM, extramedullary.
Patient characteristics and clinical outcome
| Patient characteristics . | All . | Age, 16-35 y . | Age, 36-55 y . | Age, 56-65 y . |
|---|---|---|---|---|
| No. of patients | 81 | 34 | 34 | 13 |
| Sex, n (%) | ||||
| Male | 40 (49.4) | 17 (50.0) | 14 (41.2) | 9 (69.2) |
| Female | 41 (50.6) | 17 (50.0) | 20 (58.8) | 4 (30.8) |
| Median age (range) | 39 (16-64) | 22 (16-35) | 43 (36-52) | 61 (58-64) |
| Median WBC count (range), ×109/L | 9.9 (1.0-925.3) | 10.2 (1.9-242.4) | 12.8 (1.3-925.3) | 5.6 (1.0-183.5) |
| CNS involvement at diagnosis, n | 6 | 5 | 1 | 0 |
| Immunophenotype, n (%) | ||||
| B-lineage | 66 (81.5) | 25 (73.5) | 30 (88.2) | 11 (84.6) |
| T-lineage | 13 (16.0) | 8 (23.5) | 3 (8.8) | 2 (15.4) |
| MPAL | 2 (2.5) | 1 (3.0) | 1 (3.0) | 0 (0.0) |
| MRD, n∗ | ||||
| Chimeric mRNA | ||||
| ETV::RUNX1 | 3 | 1 | 2 | 0 |
| TCF3::PBX1 | 5 | 2 | 2 | 1 |
| KMT2A::AFF1 | 6 | 2 | 4 | 0 |
| IgH | 48 | 18 | 24 | 6 |
| Igk | 3 | 1 | 1 | 1 |
| TCRβ | 7 | 3 | 4 | 0 |
| TCRγ | 5 | 4 | 1 | 0 |
| TCRδ | 17 | 7 | 4 | 6 |
| Clinical outcome | ||||
| CR rate, n (%) | 72 (89) | 31 (91) | 30 (88) | 11 (85) |
| 3-y EFS % (95% CI) | 55 (43-65) | 71 (52-83) | 50 (32-66) | 23 (6-48) |
| 3-y OS % (95% CI) | 72 (61-81) | 85 (68-94) | 69 (50-82) | 46 (20-70) |
| Patient characteristics . | All . | Age, 16-35 y . | Age, 36-55 y . | Age, 56-65 y . |
|---|---|---|---|---|
| No. of patients | 81 | 34 | 34 | 13 |
| Sex, n (%) | ||||
| Male | 40 (49.4) | 17 (50.0) | 14 (41.2) | 9 (69.2) |
| Female | 41 (50.6) | 17 (50.0) | 20 (58.8) | 4 (30.8) |
| Median age (range) | 39 (16-64) | 22 (16-35) | 43 (36-52) | 61 (58-64) |
| Median WBC count (range), ×109/L | 9.9 (1.0-925.3) | 10.2 (1.9-242.4) | 12.8 (1.3-925.3) | 5.6 (1.0-183.5) |
| CNS involvement at diagnosis, n | 6 | 5 | 1 | 0 |
| Immunophenotype, n (%) | ||||
| B-lineage | 66 (81.5) | 25 (73.5) | 30 (88.2) | 11 (84.6) |
| T-lineage | 13 (16.0) | 8 (23.5) | 3 (8.8) | 2 (15.4) |
| MPAL | 2 (2.5) | 1 (3.0) | 1 (3.0) | 0 (0.0) |
| MRD, n∗ | ||||
| Chimeric mRNA | ||||
| ETV::RUNX1 | 3 | 1 | 2 | 0 |
| TCF3::PBX1 | 5 | 2 | 2 | 1 |
| KMT2A::AFF1 | 6 | 2 | 4 | 0 |
| IgH | 48 | 18 | 24 | 6 |
| Igk | 3 | 1 | 1 | 1 |
| TCRβ | 7 | 3 | 4 | 0 |
| TCRγ | 5 | 4 | 1 | 0 |
| TCRδ | 17 | 7 | 4 | 6 |
| Clinical outcome | ||||
| CR rate, n (%) | 72 (89) | 31 (91) | 30 (88) | 11 (85) |
| 3-y EFS % (95% CI) | 55 (43-65) | 71 (52-83) | 50 (32-66) | 23 (6-48) |
| 3-y OS % (95% CI) | 72 (61-81) | 85 (68-94) | 69 (50-82) | 46 (20-70) |
Ig, immunoglobulin; MPAL, mixed phenotype acute leukemia; TCR, T-cell receptor.
