Key Points
Besides marrow involvement, no differences in outcome or transformation to higher histology between CD5+ vs CD5– MZL
CD5+ MZL has similarities to CLL in terms of immunophenotype, IGVH gene mutational status, survival, and time-to-first systemic therapy.
Visual Abstract
The prognostic relevance of CD5 expression in marginal zone lymphoma (MZL) remains poorly characterized. We aimed to compare baseline characteristics and outcomes of patients with CD5+ MZL and CD5– historical matched controls. We hypothesized that patients with CD5+ MZL may have similarities to other CD5– expressing B-cell lymphomas, which may be informative when considering alternative therapeutic approaches for this MZL subgroup. We retrospectively analyzed 64 patients with CD5+ MZL and 137 CD5– MZL controls matched on age at diagnosis and sex. The CD5+ and CD5– cases did not differ in terms of mucosa assiociated lymphoid tissue (MALT)–lymphoma International Prognostic Index or incidence of nodal involvement. Bone marrow involvement was significantly more frequent in CD5+ patients than in CD5– patients (67.5% vs 47.2%; P = .048). Mutated immunoglobulin heavy chain variable region gene was more common in CD5+ patients (80.0%) than CD5– patients (64.0%), but this association was not significant (P = .327). Overall survival was calculated until death from any cause, disease-specific survival until lymphoma-related death, and time from diagnosis to first treatment was calculated either considering all interventions or only systemic treatments. None of these outcomes were associated with CD5 expression.
Introduction
Marginal zone lymphoma (MZL) is a heterogeneous group of B-cell lymphomas and is the third most common type of non-Hodgkin lymphoma after diffuse large B-cell lymphoma (DLBCL) and follicular lymphoma (FL).1,2 The World Health Organization classifies MZL into 3 subtypes: nodal MZL, extranodal MZL, and splenic MZL.3 The diagnosis of MZL is based on immunohistochemical algorithms while taking clinical presentation into account, although cases of splenic MZL are often indirectly established based on morphologic patterns of blood and bone marrow (BM) involvement.4,5 Although MZL is generally characterized by the expression of B-cell markers and the absence of CD10 and CD5, some patients with MZL (up to 17% of nodal MZL) express CD5.6 Expression of the CD5 glycoprotein prevents B cells from activation-induced cell death and maintains tolerance in anergic B cells in vivo.7 CD5 expression occurs in a wide range of B-cell lymphomas, from indolent disorders such as chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) to more aggressive diseases such as mantle cell lymphoma (MCL) and DLBCL. In DLBCL, CD5 expression predicts poor prognosis with a 5-year overall survival (OS) of 34%.8-11
The treatment landscape in the modern era for common CD5+ lymphomas such as CLL/SLL and MCL includes B-cell receptor signaling inhibitors12,13 and B-cell lymphoma 2 inhibitors.13,14 However, the current treatment options for patients with MZL are limited. Monotherapy with rituximab, an anti-CD20 monoclonal antibody, or bendamustine with rituximab are considered standard-of-care frontline treatments for MZL.15-21 However, data presented by Hsu et al suggested that rituximab monotherapy was a suboptimal selection for patients with CD5+ MZL.22 In this retrospective case-control series, we aimed to compare baseline characteristics and outcomes of patients with CD5+ MZL vs CD5– historical matched controls. We hypothesized that patients with CD5+ MZL may have similarities to other CD5–expressing B-cell lymphomas, such as CLL/SLL or MCL, and these similarities may be informative when considering alternative therapeutic approaches and expanding treatment access for this MZL subgroup.