KMT2A::MLLT3, KMT2A::MLLT4, KMT2A::MLLT1, or SIL::TAL1 were not detected.
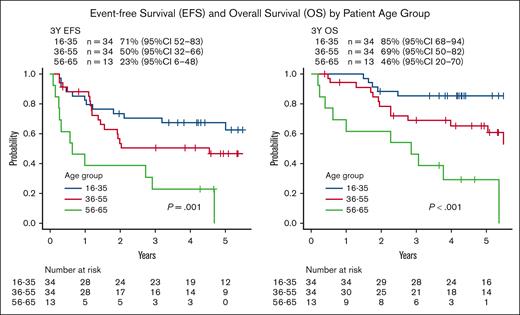
One patient died during consolidation B1 due to Bacillus bacteremia. The probability of 3-year EFS and OS in these patients with ALL was 55% (95% confidence interval [CI], 43-65) and 72% (95% CI, 61-81), respectively (Table 1; supplemental Figure 1). Analyzing the survival rates by patient age groups (16-35, 36-55, and 56-65 years), the probabilities of 3-year EFS and OS were as follows: for EFS, 71% (95% CI, 52-83) vs 50% (95% CI, 32-66) vs 23% (95% CI, 6-48; P = .001; Figure 2); and for OS, 85% (95% CI, 68-94) vs 69% (95% CI, 50-82) vs 46% (95% CI, 20-70; P < .001), respectively (Figure 2).
EFS and OS by patient age group. Analyzing the survival rates by patient age groups (16-35, 36-55, and 56-65 years), the probability of 3-year EFS and OS were as follows: for EFS, 71% (95% CI, 52-83) vs 50% (95% CI, 32-66) vs 23% (95% CI, 6-48; P = .001); and for OS, 85% (95% CI, 68-94) vs 69% (95% CI, 50-82) vs 46% (95% CI, 20-70; P < .001), respectively.
EFS and OS by patient age group. Analyzing the survival rates by patient age groups (16-35, 36-55, and 56-65 years), the probability of 3-year EFS and OS were as follows: for EFS, 71% (95% CI, 52-83) vs 50% (95% CI, 32-66) vs 23% (95% CI, 6-48; P = .001); and for OS, 85% (95% CI, 68-94) vs 69% (95% CI, 50-82) vs 46% (95% CI, 20-70; P < .001), respectively.
Relationship between MRD status and treatment outcomes
The MRD status was determined by PCR analysis of major gene rearrangements and/or chimeric mRNAs as shown in Table 1.
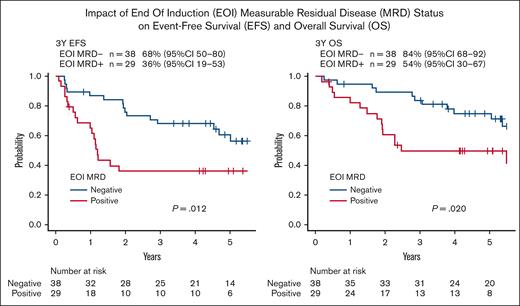
In terms of CR1 status, 3-year EFS was 68% (95% CI, 50-80) in EOI MRD-negative patients and 36% (95% CI, 19-53) in EOI MRD-positive patients (P = .012). Three-year OS was 84% (95% CI, 68-92) in EOI MRD-negative patients (n = 38) and 54% (95% CI, 30-67) in EOI MRD-positive patients (n = 29; P = .020), as shown in Figure 3. No patient proceeded to HSCT among 38 EOI MRD-negative patients in CR1. Three-year EFS was 71% (95% CI, 54-82) in EOC MRD-negative patients and 39% (95% CI, 14-63) in EOC MRD-positive patients (P = .039). Three-year OS was 88% (95% CI, 73-95) in EOC MRD-negative patients (n = 42) and 54% (95% CI, 25-76) in EOC MRD-positive patients (n = 14; P = .049), as shown in supplemental Figure 2.
Impact of EOI MRD status on EFS and OS. In terms of CR1 status, 3-year EFS was 68% (95% CI, 50-80) in EOI MRD-negative patients and 36% (95% CI, 19-53) in EOI MRD-positive patients (P = .012). Three-year OS was 84% (95% CI, 68-92) in EOI MRD-negative patients (n = 38) and 54% (95% CI, 30-67) in EOI MRD-positive patients (n = 29; P = .020).
Impact of EOI MRD status on EFS and OS. In terms of CR1 status, 3-year EFS was 68% (95% CI, 50-80) in EOI MRD-negative patients and 36% (95% CI, 19-53) in EOI MRD-positive patients (P = .012). Three-year OS was 84% (95% CI, 68-92) in EOI MRD-negative patients (n = 38) and 54% (95% CI, 30-67) in EOI MRD-positive patients (n = 29; P = .020).
Patients who were EOI MRD-positive but became EOC MRD-negative (n = 6) showed a similar 3-year EFS compared with patients who were EOI MRD-negative (67% [95% CI, 20-90] vs 68% [95% CI, 50-80]; P = .895). Fourteen patients were EOC MRD-positive and assigned to HSCT. Of these, 10 patients proceeded to HSCT in CR1, and 4 did not. Three-year EFS with or without HSCT was 50% (95% CI, 18-75) vs 0% (95% CI, 9-67; P = .022), respectively. Among 42 EOC MRD-negative patients, 13 patients relapsed (bone marrow, n = 10; isolated CNS, n = 3), and among 14 EOC MRD-positive patients, 7 patients relapsed (bone marrow, n = 6; isolated CNS, n = 1).
Table 2 shows the results of univariate and multivariate Cox regression analysis for EFS in 81 patients. The univariate analysis indicates that age (≥35 vs <35 years, hazard ratio [HR], 2.163; P = .029), EOI MRD status (positive vs negative, HR, 2.138; P = .046), and EOC MRD status (positive vs negative, HR, 2.456; P = .044) were significant prognostic factors, but WBC count (≥30 × 109/L vs <30 × 109/L, HR, 1.384; P = .337) was not. The multivariate analysis indicates that age (≥35 vs <35 years, HR, 3.966; P = .011) and EOI MRD status (positive vs negative: HR, 3.394; P = .010) remained as significant prognostic factors (Table 2).
Univariable and multivariable prognostic analysis for EFS (Cox regression model)
| Variables . | Univariable analysis . | Multivariable analysis . | ||||||
|---|---|---|---|---|---|---|---|---|
| HR . | 95% CI . | P value . | HR . | 95% CI . | P value . | |||
| Age variable, ≥35 vs <35 y | 2.126 | 1.079 | 4.189 | .029 | 3.966 | 1.378 | 11.414 | .011 |
| WBC variable, ≥30 000 vs <30 000 | 1.384 | 0.713 | 2.685 | .337 | ||||
| EOI MRD, positive vs negative | 2.138 | 1.013 | 4.512 | .046 | 3.394 | 1.343 | 8.582 | .010 |
| EOC MRD, positive vs negative | 2.456 | 1.023 | 5.897 | .044 | ||||
| Variables . | Univariable analysis . | Multivariable analysis . | ||||||
|---|---|---|---|---|---|---|---|---|
| HR . | 95% CI . | P value . | HR . | 95% CI . | P value . | |||
| Age variable, ≥35 vs <35 y | 2.126 | 1.079 | 4.189 | .029 | 3.966 | 1.378 | 11.414 | .011 |
| WBC variable, ≥30 000 vs <30 000 | 1.384 | 0.713 | 2.685 | .337 | ||||
| EOI MRD, positive vs negative | 2.138 | 1.013 | 4.512 | .046 | 3.394 | 1.343 | 8.582 | .010 |
| EOC MRD, positive vs negative | 2.456 | 1.023 | 5.897 | .044 | ||||
Toxicities
There was 1 treatment-related death, which occurred during consolidation B1 due to Bacillus bacteremia. During induction therapy, grade 3 to 4 nonhematological toxicities, which occurred in >10% of patients, included hypofibrinogenemia (28.4%), elevated transaminases (25.9%), elevated bilirubin (18%), febrile neutropenia (25.9%), and documented infections (18.5%). The frequency of events decreased markedly during postremission treatment, except for febrile neutropenia and documented infection. Overall, 5 patients (6.2%) had grade 3 to 4 hypersensitivity reactions to L-Asp throughout protocol treatment. Grade 3 pancreatitis occurred in 1 patient as shown in Table 3.