Methods
Study population
This retrospective case-control study was approved by the institutional review board at Memorial Sloan Kettering Cancer Center (MSK) who granted a waiver of consent. This study was conducted in accordance with the Declaration of Helsinki. We reviewed patients with MZL diagnosed at MSK from 1998 to 2019. The department of hematopathology at MSK confirmed histologic diagnoses of MZL according to the World Health Organization classification of lymphoid neoplasms.3 Among 1637 pathology reports that contained the words “marginal zone” and/or “MALT,” 158 reports with ambiguous diagnoses that could not be distinguished from other indolent lymphoma subtypes, including CLL/SLL, FL, lymphoplasmacytic lymphoma, or indolent MCL, were excluded, resulting in 64 potential patients with CD5+ disease and 1415 potential patients with CD5– disease. A 1:3 matching of CD5+ to CD5–patientswas attempted based on age at diagnosis and sex (Figure 1). Random matching resulted in 60 CD5+ cases with 3 matches each, 3 CD5+ cases with 1 match each, and 1 CD5+ case with no matches, resulting in a total of 183 potential CD5–patients. Full manual chart review was performed on the 64 CD5+ cases and 183 CD5– matched controls. All 64 CD5+ cases were confirmed to have MZL diagnoses and received treatment and follow-up at MSK. Of the 183 CD5– matched controls, 13 patients had alternative diagnoses (including FL, CLL, adenocarcinoma, and chronic myelomonocytic leukemia), and 33 patients were pathology consults only with no available follow-up at MSK. These 46 patients were excluded, resulting in 137 CD5– MZL matched controls.
CONSORT diagram of inclusion and exclusion criteria for patients with CD5+ MZL (n = 64) and CD5– MZL matched controls (n = 137). cMMoL, chronic myelomonocytic leukemia.
CONSORT diagram of inclusion and exclusion criteria for patients with CD5+ MZL (n = 64) and CD5– MZL matched controls (n = 137). cMMoL, chronic myelomonocytic leukemia.
Statistical analysis
Patient and disease characteristics were summarized using descriptive statistics. The comparison of baseline factors between the CD5+ and CD5–patientswas done using the Wilcoxon rank-sum test for continuous variables and the Fisher exact test for categorical variables. OS was defined as the time from disease diagnosis until death from any cause. Disease-specific OS was defined as the time from disease diagnosis until lymphoma-related death. Patients alive were censored at their last follow-up. OS was estimated using the Kaplan-Meier method, and the standard Cox proportional hazards model was used to compare the hazard of death. Time-to-first treatment (TT1T) was calculated from the time of disease diagnosis until the start of first local or systemic treatment as well as until first systemic treatment only. Patients alive without receiving treatment were censored at their last follow-up. TT1T was estimated using the subdistribution function to adjust for the semicompeting risk of death on treatment initiation.23,24 A modified proportional hazards model was used to compare the subdistribution hazard of initiating therapy.25 The median follow-up was estimated using the reverse Kaplan-Meier method. A 2-sided P value <.05 was considered statistically significant. Statistical analyses were done using SAS 9.4 (SAS Institute Inc, Cary, NC). Although there may be some interphysician variability in treatment initiation, the decision for starting treatment at MSK is discussed as a group and recommended based on Groupe d'Etude des Lymphoms Folliculaires (GELF) criteria.
Results
Patient characteristics
In our study population of 201 patients with MZL (64 CD5+ and 137 CD5–), the median age at diagnosis was 64 years (range, 22-83) with a slight male predominance (52.7%). At baseline, 168 patients (83.5%) had extranodal disease; sites of extranodal disease are the spleen (n = 35 [20.8%]), stomach (n = 29 [17.3%]), and lungs (n = 19 [11.3%]). Among 179 patients with available data to calculate the MALT–lymphoma International Prognostic Index (MALT-IPI),26 the distribution was as follows: low risk 38.5% (n = 69), intermediate risk 40.2% (n = 72), and high risk 21.2% (n = 38); and 9 of 179 (5.0%) experienced transformation to an aggressive lymphoma. Among 112 patients who underwent BM assessment, 61 (54.5%) had BM involvement. Of the 45 patients with immunoglobulin heavy chain variable region gene (IGHV) mutational status available, 32 (71.1%) had IGHV mutated genes. At a median follow-up of 5.7 years (95% confidence interval [CI], 4.9-7.3), 150 patients (74.6%) had received first-line treatment for MZL, and 96 patients (47.8%) had received systemic treatment for MZL. Of 201 patients with MZL, 9 experienced transformation to DLBCL. The median time from MZL diagnosis to transformation was 4.2 years (range, 0.2-15.4). Of the 64 CD5+patients, 4 experienced transformation, 2 of which occurred in untreated patients; the median time from MZL diagnosis to transformation was 0.7 years (range, 0.2-4.4). Of the 137 CD5– patients, 5 experienced transformation, all of which occurred in previously treated patients; the median time from MZL diagnosis to transformation was 6.5 years (range, 1.1-15.4).