Adverse events
| Adverse event . | Induction (A1; n = 81) . | Consolidation (B1; n = 78) . | Consolidation (C1; n = 70) . | Consolidation (A2; n = 62) . | Consolidation (B2; n = 58) . | Consolidation (C2; n = 50) . | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grade 3 . | Grade 4 . | Grade 5 . | Grade 3 . | Grade 4 . | Grade 5 . | Grade 3 . | Grade 4 . | Grade 5 . | Grade 3 . | Grade 4 . | Grade 5 . | Grade 3 . | Grade 4 . | Grade 5 . | Grade 3 . | Grade 4 . | Grade 5 . | |
| Allergic/hypersensitivity | 0 | 0 | 0 | 1 1.3% | 1 1.3% | 0 | 0 | 1 1.4% | 0 | 1 1.6% | 1 1.6% | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| AST/ALT | 21 25.9% | 2 2.5% | 0 | 4 5.1% | 0 | 0 | 1 1.4% | 0 | 0 | 5 6.5% | 1 1.6% | 0 | 0 | 0 | 0 | 3 6.0% | 0 | 0 |
| Bilirubin | 8 9.9% | 1 1.2% | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Febrile neutropenia | 20 24.7% | 1 1.2% | 0 | 25 32.0% | 1 1.3% | 0 | 8 11.4% | 0 | 0 | 12 19.4% | 0 | 0 | 16 27.6% | 1 1.7% | 0 | 5 10.0% | 0 | 0 |
| Infection | 13 16.0% | 2 2.5% | 0 | 13 16.7% | 0 | 1 1.3% | 3 4.3% | 0 | 0 | 6 9.7% | 0 | 0 | 9 15.5% | 3 5.2% | 0 | 2 4.0% | 0 | 0 |
| Stomatitis | 1 1.2% | 0 | 0 | 0 | 0 | 0 | 4 5.7% | 0 | 0 | 0 | 0 | 0 | 1 1.7% | 0 | 0 | 1 2.0% | 0 | 0 |
| Pancreatitis | 1 1.2% | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hypofibrinogenemia | 21 25.9% | 2 2.5% | 0 | 0 | 0 | 0 | 2 2.9% | 0 | 0 | 10 16.0% | 1 1.6% | 0 | 0 | 0 | 0 | 2 4.0% | 0 | 0 |
| Peripheral neuropathy | 4 4.9% | 0 | 0 | 2 2.6% | 0 | 0 | 4 5.7% | 0 | 0 | 3 4.8% | 0 | 0 | 2 3.4% | 0 | 0 | 5 10.0% | 0 | 0 |
| Creatinine | 0 | 0 | 0 | 0 | 0 | 0 | 3 4.3% | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cardiac | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Adverse event . | Induction (A1; n = 81) . | Consolidation (B1; n = 78) . | Consolidation (C1; n = 70) . | Consolidation (A2; n = 62) . | Consolidation (B2; n = 58) . | Consolidation (C2; n = 50) . | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grade 3 . | Grade 4 . | Grade 5 . | Grade 3 . | Grade 4 . | Grade 5 . | Grade 3 . | Grade 4 . | Grade 5 . | Grade 3 . | Grade 4 . | Grade 5 . | Grade 3 . | Grade 4 . | Grade 5 . | Grade 3 . | Grade 4 . | Grade 5 . | |
| Allergic/hypersensitivity | 0 | 0 | 0 | 1 1.3% | 1 1.3% | 0 | 0 | 1 1.4% | 0 | 1 1.6% | 1 1.6% | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| AST/ALT | 21 25.9% | 2 2.5% | 0 | 4 5.1% | 0 | 0 | 1 1.4% | 0 | 0 | 5 6.5% | 1 1.6% | 0 | 0 | 0 | 0 | 3 6.0% | 0 | 0 |
| Bilirubin | 8 9.9% | 1 1.2% | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Febrile neutropenia | 20 24.7% | 1 1.2% | 0 | 25 32.0% | 1 1.3% | 0 | 8 11.4% | 0 | 0 | 12 19.4% | 0 | 0 | 16 27.6% | 1 1.7% | 0 | 5 10.0% | 0 | 0 |
| Infection | 13 16.0% | 2 2.5% | 0 | 13 16.7% | 0 | 1 1.3% | 3 4.3% | 0 | 0 | 6 9.7% | 0 | 0 | 9 15.5% | 3 5.2% | 0 | 2 4.0% | 0 | 0 |
| Stomatitis | 1 1.2% | 0 | 0 | 0 | 0 | 0 | 4 5.7% | 0 | 0 | 0 | 0 | 0 | 1 1.7% | 0 | 0 | 1 2.0% | 0 | 0 |
| Pancreatitis | 1 1.2% | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hypofibrinogenemia | 21 25.9% | 2 2.5% | 0 | 0 | 0 | 0 | 2 2.9% | 0 | 0 | 10 16.0% | 1 1.6% | 0 | 0 | 0 | 0 | 2 4.0% | 0 | 0 |
| Peripheral neuropathy | 4 4.9% | 0 | 0 | 2 2.6% | 0 | 0 | 4 5.7% | 0 | 0 | 3 4.8% | 0 | 0 | 2 3.4% | 0 | 0 | 5 10.0% | 0 | 0 |
| Creatinine | 0 | 0 | 0 | 0 | 0 | 0 | 3 4.3% | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cardiac | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
ALT, alanine aminotransferase; AST, aspartate aminotransferase.