CD5+ vs CD5– MZL baseline characteristics
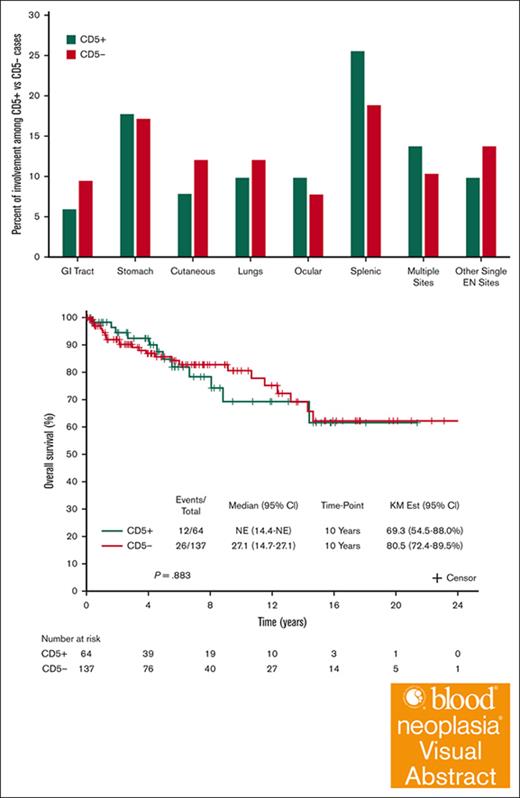
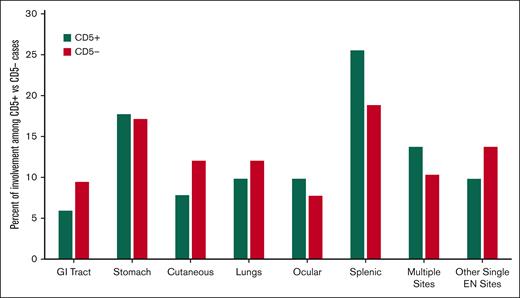
Comparison of baseline characteristics between CD5+ and CD5– MZL are summarized in Table 1 and Figure 2. CD5+ and CD5– cases did not differ in terms MALT-IPI or by the 3 MZL subtypes. Nodal involvement appeared more common in CD5+ patients (20.3%) than CD5–patients (14.6%). The most common extranodal site was the spleen (n = 35). Spleen involvement appeared more frequent among CD5+ (25.5%) than CD5–(18.8%) patients. Advanced stage disease appeared more frequent among CD5+ (59%) than CD5– patients (43%). This difference was most pronounced in the extranodal MZL subtype. Among 112 patients who underwent BM assessment, the incidence of BM involvement was higher in CD5+ than in CD5– patients (67.5% vs 47.2%, respectively). Among the 45 patients with available IGHV mutational status, mutated IGHV appeared more common in the CD5+ patients (80.0%) than in CD5– patients (64.0%). Although the data showed most of these associations as nonsignificant, the small sample size of our cohort likely does not have the statistical power to demonstrate such differences.