Effects of truncations of L-Asp
We investigated the impact of early truncations of L-Asp on EFS and OS. For this analysis, we selected 50 patients who achieved CR and completed 5 courses of consolidation therapies. Among them, 34 patients completed L-Asp administration without modification, whereas 16 patients experienced truncations. There were no significant differences in patient or disease characteristics among these subgroups with regard to sex (P = .546), grouped age (P = .257), age (P = .738), immunophenotype (P = .137), or WBC count at diagnosis (P = .200). The main reasons for L-Asp truncation were grade 3 to 4 liver dysfunctions in 11 patients (elevated transaminases in 5 patients and elevated bilirubin in 6 patients), grade 3 to 4 allergy/anaphylaxis in 4 patients, and grade 3 pancreatitis in 1 patient. Among them, 6 were treated with L-Asp only during the induction therapy, and 10 received a truncated dose of L-Asp but completed 5 consolidation therapies (Table 4).
Patients and disease characteristics between L-Asp fully administered and L-Asp truncated
| n . | Total . | L-Asp fully administered . | L-Asp truncated . | P value . |
|---|---|---|---|---|
| 50 . | 34 . | 16 . | ||
| No. of L-Asp treatments | ||||
| Median | 6 | 6 | 6 | |
| Range | 1-6 | 6 | 1-6 | |
| Immunophenotype, n (%) | .137 | |||
| B cell | 41 (82.0) | 30 (88.2) | 11 (68.8) | |
| T cell | 8 (16.0) | 4 (11.8) | 4 (25.0) | |
| MPAL | 1 (2.0) | 0 (0.0) | 1 (6.2) | |
| WBC, n (%) | .200 | |||
| ≤30 | 33 (66.0) | 20 (58.8) | 13 (81.3) | |
| >30 | 17 (34.0) | 14 (41.2) | 3 (18.7) | |
| Sex, n (%) | .546 | |||
| Male | 23 (46.0) | 17 (50.0) | 6 (37.5) | |
| Female | 27 (54.0) | 17 (50.0) | 10 (62.5) | |
| Grouped age, n (%), y | .735 | |||
| 16-35 | 18 (36.0) | 11 (32.4) | 7 (43.8) | |
| 36-55 | 22 (44.0) | 16 (47.1) | 6 (37.5) | |
| 56-65 | 10 (20.0) | 7 (20.5) | 3 (28.7) | |
| Age, y | .257 | |||
| Median | 39 | 41.5 | 37.5 | |
| Range | 16-64 | 18-64 | 16-63 | |
| Cause of death, n (%) | .738 | |||
| N/A (alive) | 38 (76.0) | 26 (76.5) | 12 (75.0) | |
| Dead | ||||
| Not related | 1 (2.0) | 0 (0.0) | 1 (6.3) | |
| Disease related | 6 (12.0) | 5 (14.7) | 1 (6.3) | |
| Transplant related | 5 (10.0) | 3 (8.8) | 2 (12.5) |
| n . | Total . | L-Asp fully administered . | L-Asp truncated . | P value . |
|---|---|---|---|---|
| 50 . | 34 . | 16 . | ||
| No. of L-Asp treatments | ||||
| Median | 6 | 6 | 6 | |
| Range | 1-6 | 6 | 1-6 | |
| Immunophenotype, n (%) | .137 | |||
| B cell | 41 (82.0) | 30 (88.2) | 11 (68.8) | |
| T cell | 8 (16.0) | 4 (11.8) | 4 (25.0) | |
| MPAL | 1 (2.0) | 0 (0.0) | 1 (6.2) | |
| WBC, n (%) | .200 | |||
| ≤30 | 33 (66.0) | 20 (58.8) | 13 (81.3) | |
| >30 | 17 (34.0) | 14 (41.2) | 3 (18.7) | |
| Sex, n (%) | .546 | |||
| Male | 23 (46.0) | 17 (50.0) | 6 (37.5) | |
| Female | 27 (54.0) | 17 (50.0) | 10 (62.5) | |
| Grouped age, n (%), y | .735 | |||
| 16-35 | 18 (36.0) | 11 (32.4) | 7 (43.8) | |
| 36-55 | 22 (44.0) | 16 (47.1) | 6 (37.5) | |
| 56-65 | 10 (20.0) | 7 (20.5) | 3 (28.7) | |
| Age, y | .257 | |||
| Median | 39 | 41.5 | 37.5 | |
| Range | 16-64 | 18-64 | 16-63 | |
| Cause of death, n (%) | .738 | |||
| N/A (alive) | 38 (76.0) | 26 (76.5) | 12 (75.0) | |
| Dead | ||||
| Not related | 1 (2.0) | 0 (0.0) | 1 (6.3) | |
| Disease related | 6 (12.0) | 5 (14.7) | 1 (6.3) | |
| Transplant related | 5 (10.0) | 3 (8.8) | 2 (12.5) |
MPAL, mixed phenotype acute leukemia; N/A, not applicable.