Comparing baseline characteristics and treatment between CD5+ vs CD5– MZL
| Characteristic . | CD5+ (n = 64) . | CD5– (n = 137) . | P value∗ . |
|---|---|---|---|
| Age at diagnosis, y | |||
| Median (range) | 64 (24-83) | 64 (22-82) | .716 |
| Q1, Q3 | 51, 71 | 55, 70 | |
| Sex, n | |||
| Male | 35/64 (54.7%) | 71/137 (51.8%) | .763 |
| Female | 29/64 (45.3%) | 66/137 (48.2%) | |
| MZL subtype, n | |||
| NMZL | 13/64 (20.3%) | 20/137 (14.6%) | .379 |
| ENMZL | 38/64 (59.4%) | 95/137 (69.3%) | |
| SMZL | 13/64 (20.3%) | 22/137 (16.1%) | |
| Nodal vs extranodal disease | |||
| Nodal disease | 13/64 (20.3%) | 20/137 (14.6%) | .314 |
| Extranodal disease | 51/64 (79.7%) | 117/137 (85.4%) | |
| Spleen involvement, n | |||
| Yes | 13/51 (25.5%) | 22/117 (18.8%) | .409 |
| No | 38/51 (74.5%) | 95/117 (81.2%) | |
| Stage, n | |||
| I-II | 26/64 (41%) | 78/137 (57%) | .035 |
| III-IV | 38/64 (59%) | 59/137 (43%) | |
| NMZL stage I-II | 3/13 (23%) | 6/20 (30%) | .999 |
| Stage III-IV | 10/13 (77%) | 14/20 (70%) | |
| ENMZL stage I-II | 22/38 (58%) | 71/95 (75%) | .063 |
| Stage III-IV | 16/38 (42%) | 24/95 (25%) | |
| SMZL stage I-II | 1/13 (8%) | 1/22 (5%) | .999 |
| Stage III-IV | 12/13 (92%) | 21/22 (95%) | |
| MALT-IPI score, n | |||
| Low risk (0 points) | 19/58 (32.8%) | 50/121 (41.3%) | .451 |
| Intermediate risk (1 point) | 27/58 (46.6%) | 45/121 (37.2%) | |
| High risk (2-3 points) | 12/58 (20.7%) | 26/121 (21.5%) | |
| Transformation to DLBCL, n | |||
| Yes | 4/58 (6.9%) | 5/121 (4.1%) | .474 |
| No | 54/58 (93.1%) | 5/21 (95.9%) | |
| BM involvement, n | |||
| Yes | 27/40 (67.5%) | 34/72 (47.2%) | .048 |
| No | 13/40 (32.5%) | 38/72 (52.8%) | |
| IGHV mutational status, n | |||
| Mutated | 16/20 (80.0%) | 16/25 (64.0%) | .327 |
| unmutated | 4/20 (20.0%) | 9/25 (36.0%) | |
| LDH > ULN, n | |||
| Yes | 6/8 (10.3%) | 21/121 (17.4%) | .269 |
| No | 52/58 (89.7%) | 100/121 (82.6%) | |
| First-line treatment received, n | |||
| Chemoimmunotherapy | 10/64 (16%) | 26/137 (19%) | .347 |
| R-monotherapy | 16/64 (25%) | 30/137 (22%) | |
| Radiation | 16/64 (25%) | 36/137 (26%) | |
| Excision | 2/64 (3%) | 14/137 (10%) | |
| Never treated | 20/64 (31%) | 31/137 (23 %) |
| Characteristic . | CD5+ (n = 64) . | CD5– (n = 137) . | P value∗ . |
|---|---|---|---|
| Age at diagnosis, y | |||
| Median (range) | 64 (24-83) | 64 (22-82) | .716 |
| Q1, Q3 | 51, 71 | 55, 70 | |
| Sex, n | |||
| Male | 35/64 (54.7%) | 71/137 (51.8%) | .763 |
| Female | 29/64 (45.3%) | 66/137 (48.2%) | |
| MZL subtype, n | |||
| NMZL | 13/64 (20.3%) | 20/137 (14.6%) | .379 |
| ENMZL | 38/64 (59.4%) | 95/137 (69.3%) | |
| SMZL | 13/64 (20.3%) | 22/137 (16.1%) | |
| Nodal vs extranodal disease | |||
| Nodal disease | 13/64 (20.3%) | 20/137 (14.6%) | .314 |
| Extranodal disease | 51/64 (79.7%) | 117/137 (85.4%) | |
| Spleen involvement, n | |||
| Yes | 13/51 (25.5%) | 22/117 (18.8%) | .409 |
| No | 38/51 (74.5%) | 95/117 (81.2%) | |
| Stage, n | |||
| I-II | 26/64 (41%) | 78/137 (57%) | .035 |
| III-IV | 38/64 (59%) | 59/137 (43%) | |
| NMZL stage I-II | 3/13 (23%) | 6/20 (30%) | .999 |
| Stage III-IV | 10/13 (77%) | 14/20 (70%) | |
| ENMZL stage I-II | 22/38 (58%) | 71/95 (75%) | .063 |
| Stage III-IV | 16/38 (42%) | 24/95 (25%) | |
| SMZL stage I-II | 1/13 (8%) | 1/22 (5%) | .999 |
| Stage III-IV | 12/13 (92%) | 21/22 (95%) | |
| MALT-IPI score, n | |||
| Low risk (0 points) | 19/58 (32.8%) | 50/121 (41.3%) | .451 |
| Intermediate risk (1 point) | 27/58 (46.6%) | 45/121 (37.2%) | |
| High risk (2-3 points) | 12/58 (20.7%) | 26/121 (21.5%) | |
| Transformation to DLBCL, n | |||
| Yes | 4/58 (6.9%) | 5/121 (4.1%) | .474 |
| No | 54/58 (93.1%) | 5/21 (95.9%) | |
| BM involvement, n | |||
| Yes | 27/40 (67.5%) | 34/72 (47.2%) | .048 |
| No | 13/40 (32.5%) | 38/72 (52.8%) | |
| IGHV mutational status, n | |||
| Mutated | 16/20 (80.0%) | 16/25 (64.0%) | .327 |
| unmutated | 4/20 (20.0%) | 9/25 (36.0%) | |
| LDH > ULN, n | |||
| Yes | 6/8 (10.3%) | 21/121 (17.4%) | .269 |
| No | 52/58 (89.7%) | 100/121 (82.6%) | |
| First-line treatment received, n | |||
| Chemoimmunotherapy | 10/64 (16%) | 26/137 (19%) | .347 |
| R-monotherapy | 16/64 (25%) | 30/137 (22%) | |
| Radiation | 16/64 (25%) | 36/137 (26%) | |
| Excision | 2/64 (3%) | 14/137 (10%) | |
| Never treated | 20/64 (31%) | 31/137 (23 %) |
ENMZL, extranodal MZL; LDH, lactate dehydrogenase; NMZL, nodal MZL; Q, quartile; R-IPI, revised IPI; R-monotherapy, Rituximab-monotherapy; SMZL, splenic marginal zone lymphoma; UNL, upper normal limit.
Comparison was done using the Wilcoxon rank-sum test for continuous variables and the Fisher exact test for categorical variables.
Localization of extranodal (EN) disease among patients with CD5+ vs CD5– MZL. GI, gastrointestinal.
Localization of extranodal (EN) disease among patients with CD5+ vs CD5– MZL. GI, gastrointestinal.
Survival outcomes and prognosis
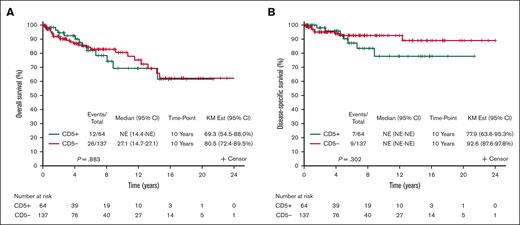
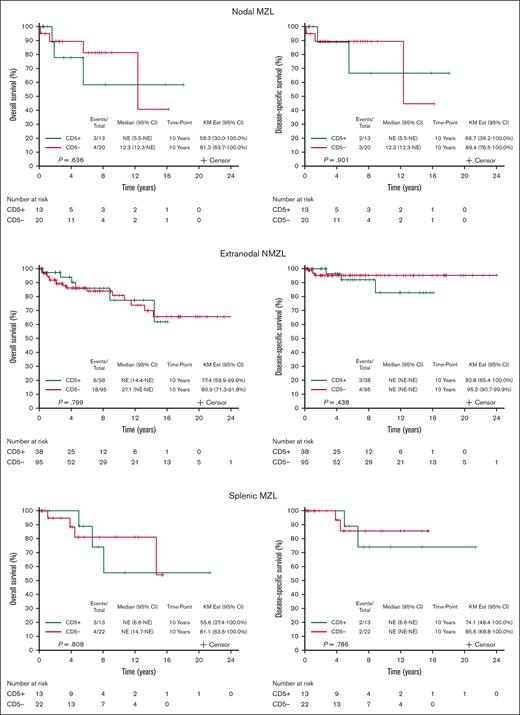
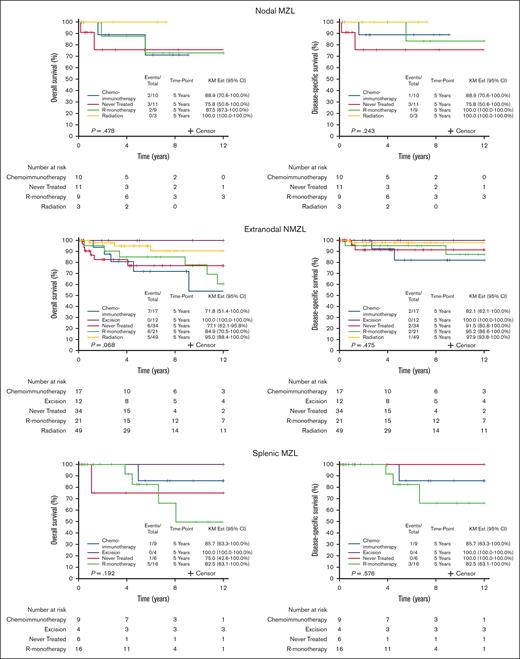