Our analysis found no significant difference in 3-year EFS (64% vs 81%; P = .710) or 3-year OS (85% vs 88%; P = .852) between patients who received full administration of L-Asp and those who received truncated administration of L-Asp (supplemental Figure 3).
Discussion
Survival rates for adult patients with ALL have improved over the decades.27 However, treatment results for adult ALL have been unsatisfactory compared with those for childhood ALL.28,29
The treatment outcomes for AYA patients with ALL have shown improvement through the adoption of a pediatric-inspired chemotherapy regimen rather than an adult ALL protocol.
CALGB 10403 is a prospective study involving 295 AYAs with ALL aged 17 to 39 years, mirroring the control arm of Children's Oncology Group (COG) protocol AALL0232. It demonstrated superior outcomes for AYAs treated with the pediatric-inspired protocol, compared with a standard adult ALL protocol. The estimated 3-year OS was 73%, compared with a historical rate of 58% for CALGB patients aged 16 to 29 years.18
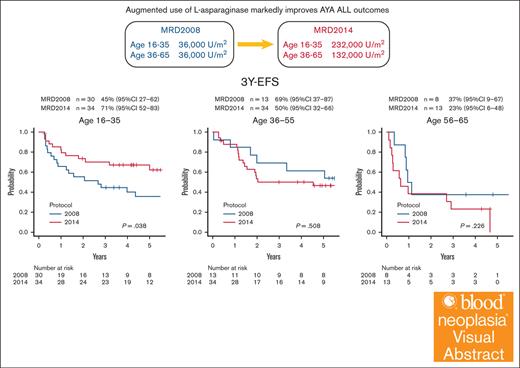
The improvement in treatment outcomes observed when transitioning from adult to pediatric protocols may be attributed to several key elements.30 According to the meta-analysis by Ram et al, pediatric-inspired protocols used higher median doses of corticosteroids (ratio of 2.2; range, 0.5-3.8), VCR (ratio of 2.5; range, 0.9-16), Asp (ratio of 2.3; range 1-420), and intrathecal MTX (ratio of 1.8; range 0.2-2.8) than conventional adult regimens. Conversely, pediatric-inspired regimens had lower median doses of daunorubicin (ratio of 0.8; range, 0.6-1.3), AraC (ratio of 0.9; range, 0.1-1.0), and etoposide (ratio of 0.1; range, 0-0.3).31 Further research and analysis are necessary to identify and understand the precise contributions of each of these elements in improving the efficacy of the treatment protocol. In this MRD2014 study, we have modified our protocol to include an augmented dose of native E. coli L-Asp, in contrast to the previous MRD200210 and MRD200811 protocols. During this clinical trial, only native E. coli L-Asp was available, and pegylated (PEG) Asp was launched in Japan in 2023. Compared with MRD2008, the total dose of L-Asp has been raised from 36 000 U/m2 to 232 000 U/m2 in patients aged 16 to 35 years and from 36 000 U/m2 to 132 000 U/m2 in patients aged 36 to 65 years.
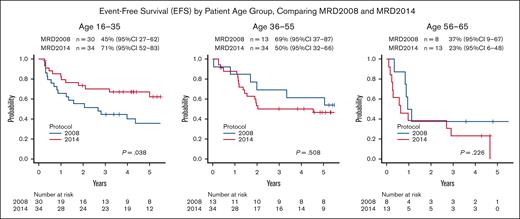
With this modification, the treatment outcomes of patients aged 16 to 35 were remarkably improved. Compared with the 3-year EFS of MRD2008 (n = 30), which was 45% (95% CI, 27-62), the 3-year EFS of MRD2014 (n = 34) was significantly improved at 71% (95% CI, 52-85; P = .038; Figure 4). The 3-year OS was also significantly improved from the 63% (95% CI, 44-78) with MRD2008 to 85% (95% CI, 68-94) with MRD2014 (P = .014), as shown in supplemental Figure 4. Thus, our study clearly demonstrated the beneficial effects of augmented use of L-Asp in this AYA population.