The survival outcomes are reported after a median follow-up of 5.7 years (95% CI, 4.9-7.3). Among 179 patients with available data to calculate the MALT-IPI, CD5 expression was not associated with transformation to an aggressive lymphoma: 4 patients with CD5+ MZL (6.9%) and 5 patients with CD5– MZL (4.1%) experienced transformation to DLBCL (P = .474; Table 1). When considering death from any cause, the 10-year OS was 69.3% (95% CI, 54.5-88.0) and 80.5% (95% CI, 72.4-89.5) for CD5+ and CD5– MZL, respectively; OS, considering death from any cause, did not differ by CD5 expression (P = .883; Figure 3A). The 10-year disease-specific survival was 77.9% (95% CI, 63.6-95.3) and 92.6% (95% CI, 87.6-97.8) for CD5+ and CD5– MZL, respectively (P = .302; Figure 3B). Comparing outcomes between CD5+ vs CD5– separately in the 3 MZL subtypes also shows no differences within the 3 subtypes (Figure 4). Outcome in the 3 MZL subtypes by treatment is shown in Figure 5.
Survival. (A) OS of CD5+ vs CD5– MZL. (B) Disease-specific survival of CD5+ vs CD5– MZL. KM Est, Kaplan Meier estimate).
Survival. (A) OS of CD5+ vs CD5– MZL. (B) Disease-specific survival of CD5+ vs CD5– MZL. KM Est, Kaplan Meier estimate).
OS and disease-specific survival of CD5+ vs CD5– MZL in the 3 MZL subtypes: nodal MZL (NMZL), extranodal MZL (ENMZL), and splenic MZL (SMZL). KM Est, Kaplan Meier estimate.
OS and disease-specific survival of CD5+ vs CD5– MZL in the 3 MZL subtypes: nodal MZL (NMZL), extranodal MZL (ENMZL), and splenic MZL (SMZL). KM Est, Kaplan Meier estimate.
OS and disease-specific survival by treatment in the 3 MZL subtypes: NMZL, ENMZL, and SMZL. R-monotherapy, rituximab-monotherapy.
OS and disease-specific survival by treatment in the 3 MZL subtypes: NMZL, ENMZL, and SMZL. R-monotherapy, rituximab-monotherapy.
TT1T
TT1T was calculated from the time of disease diagnosis until the start of the first systemic or local treatment (including radiotherapy, excisional biopsies, and topical treatments) as well as until first systemic treatment only (excluding topical and localized treatments). When considering all treatments, the median TT1T was 1.5 years (95% CI, 0.4-3.1) and 0.4 years (95% CI, 0.3-0.9), and the 10-year risk of needing first treatment was 70.6% (95% CI, 58.7-85.0) and 86.2% (95% CI, 78.9-94.1) for CD5+ and CD5– MZL, respectively. TT1T, considering all treatments, did not differ by CD5 expression (P = .150). When considering systemic treatment only, the median TT1T was 4.7 years (95% CI, 1.9-12.9) and 8.0 years (95% CI, 3.5 to not reached), and the 10-year risk of needing first systemic treatment was 60.4% (95% CI, 46.2-79.1) and 56.6% (95% CI, 46.4-69.1) for CD5+ and CD5– MZL, respectively; TT1T, considering systemic treatment only, did not differ by CD5 expression (P = .609; Figure 6).
Comparing time-to-first systemic treatment between patients with CD5+ vs CD5– MZL. NE, not estimable.
Comparing time-to-first systemic treatment between patients with CD5+ vs CD5– MZL. NE, not estimable.
Discussion
To our knowledge, this is the first retrospective case-control analysis in MZL exploring the association between CD5 expression and clinical presentation, disease prognosis, and histologic transformation to more aggressive lymphoma. Similar to previous reports, our data did not demonstrate any association between CD5 expression and disease prognosis. We observed an increased frequency of BM involvement in CD5+ MZL, which has been noted in other studies,6,22,27,28 but no associations between CD5 expression and other clinicopathologic characteristics were observed. The small sample size of our cohort likely does not have the statistical power to demonstrate such differences.
As seen in Figure 5, the outcome was generally quite good in our cohort. However, treatment selection for individual patients was biased by the clinical presentation. Patients with localized disease tended to have an excision or treated with radiation, which likely reflected the better outcome with these patients. However, patients who received rituximab monotherapy and chemoimmunotherapy tended to have systemic disease, which may account for some of the outcome differences observed. We believe that the varying outcome by treatment more likely reflects selection bias than treatment effect.
In this study, we extend observations from prior series regarding the lack of prognostic impact of CD5 expression on survival outcomes. Baseggio et al compared 24 CD5+ and 42 unmatched CD5– patients with MZL and found no differences in clinical outcome.28 In a retrospective unmatched case series by Hsu et al22 with 244 patients with MZL, of whom 25 were CD5+, CD5 expression was not associated with progression-free survival or OS. Similarly, Xia et al29 identified 204 patients with MZL, of whom 48 were CD5+, and observed no difference in disease prognosis between CD5+ and CD5– patients. In our case-control series of 64 CD5+ patients and 137 CD5– historical matched controls, OS was calculated until death from any cause, disease-specific survival until lymphoma-related death, and TT1T was calculated either considering all interventions or only systemic treatments. None of these outcomes were associated with CD5 expression.
CD5+ expression typically serves as a distinctive marker differentiating CLL/SLL and MCL from MZL. The expression of CD5 in MZL warrants its comparison with other CD5+ small B-cell lymphomas, particularly CLL/SLL and MCL, in terms of their clinical characteristics and prognosis. BM involvement is nearly universal in CLL/SLL, whereas MCL involves the BM in 40% to 80% of cases. The prevalence of mutated IGHV genes (<97% to 98% germ line identity) is ∼60% among newly diagnosed and asymptomatic patients with CLL30 and ∼36% among patients with MCL.31 In CLL/SLL, the median TT1T (which is predominantly systemic therapy) has been reported to be ∼5 to 7 years, with the 10-year OS (death from any cause) to be ∼70% to 75%.32 In MCL, the median TT1T (standard-of-care is systemic therapy) has been reported to be >1 year and the 10-year OS (death from any cause) ∼50%.33,34 Among the patients with CD5+ MZL in our present series, 68% had BM involvement, 80% had mutated IGHV, the median time-to-first systemic treatment was 4.7 years, and the 10-year OS (death from any cause) was 69%. We propose that CD5+ MZL is more similar to CLL than to MCL in terms of clinicopathologic features and disease outcomes.
This study is limited by its relatively small sample size and retrospective study design. Further research with larger cohorts is necessary to confirm our findings of increased frequency of BM involvement in CD5+ MZL and lack of prognostic association between CD5 expression and clinical outcome. The current standard first-line treatment for MZL include bendamustine with rituximab and rituximab monotherapy.15-21 However, data presented by Hsu et al suggested that rituximab monotherapy is a suboptimal selection for patients with CD5+ MZL.22 The similarities between CD5+ MZL and CLL/SLL raise the question of whether first-line therapeutic options for CD5+ MZL can be expanded to include targeted CLL treatment paradigms involving B-cell receptor signaling inhibitors and B-cell lymphoma 2 inhibitors. At MSK, we are conducting a multicenter randomized trial in MZL exploring the efficacy of combining rituximab with Bruton tyrosine kinase inhibitors compared with rituximab alone. If there are adequate patient numbers, analysis of the CD5+ cases may be informative.