EFS by patient age group, comparing MRD2008 and MRD2014. The treatment outcomes of patients aged 16 to 35 years were remarkably improved. Compared with the 3-year EFS of MRD2008 (n = 30) of 45% (95% CI, 27-62), the 3-year EFS of MRD2014 (n = 34) of 71% (95% CI, 52-83) was significantly improved (P = .038). In contrast, there were no significant difference in 3-year EFS in patients aged 36 to 55 years, 69% (95% CI, 37-87) vs 50% (95% CI, 32-66; P = .508); and with patient aged 56 to 65 years, 37% (95% CI, 9-67) vs 23% (95% CI, 6-48; P = .226), respectively.
EFS by patient age group, comparing MRD2008 and MRD2014. The treatment outcomes of patients aged 16 to 35 years were remarkably improved. Compared with the 3-year EFS of MRD2008 (n = 30) of 45% (95% CI, 27-62), the 3-year EFS of MRD2014 (n = 34) of 71% (95% CI, 52-83) was significantly improved (P = .038). In contrast, there were no significant difference in 3-year EFS in patients aged 36 to 55 years, 69% (95% CI, 37-87) vs 50% (95% CI, 32-66; P = .508); and with patient aged 56 to 65 years, 37% (95% CI, 9-67) vs 23% (95% CI, 6-48; P = .226), respectively.
Regarding the schedule of L-Asp administration, in MRD2014, L-Asp was administered continuously from induction to the middle of maintenance. In contrast, in MRD2008, L-Asp was administered intermittently, specifically during the A1 induction and A2 consolidation phases. For native E. coli L-Asp, intermittent use was reported to be associated with a higher frequency of hypersensitivity reactions than continuous use.32 For PEG-Asp, a meta-analysis conducted by the Ponte di Legno Toxicity Working Group revealed that allergic reactions almost exclusively occurred after an Asp-free interval. The incidence of allergic reactions was 2% during induction and 8% during postinduction. Furthermore, the study indicated that the frequency of Asp-free intervals is an important determinant of allergic reactions.33
In the Dutch Childhood Oncology Group ALL11 randomized trial, 312 patients in the medium-risk arm were randomly assigned to receive 14 individualized PEG-Asp doses once every 2 weeks in either a noncontinuous or continuous schedule after the first 3 doses in induction. There were notable differences in hypersensitivity reactions. The 2 reactions (1.3%) in the continuous arm and 13 reactions (8.3%) in the noncontinuous arm were inactivating reactions (P < .01).34 In our study, 61 of 81 patients (75.3%) were able to complete the scheduled dose of L-Asp during induction and consolidation. Among 50 CR patients who completed consolidation therapies, 16 patients (32%) were treated with truncated L-Asp. This figure is comparable with the early discontinuation rate of PEG-Asp of 32% in the CALGB 10403 study. They reported that patients who discontinued early suffered from a nonsignificant reduction in survival.35 Although the number of patients was small, we did not find a negative impact of truncated L-Asp on EFS or OS (supplemental Figure 3). Of 16 patients, 10 completed 5 rounds of consolidation therapies with a reduced dose of L-Asp, and 6 patients were treated with L-Asp only during induction therapy. Therefore, the relatively sustained administration of L-Asp may contribute to favorable survival outcomes in patients receiving truncated L-Asp.