Acknowledgment
This research was funded in part by the National Institutes of Health/National Cancer Institute Cancer Center Support Grant (P30 CD008748).
Authorship
Contribution: A.D.Z. and P.G. idealized the project; P.G., A.D.Z., K.S.B., and E.J. designed the study; A.D. reviewed the pathology of the cases; P.G., K.S.B., and A.D.Z. wrote the paper; K.S.B. analyzed the data; A.D.Z., M.L.P., A.N., P.C., P.H., A.K., M.M., C.O., A.M., L.F., D.S., and S.H. clinically treated and followed the patients; and all authors approved the final version of the manuscript.
Conflict-of-interest disclosure: E.J. reports membership on advisory board of Epizyme and AstraZeneca. M.L.P. reports honoraria from Pharmacyclics, Celgene, Merck, Novartis, Regeneron, and Juno Therapeutics, a Bristol Myers Squibb company; and research funding from Genentech and Juno Therapeutics. A.N. reports research funding from Rafael Pharma, Janssen, and National Institutes of Health; and consultancy fees from Pharmacyclics, Medscape, Targeted Oncology, MorphoSys, and Pharmacyclics. M.M. reports consultancy fees, honoraria, and research funding from Genentech Inc, Bayer, and Immunovaccine; and consultancy fees from Merck, Bayer, Juno Therapeutics, F. Hoffmann-La Roche Ltd, Technologies, Daiichi Sankyo, Seattle Genetics IGM Biosciences, Janssen, Pharmacyclics, Rocket Medical, Takeda, GlaxoSmithKline, and Teva. P.H. reports research funding from J&J Pharmaceuticals, Portola, Molecular Templates, and Incyte; and consultancy fees from Celgene, Karyopharm, and Juno Therapeutics. A.K. reports honoraria from Kite Pharmaceuticals, AstraZeneca, Celgene, and Seattle Genetics; and research funding from Pharmacyclics, Celgene, Adaptive Biotechnologies, and AbbVie. A.M. reports consultancy fees from Seattle Genetics, miRagen Therapeutics, Imbrium Therapeutics, L.P., and Merck; and research funding from Bristol Myers Squibb, Incyte, Seattle Genetics, Merck. L.F. reports research funding from Genmab and Roche. D.S. reports consultancy fees and speakers bureau fees from Targeted Oncology, Seattle Genetics, Elsevier, and OncLive; research funding from Takeda Pharmaceuticals; and consultancy fees from Imedex, Inc and Karyopharm Therapeutics. S.H. reports consultancy and research funding from Aileron, ADCT Therapeutics, Celgene, Forty Seven, Infinity/Verastem Oncology, Kyowa Hakka Kirin, Millenium/Takeda, Seattle Genetics, Trillium, Corvus, Innate Pharma, Mundipharma, Portola, BeiGene, C4 Therapeutics, Daiichi Sankyo, GlaxoSmithKline, Janssen, Kura Oncology, miRagen, Myeloid Therapeutics, Verastem Oncology, Vividion Therapeutics, Affirmed, and Astex. A.D. reports consultancy fees from Takeda and Roche; research funding from Physicians Education Resource, Corvus Pharmaceuticals, Seattle Genetics, EUSA Pharma, AbbVie, and National Cancer Institute. A.D.Z. serves on the board of directors or advisory committees for BeiGene; has received consultancy fees from Adaptive Biotechnology, Novartis, Amgen, Janssen, Celgene, Genentech/Roche, and Gilead; and research funding from Roche, Celgene, Sandoz, MorphoSys, and MEI Pharma. The remaining authors declare no competing financial interests.
Correspondence: Paola Ghione, Memorial Sloan Kettering Cancer Center. 530 East 74th St, New York, NY 10021; email: ghionep@mskcc.org.
References
Author notes
A.D. and A.D.Z. are joint senior authors.
Data are available on request from the corresponding author, Paola Ghione (ghionep@mskcc.org).