Although L-Asp activity was not specifically examined in our MRD2014 study, the continuous use of L-Asp, along with an increased total dose, may have contributed to the low incidence of hypersensitivity reactions and the excellent outcomes observed in AYA patients with ALL in this study. In patients aged 36 to 65 years, the increased dosage of L-Asp did not show evident efficacy. This lack of efficacy could be attributed to factors such as a higher incidence of biologically aggressive ALL or increased L-Asp toxicity. However, in this study, there was no observed tendency for heightened L-Asp toxicity among older patients. The 3-year EFS of 50% (95% CI, 32-66) and 3-year OS of 69% (95% CI, 50-82) in patients aged 35 to 55 years were not significantly improved compared with MRD2008 (Figure 2). The 3-year EFS of 85% (95% CI, 51-96) in a smaller cohort of 13 patients in MRD2008 was fairly good. Therefore, the effects of augmented use of L-Asp should be evaluated in a larger number of patients in this population. There was no significant improvement of EFS or OS in patients aged 56 to 65 years. The 3-year EFS of 23% (95% CI, 6-48) and 3-year OS of 46% (95% CI, 20-70; Figure 2) were unsatisfactory. The major cause of treatment failure in this group was relapse. Even in the 5 EOC MRD-negative patients, 3 suffered from relapse. Pediatric-inspired therapy was reported to be superior to nonintensive therapy in older people with ALL.36-38 However, the survival rate for patients age >60 years remains <30%.39 New therapeutic approaches for older adults with ALL involve integrating novel targeted agents, including monoclonal antibody–based therapies such as blinatumomab40,41 and inotuzumab ozogamicin,42,43 as frontline treatment. Thus, for these older patients with ALL, innovative approaches are strongly recommended.
Our study has several limitations. The sample size of 83 MRD-evaluable adult patients with non-Ph ALL was small, lacking sufficient statistical power to examine various prognostic factors, including the truncated use of L-Asp. The impact of L-Asp usage was assessed not through a prospective clinical trial but by comparing the results of MRD2014 study with historical controls from MRD2008 study.
We are currently conducting studies ALL MRD2019 and MRD2023, incorporating more frequent CNS prophylaxis and early use of blinatumomab for B-cell ALL. We anticipate that these studies could demonstrate the beneficial effect of early use of blinatumomab in older patients as well as AYA patients.
In conclusion, our study unequivocally demonstrated the beneficial effects of augmented use of L-Asp in this AYA population.
Authorship
Contribution: All authors recruited and treated patients for this study; K.N. designed the study, led the study, conducted correlative analysis, analyzed the data, and wrote the manuscript; K.Y. provided the statistical design and edited the manuscript; K.A. coordinated the study; and all authors reviewed and approved the final draft of the manuscript.
Conflict-of-interest disclosure: K.N. has received honoraria/fees from Kyowa Kirin Co, Chugai Pharmaceutical Co, and Asahi Kasei Pharma Co. T.M. has received honoraria/fees from Takeda Pharmaceutical Co, Otsuka Pharmaceutical Co, MSD K.K., Astellas Pharma Inc, Janssen Pharmaceutical K.K., AbbVie Inc, and Kyowa Kirin Co; and research funding from Kyowa Kirin Co and Chugai Pharmaceutical Co. K. Kato has received honoraria/fees from Novartis Pharmaceuticals, Chugai Pharmaceutical Co, and Meiji Seika Pharma Co; and research funding from Chugai Pharmaceutical Co, Takeda Pharmaceutical Co, Kyowa Kirin Co, AbbVie Inc, Novartis Pharmaceuticals, Eisai Co Ltd, Janssen Pharmaceutical K.K., Ono Pharmaceutical Co, Meiji Seika Pharma Co, Daiichi Sankyo, MSD K.K., Bristol Myers Squibb Co, Gilead Sciences Inc, and Astellas Pharma Inc. T. Kamimura has received honoraria/fees from Janssen Pharmaceutical K.K., Ono Pharmaceutical Co, and AbbVie Inc. K.A. has received honoraria/fees from Asahi Kasei Pharma Co, Astellas Pharma Inc, AstraZeneca K.K., AbbVie Inc, Kyowa Kirin Co, Chugai Pharmaceutical Co, Bristol Myers Squibb Co, and Janssen Pharmaceutical K.K.; research funding from AbbVie Inc and Kyowa Kirin Co; and scholarship endowments/academic research funding from Otsuka Pharmaceutical Co, Nippon Shinyaku Co Ltd, Taiho Pharmaceutical Co Ltd, Asahi Kasei Pharma Co, Kyowa Kirin Co, Chugai Pharmaceutical Co, Sumitomo Pharmaceutical Co Ltd, AbbVie Inc, Eisai Co Ltd, and Takeda Pharmaceutical Co. The remaining authors declare no competing financial interests.
Correspondence: Koji Nagafuji, Division of Hematology and Oncology, Department of Medicine, Kurume University School of Medicine, 67 Asahi-machi, Kurume 830-0011, Japan; email: knagafuji@med.kurume-u.ac.jp.
References
Author notes
Data are available on request from the corresponding author, Koji Nagafuji (knagafuji@med.kurume-u.ac.jp).
The full-text version of this article contains a data supplement.